Tumoral C2 Regulates the Tumor Microenvironment by Increasing the Ratio of M1/M2 Macrophages and Tertiary Lymphoid Structures to Improve Prognosis in Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Preprocessing
2.2. Cell Culture and Transfection
2.3. RNA-Seq Data Analysis
2.4. Single-Cell RNA-Seq Filtrating and Processing
2.5. Tumor Microenvironment and Immune Infiltrate Analysis
2.6. Cell–Cell Interaction Analysis
2.7. Pharmacotherapy Prediction Response
2.8. Immunohistochemical Staining, Immunofluorescence, and Immunocytochemistry
2.9. Western Blot
2.10. Mouse Models
2.11. RT-PCR
2.12. Cell Counting Kit-8 (CCK8) Assay
2.13. Flow Cytometry
2.14. Preparation of Conditioned Medium (CM) and Macrophage Induction
2.15. Statistical Analysis
3. Results
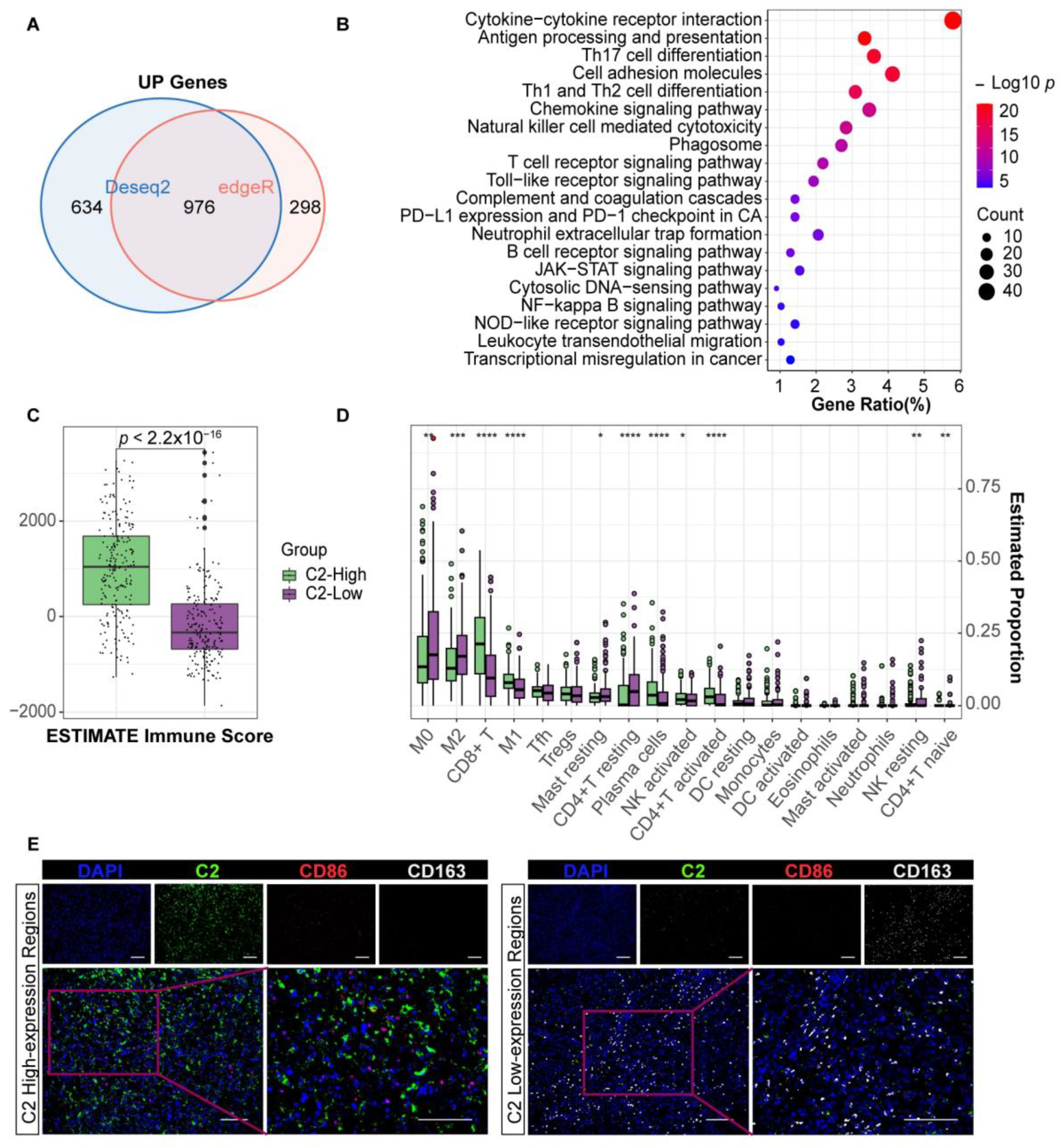
3.1. The Clinical Value of Complement C2 in Melanoma
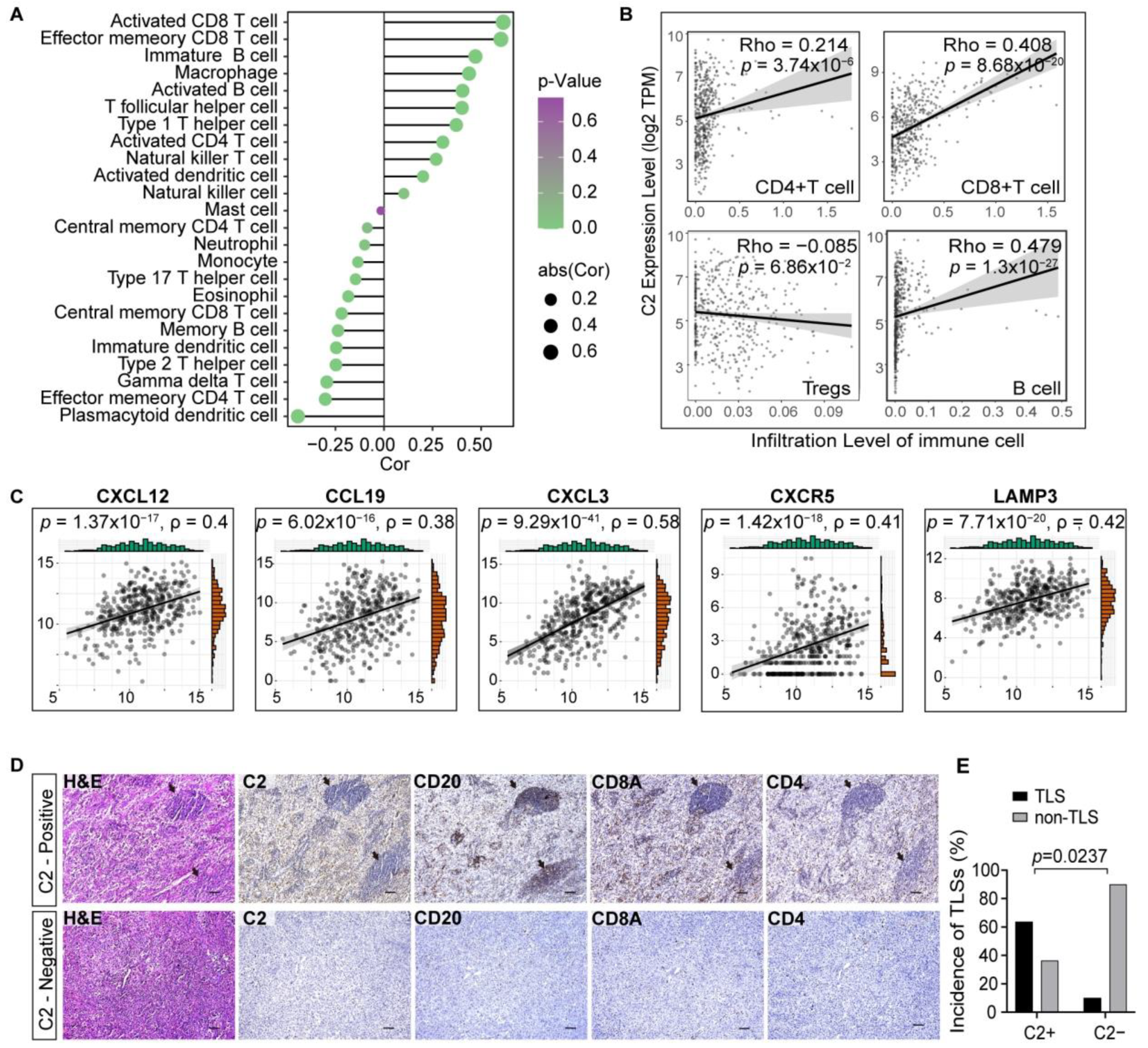
3.2. Complement C2 Implies a More Advantageous Immune Status
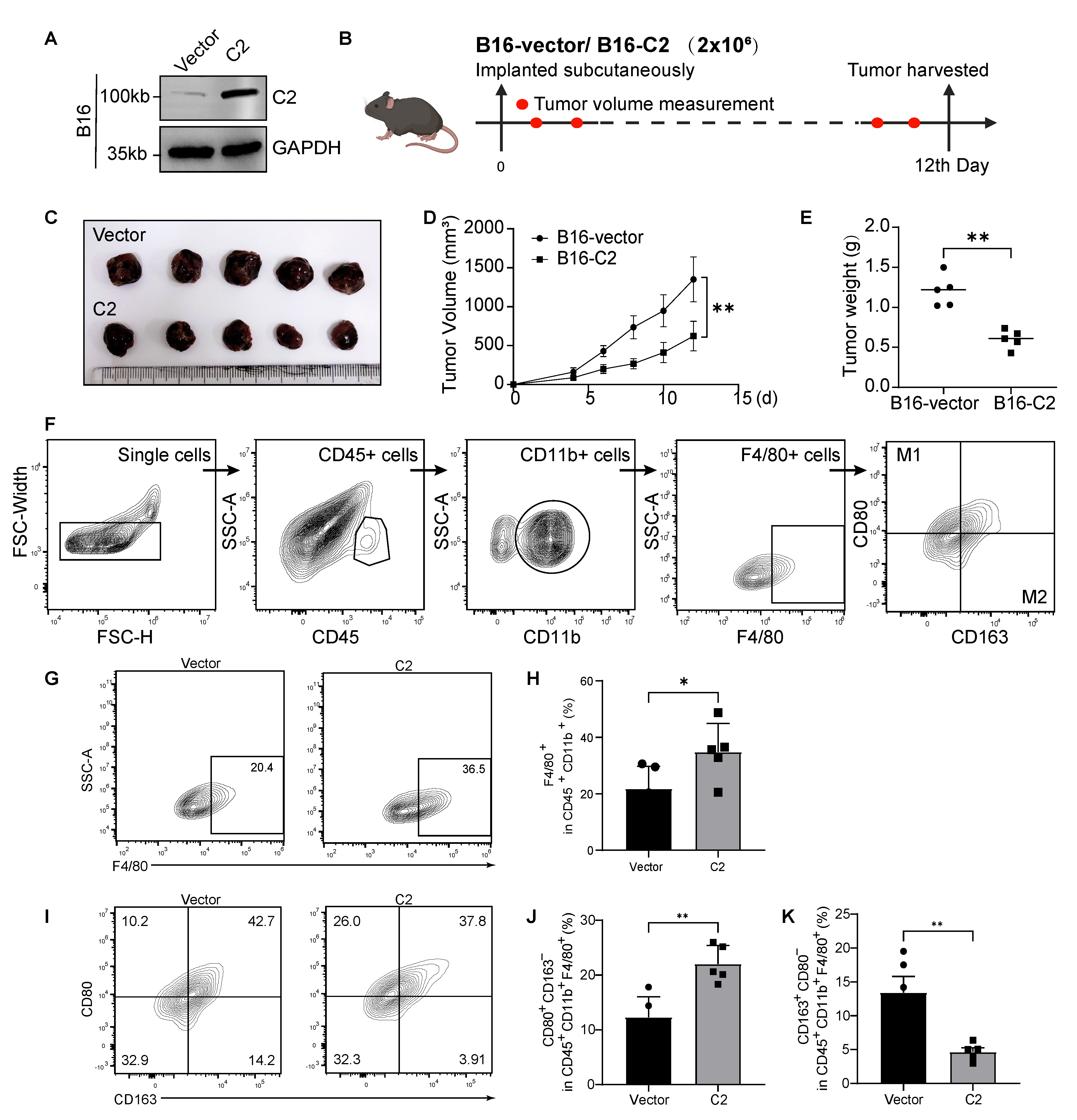
3.3. C2 Inhibits Melanoma Growth and Regulates M1/M2 Macrophage Ratio In Vivo
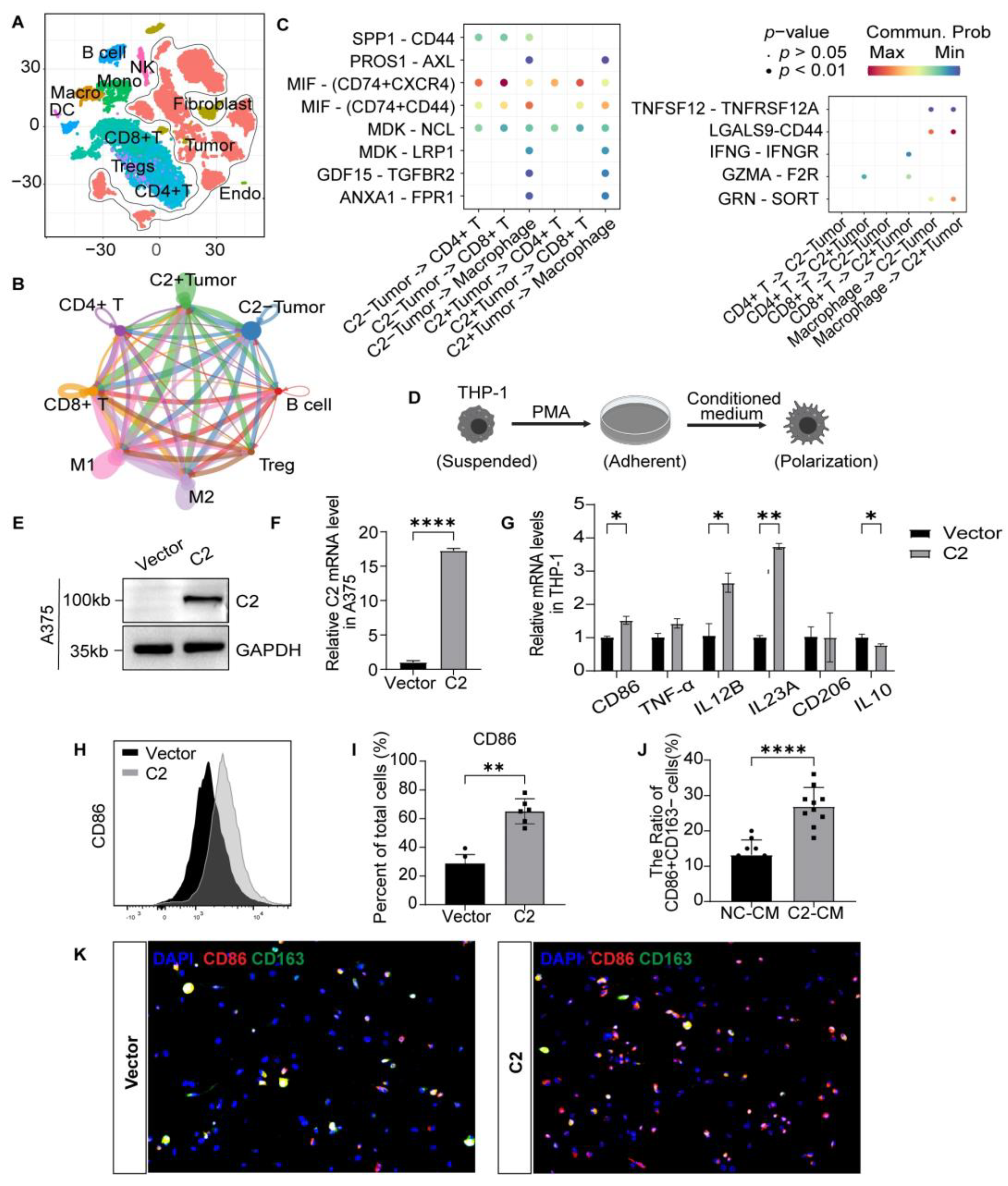
3.4. Tumoral C2 Promotes Macrophages Differentiation into the M1 Subtype In Vitro
3.5. C2 Influences the Formation of Tertiary Lymphoid Structures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef]
- Marzagalli, M.; Ebelt, N.D.; Manuel, E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin. Cancer Biol. 2019, 59, 236–250. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Clark, W.H., Jr.; Elder, D.E.; Guerry, I.V.D.; Braitman, L.E.; Trock, B.J.; Schultz, D.; Synnestvedt, M.; Halpern, A.C. Model predicting survival in stage I melanoma based on tumor progression. J. Natl. Cancer Inst. 1989, 81, 1893–1904. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef]
- Brandner, J.M.; Haass, N.K. Melanoma’s connections to the tumour microenvironment. Pathology 2013, 45, 443–452. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Bulla, R.; Tripodo, C.; Rami, D.; Ling, G.S.; Agostinis, C.; Guarnotta, C.; Zorzet, S.; Durigutto, P.; Botto, M.; Tedesco, F. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat. Commun. 2016, 7, 10346. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, J.A.; Manthey, H.D.; Steyn, F.J.; Chen, W.; Widiapradja, A.; Md Akhir, F.N.; Boyle, G.M.; Taylor, S.M.; Woodruff, T.M.; Rolfe, B.E. The Complement C3a Receptor Contributes to Melanoma Tumorigenesis by Inhibiting Neutrophil and CD4+ T Cell Responses. J. Immunol. 2016, 196, 4783–4792. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Bauer, A.T.; Kirschfink, M.; Ding, P.; Gebhardt, C.; Borsig, L.; Tüting, T.; Renné, T.; Häffner, K.; et al. Neutrophils activated by membrane attack complexes increase the permeability of melanoma blood vessels. Proc. Natl. Acad. Sci. USA 2022, 119, e2122716119. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Wang, P.; Zenklusen, J.C. SnapShot: TCGA-Analyzed Tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef] [PubMed]
- Cirenajwis, H.; Ekedahl, H.; Lauss, M.; Harbst, K.; Carneiro, A.; Enoksson, J.; Rosengren, F.; Werner-Hartman, L.; Törngren, T.; Kvist, A.; et al. Molecular stratification of metastatic melanoma using gene expression profiling: Prediction of survival outcome and benefit from molecular targeted therapy. Oncotarget 2015, 6, 12297–12309. [Google Scholar] [CrossRef] [PubMed]
- Smalley, I.; Chen, Z.; Phadke, M.; Li, J.; Yu, X.; Wyatt, C.; Evernden, B.; Messina, J.L.; Sarnaik, A.; Sondak, V.K.; et al. Single-Cell Characterization of the Immune Microenvironment of Melanoma Brain and Leptomeningeal Metastases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4109–4125. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Havel, J.J.; Makarov, V.; Desrichard, A.; Urba, W.J.; Sims, J.S.; Hodi, F.S.; Martín-Algarra, S.; Mandal, R.; Sharfman, W.H.; et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017, 171, 934–949.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, H.; Yang, T.; Li, T.; Liu, X.; Wang, J.; Liao, Z.; Wei, J.; Lu, J.; Liu, H.; et al. A single-cell analysis reveals tumor heterogeneity and immune environment of acral melanoma. Nat. Commun. 2022, 13, 7250. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T.; Kang, Z.; Guo, G.; Sun, Y.; Lin, K.; Huang, Q.; Shi, X.; Ni, Z.; Ding, N.; et al. Tumor-Infiltrating Immune Cells Act as a Marker for Prognosis in Colorectal Cancer. Front. Immunol. 2019, 10, 2368. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Maeser, D.; Gruener, R.F.; Huang, R.S. oncoPredict: An R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief. Bioinform. 2021, 22, bbab260. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.A. Complement. Bailliere’s Clin. Haematol. 1991, 4, 927–955. [Google Scholar] [CrossRef] [PubMed]
- Truedsson, L. Classical pathway deficiencies—A short analytical review. Mol. Immunol. 2015, 68, 14–19. [Google Scholar] [CrossRef]
- Lundtoft, C.; Sjöwall, C.; Rantapää-Dahlqvist, S.; Bengtsson, A.A.; Jönsen, A.; Pucholt, P.; Wu, Y.L.; Lundström, E.; Eloranta, M.L.; Gunnarsson, I.; et al. Strong Association of Combined Genetic Deficiencies in the Classical Complement Pathway With Risk of Systemic Lupus Erythematosus and Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2022, 74, 1842–1850. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Ning, G.; Huang, Y.L.; Zhen, L.M.; Xu, W.X.; Li, X.J.; Wu, L.N.; Liu, Y.; Xie, C.; Peng, L. Prognostic Value of Complement Component 2 and Its Correlation with Immune Infiltrates in Hepatocellular Carcinoma. BioMed Res. Int. 2020, 2020, 3765937. [Google Scholar] [CrossRef] [PubMed]
- Gogas, H.; Eggermont, A.M.; Hauschild, A.; Hersey, P.; Mohr, P.; Schadendorf, D.; Spatz, A.; Dummer, R. Biomarkers in melanoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20 (Suppl. S6), vi8–vi13. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Bolick, N.L.; Geller, A.C. Epidemiology of Melanoma. Hematol./Oncol. Clin. N. Am. 2021, 35, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Bahri, R.; Kiss, O.; Prise, I.; Garcia-Rodriguez, K.M.; Atmoko, H.; Martínez-Gómez, J.M.; Levesque, M.P.; Dummer, R.; Smith, M.P.; Wellbrock, C.; et al. Human Melanoma-Associated Mast Cells Display a Distinct Transcriptional Signature Characterized by an Upregulation of the Complement Component 3 That Correlates with Poor Prognosis. Front. Immunol. 2022, 13, 861545. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L., Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, H.; Vilain, R.E.; Wilmott, J.S.; Burke, H.; Yearley, J.H.; Thompson, J.F.; Hersey, P.; Long, G.V.; Scolyer, R.A. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2015, 28, 1535–1544. [Google Scholar] [CrossRef]
- Piras, F.; Colombari, R.; Minerba, L.; Murtas, D.; Floris, C.; Maxia, C.; Corbu, A.; Perra, M.T.; Sirigu, P. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 2005, 104, 1246–1254. [Google Scholar] [CrossRef]
- Van Houdt, I.S.; Sluijter, B.J.; Moesbergen, L.M.; Vos, W.M.; de Gruijl, T.D.; Molenkamp, B.G.; van den Eertwegh, A.J.; Hooijberg, E.; van Leeuwen, P.A.; Meijer, C.J.; et al. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int. J. Cancer 2008, 123, 609–615. [Google Scholar] [CrossRef]
- Ladányi, A.; Mohos, A.; Somlai, B.; Liszkay, G.; Gilde, K.; Fejős, Z.; Gaudi, I.; Tímár, J. FOXP3+ cell density in primary tumor has no prognostic impact in patients with cutaneous malignant melanoma. Pathol. Oncol. Res. POR 2010, 16, 303–309. [Google Scholar] [CrossRef]
- Ladányi, A.; Kiss, J.; Mohos, A.; Somlai, B.; Liszkay, G.; Gilde, K.; Fejős, Z.; Gaudi, I.; Dobos, J.; Tímár, J. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol. Immunother. CII 2011, 60, 1729–1738. [Google Scholar] [CrossRef]
- Messina, J.L.; Fenstermacher, D.A.; Eschrich, S.; Qu, X.; Berglund, A.E.; Lloyd, M.C.; Schell, M.J.; Sondak, V.K.; Weber, J.S.; Mulé, J.J. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: Potential for patient selection for immunotherapy? Sci. Rep. 2012, 2, 765. [Google Scholar] [CrossRef] [PubMed]
- Cipponi, A.; Mercier, M.; Seremet, T.; Baurain, J.F.; Théate, I.; van den Oord, J.; Stas, M.; Boon, T.; Coulie, P.G.; van Baren, N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012, 72, 3997–4007. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef]
- West, E.E.; Kolev, M.; Kemper, C. Complement and the Regulation of T Cell Responses. Annu. Rev. Immunol. 2018, 36, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. The three-dimensional human skin reconstruct model: A tool to study normal skin and melanoma progression. J. Vis. Exp. JoVE 2011, 54, e2937. [Google Scholar]

| Variables | TCGA Cohort (n = 326) | High C2 Expression Group | p-Value |
|---|---|---|---|
| Age (years) | 0.848 | ||
| <55 | 114 (35.0%) | 58 (35.6%) | |
| ≥55 | 211 (64.7%) | 105 (64.4%) | |
| NA | 1 (0.3%) | ||
| Gender | 0.172 | ||
| Male | 200 (61.3%) | 94 (57.7%) | |
| Female | 126 (38.7%) | 69 (42.3%) | |
| Stage | 0.566 | ||
| I–II | 197 (60.4%) | 101 (62.0%) | |
| III–IV | 123 (37.7%) | 59 (36.2%) | |
| NA | 6 (1.9%) | 3 (1.8%) | |
| Breslow (mm) | 0.065 | ||
| <2 | 110 (33.8%) | 63 (38.7%) | |
| ≥2 | 209 (64.1%) | 97 (59.5%) | |
| NA | 7 (2.1%) | 3 (1.8%) | |
| Clark level | 0.106 | ||
| I–III | 88 (27.0%) | 52 (31.9%) | |
| IV–V | 199 (61.0%) | 97 (59.5%) | |
| NA | 39 (12.0%) | 14 (8.6%) | |
| Ulceration | 0.045 * | ||
| No | 129 (39.6%) | 71 (43.5%) | |
| Yes | 151 (46.3%) | 65 (39.9%) | |
| NA | 46 (14.1%) | 27 (16.6%) | |
| Mitotic index (/mm2) | 0.625 | ||
| ≤6 | 101 (31.0%) | 50 (30.7%) | |
| >6 | 56 (17.2%) | 30 (18.4%) | |
| NA | 169 (51.8%) | 83 (50.9%) | |
| C2 expression | |||
| High | 163 (50%) | ||
| Low | 163 (50%) |
| Characteristics | Univariate Cox Regression | Multivariate Cox Regression | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Age | 1.94 | 1.29–2.92 | 0.001 | 2.20 | 1.13–4.28 | 0.020 * |
| (≥55 vs. <55) | ||||||
| Breslow | 1.71 | 1.15–2.54 | 0.008 | 0.97 | 0.47–1.98 | 0.926 |
| (≥2 vs. <2) | ||||||
| C2 | 0.63 | 0.43–0.93 | 0.019 | 0.53 | 0.28–0.99 | 0.047 * |
| (High vs. Low) | ||||||
| Clark | 1.98 | 1.29–3.05 | 0.002 | 1.12 | 0.57–2.21 | 0.748 |
| (IV–V vs. I–III) | ||||||
| Gender | 0.97 | 0.64–1.45 | 0.868 | |||
| (Female vs. Male) | ||||||
| Mitotic | 1.67 | 1–2.8 | 0.051 | 1.7 | 0.84–3.43 | 0.138 |
| (>6 vs. ≤6) | ||||||
| Stage | 2.44 | 1.61–3.69 | 0.000 | 2.93 | 1.58–5.44 | 0.001 * |
| (III–IV vs. I–II) | ||||||
| Ulceration | 2.13 | 1.38–3.27 | 0.001 | 1.34 | 0.72–2.5 | 0.351 |
| (Yes vs. No) | ||||||
| C2 Expression | |||||
|---|---|---|---|---|---|
| Low | High | Total | p | ||
| − | 7 | 2 | 9 | ||
| CD86 | + | 3 | 9 | 12 | 0.03 |
| Total | 10 | 11 | 21 | ||
| −/+ | 1 | 7 | 8 | ||
| CD163 | ++ | 9 | 4 | 13 | 0.0237 |
| Total | 10 | 11 | 21 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Li, S.; Xiao, W.; Zhang, C.; Li, T.; Liao, Z.; Liu, H.; Xing, R.; Yao, W.; Yang, J. Tumoral C2 Regulates the Tumor Microenvironment by Increasing the Ratio of M1/M2 Macrophages and Tertiary Lymphoid Structures to Improve Prognosis in Melanoma. Cancers 2024, 16, 908. https://doi.org/10.3390/cancers16050908
Zhang G, Li S, Xiao W, Zhang C, Li T, Liao Z, Liu H, Xing R, Yao W, Yang J. Tumoral C2 Regulates the Tumor Microenvironment by Increasing the Ratio of M1/M2 Macrophages and Tertiary Lymphoid Structures to Improve Prognosis in Melanoma. Cancers. 2024; 16(5):908. https://doi.org/10.3390/cancers16050908
Chicago/Turabian StyleZhang, Gengpu, Shengnan Li, Wanyi Xiao, Chao Zhang, Ting Li, Zhichao Liao, Haotian Liu, Ruwei Xing, Wei Yao, and Jilong Yang. 2024. "Tumoral C2 Regulates the Tumor Microenvironment by Increasing the Ratio of M1/M2 Macrophages and Tertiary Lymphoid Structures to Improve Prognosis in Melanoma" Cancers 16, no. 5: 908. https://doi.org/10.3390/cancers16050908
APA StyleZhang, G., Li, S., Xiao, W., Zhang, C., Li, T., Liao, Z., Liu, H., Xing, R., Yao, W., & Yang, J. (2024). Tumoral C2 Regulates the Tumor Microenvironment by Increasing the Ratio of M1/M2 Macrophages and Tertiary Lymphoid Structures to Improve Prognosis in Melanoma. Cancers, 16(5), 908. https://doi.org/10.3390/cancers16050908






