Thermal Ablation Combined with Immune Checkpoint Blockers: A 10-Year Monocentric Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Patient, Tumor, and Treatment Characteristics
3.3. Feasibility and Safety Analysis
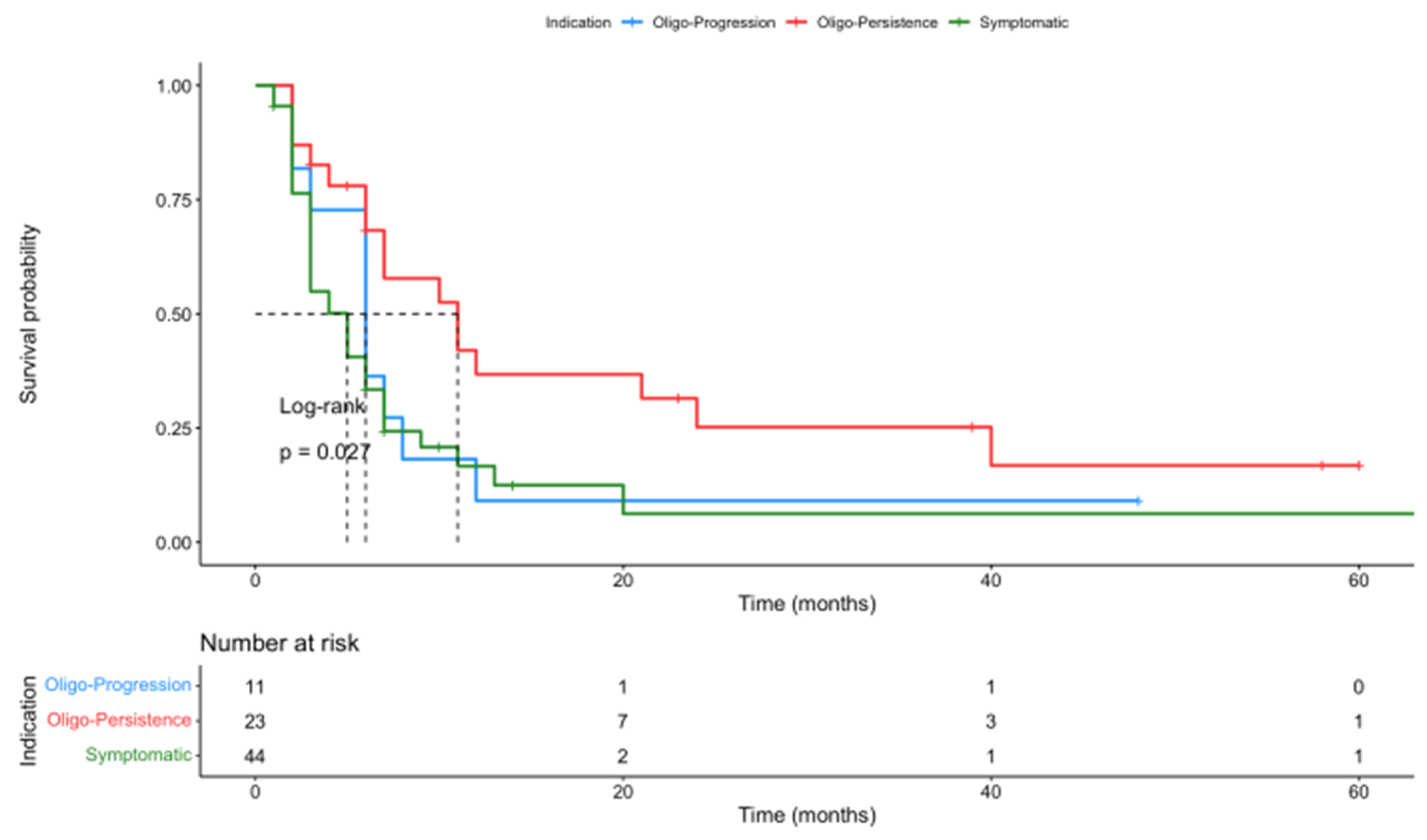
3.4. Outcome Analysis
3.5. Abscopal Effect Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Cryoablation |
| CTCAE | Common Terminology Criteria for Adverse Events v5.0 |
| ICB | Immune checkpoint blocker |
| IQR | Interquartile ratio |
| irAEs | Immune-related adverse events |
| LDH | Lactate dehydrogenase |
| NLR | Neutrophil-to-lymphocyte ratio |
| OS | Overall survival |
| PD1 | Programmed cell death 1 |
| PD(L)1 | Programmed cell death ligand 1 |
| LPFS | Local progression-free survival |
| PFS | Progression-free survival |
| PTA | Percutaneous thermal ablation |
| RFA | Radiofrequency ablation |
| SD | Standard deviation |
| SRE | Skeletal-related events |
References
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef]
- Broderick, S.R. Adjuvant and Neoadjuvant Immunotherapy in Non-small Cell Lung Cancer. Thorac. Surg. Clin. 2020, 30, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Tselikas, L.; Bazzocchi, A.; Efthymiou, E.; Kelekis, A.; Yevich, S. Percutaneous Management of Cancer Pain. Curr. Oncol. Rep. 2020, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Arrigoni, F.; Zugaro, L.; Barile, A.; Masciocchi, C.; et al. Percutaneous image-guided cryoablation: Current applications and results in the oncologic field. Med. Oncol. 2016, 33, 140. [Google Scholar] [CrossRef]
- De Baère, T.; Tselikas, L.; Deschamps, F.; Soria, J.C.; Marabelle, A. Immuno-Oncology in Cancer Care is a Fantastic Opportunity for Interventional Oncology: IO4IO (Interventional Oncology for Immuno-Oncology) Initiative. Cardiovasc. Intervent. Radiol. 2018, 41, 825–827. [Google Scholar] [CrossRef]
- Arina, A.; Gutiontov, S.I.; Weichselbaum, R.R. Radiotherapy and Immunotherapy for Cancer: From “Systemic” to “Multisite”. Clin. Cancer Res. 2020, 26, 2777–2782. [Google Scholar] [CrossRef]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy-immunotherapy combinations—Perspectives and challenges. Mol. Oncol. 2020, 14, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Pitroda, S.P.; Chmura, S.J.; Weichselbaum, R.R. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019, 20, e434–e442. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Marabelle, A.; Zitvogel, L.; Kroemer, G. Oncolysis without viruses—Inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 2020, 17, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Yakkala, C.; Chiang, C.L.; Kandalaft, L.; Denys, A.; Duran, R. Cryoablation and Immunotherapy: An Enthralling Synergy to Confront the Tumors. Front. Immunol. 2019, 10, 2283. [Google Scholar] [CrossRef] [PubMed]
- Den Brok, M.H.; Sutmuller, R.P.; van der Voort, R.; Bennink, E.J.; Figdor, C.G.; Ruers, T.J.; Adema, G.J. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004, 64, 4024–4029. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.; Wu, S.; Sterman, D.H. Immunological Aspects of Cryoablation of Non-Small Cell Lung Cancer: A Comprehensive Review. J. Thorac. Oncol. 2018, 13, 624–635. [Google Scholar] [CrossRef]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef]
- Singh, M.; Singh, T.; Soni, S. Pre-operative Assessment of Ablation Margins for Variable Blood Perfusion Metrics in a Magnetic Resonance Imaging Based Complex Breast Tumour Anatomy: Simulation Paradigms in Thermal Therapies. Comput. Methods Programs Biomed. 2021, 198, 105781. [Google Scholar] [CrossRef]
- Callstrom, M.R.; Woodrum, D.A.; Nichols, F.C.; Palussiere, J.; Buy, X.; Suh, R.D.; Abtin, F.G.; Pua, B.B.; Madoff, D.C.; Bagla, S.L.; et al. Multicenter Study of Metastatic Lung Tumors Targeted by Interventional Cryoablation Evaluation (SOLSTICE). J. Thorac. Oncol. 2020, 15, 1200–1209. [Google Scholar] [CrossRef]
- Cazzato, R.L.; De Marini, P.; Leonard-Lorant, I.; Leclerc, L.; Auloge, P.; Tricard, T.; Dalili, D.; Garnon, J.; Lang, H.; Gangi, A. Safety and Oncologic Outcomes of Magnetic Resonance Imaging-Guided Cryoablation of Renal Cell Carcinoma: A 10-Year Single-Center Experience. Investig. Radiol. 2021, 56, 153–162. [Google Scholar] [CrossRef]
- Li, F.; Tian, Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol 2013, 10, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, K.E.; Lujambio, A. Liver metastases inhibit immunotherapy efficacy. Nat. Med. 2021, 27, 25–27. [Google Scholar] [CrossRef]
- Singh, M. Modified Pennes bioheat equation with heterogeneous blood perfusion: A newer perspective. Int. J. Heat Mass Transf. 2024, 218, 124698. [Google Scholar] [CrossRef]
- Madani, K.; Najafi, A.; Boticella, A.; Roux, C.; Tselikas, L.; Delpla, A.; Al Ahmar, M.; de Baere, T.; Deschamps, F. Combined local treatments for vertebral metastases with limited epidural extension. Support Care Cancer, 2021; ahead-of-print. [Google Scholar] [CrossRef]
- Najafi, A.; de Baere, T.; Purenne, E.; Bayar, A.; Al Ahmar, M.; Delpla, A.; Roux, C.; Madani, K.; Assouline, J.; Deschamps, F.; et al. Risk factors for local tumor progression after RFA of pulmonary metastases: A matched case-control study. Eur. Radiol. 2021, 31, 5361–5369. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019, 10, 5421. [Google Scholar] [CrossRef]
- Chen, J.; Qian, W.; Mu, F.; Niu, L.; Du, D.; Xu, K. The future of cryoablation: An abscopal effect. Cryobiology. 2020, 97, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The abscopal effect of radiation therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Palussière, J.; Mathoulin-Pelissier, S.; Italiano, A. Cryoablation in Combination (or Not) with Pembrolizumab and Pemetrexed-carboplatin in 1st-line Treatment for Patients with Metastatic Lung Adenocarcinoma (CRYOMUNE). Available online: https://clinicaltrials.gov/study/NCT04339218 (accessed on 11 February 2024).
- Nahon, P.; Seror, O.; Ganne, N. Neoadjuvant and Adjuvant Nivolumab in HCC Patients Treated by Electroporation (NIVOLEP). Available online: https://clinicaltrials.gov/study/NCT03630640 (accessed on 30 November 2023).


| Patient Characteristics [n (%) or Median (IQR)] | N = 78 |
|---|---|
| Demographic and morphometric data | |
| Age (years) | 61 (54–67) |
| Sex | |
| Male | 41 (53) |
| Female | 37 (47) |
| Weight (kg) | 70 (57–85) |
| Height (cm) | 171 (165–178) |
| ECOG performance status | |
| 0–1 | 52 (80) |
| 2–3 | 16 (20) |
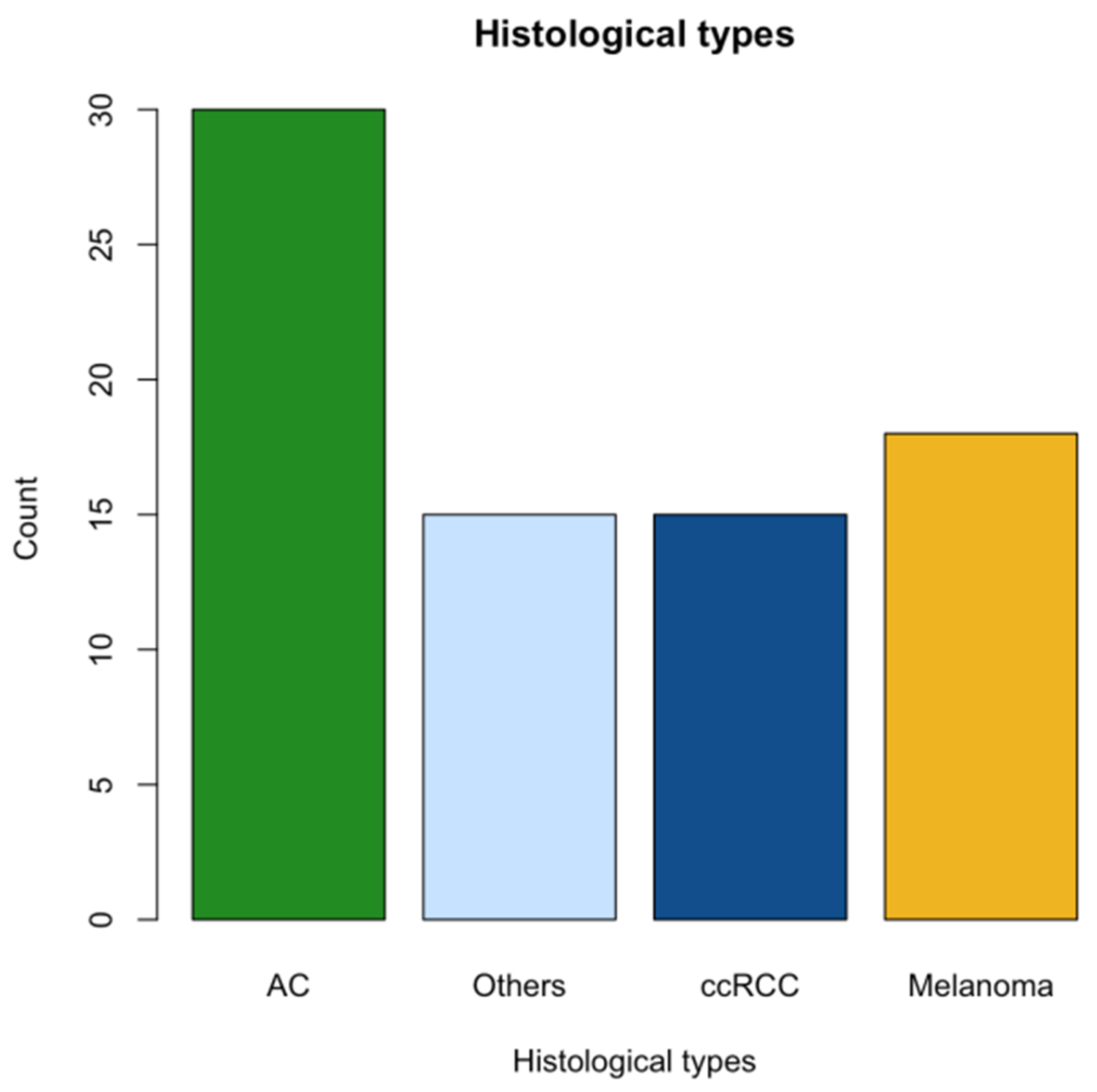
| Primary tumor location | |
| Lung (NSCLC) | 23 (29) |
| Skin | 20 (26) |
| Kidney | 17 (22) |
| Other | 18 (23) |
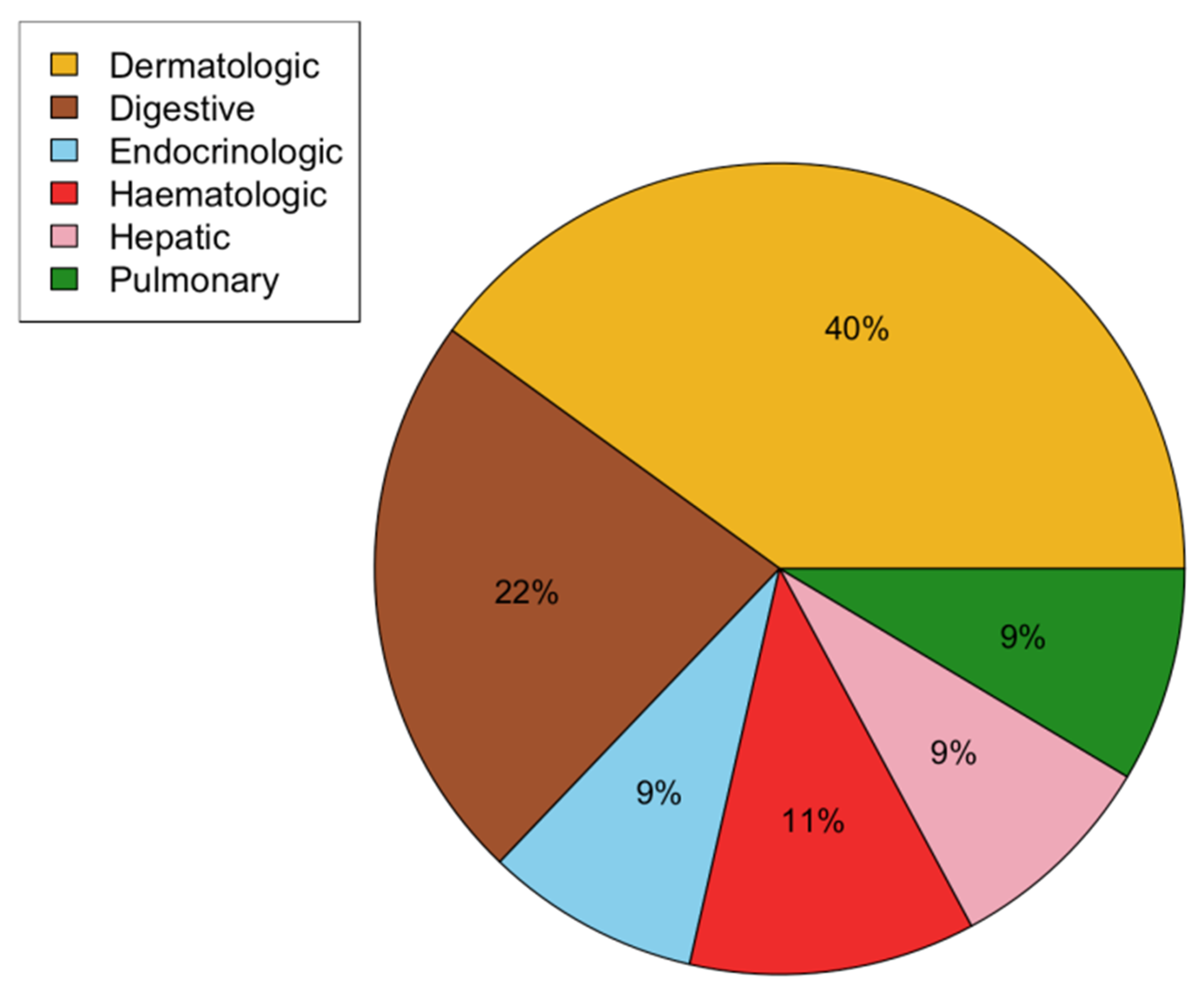
| Metastasis location | |
| Bone | 50 (64) |
| Lymphadenopathy | 40 (51) |
| Lung | 35 (45) |
| Liver | 19 (24) |
| ICB therapy | |
| Pembrolizumab | 35 (45) |
| Nivolumab | 24 (31) |
| Nivolumab + ipilimumab | 8 (10) |
| Ipilimumab | 3 (4) |
| Other | 8 (10) |
| Other systemic treatment | |
| None | 63 (81) |
| Chemotherapies | 8 (10) |
| Targeted therapies (TKI, anti-EGFR) | 7 (9) |
| PTA Characteristics [n (%) or Median (IQR)] | N = 78 |
|---|---|
| Indication | |
| Oligo-persistence | 23 (29) |
| Oligo-progression | 11 (14) |
| Symptomatic lesions or prevention of SRE | 44 (56) |
| Ablation technique | |
| CA | 51 (65) |
| RF | 24 (31) |
| MWA | 3 (4) |
| PTA location | |
| Bone | 43 (55) |
| Lung | 13 (17) |
| Liver | 7 (9) |
| Others | 15 (19) |
| Previous treatment of ablated lesion | |
| RT | 6 (8) |
| Surgery | 4 (5) |
| None | 68 (87) |
| Number of ablated lesion(s) during PTA | |
| 1 | 63 (81) |
| 2–4 | 15 (19) |
| Lesion size (cm) | |
| 0–2 | 27 (35) |
| 2–3 | 31 (40) |
| ≥3 | 20 (26) |
| PTA considered technically complete | |
| Yes | 48 (62) |
| No | 29 (37) |
| Associated locoregional treatment | |
| Radiation therapy | 17 (22) |
| Embolization | 5 (6) |
| None | 56 (72) |
| Biological data | |
| NLR pre-ablation | 2.9 (1.9–5.3) |
| NLR post-ablation | 2.6 (1.7–4.2) |
| LDH pre-ablation (U/L) | 200 (175–222) |
| LDH post-ablation (U/L) | 224 (185–277) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnet, B.; Tournier, L.; Deschamps, F.; Yevich, S.; Marabelle, A.; Robert, C.; Albiges, L.; Besse, B.; Bonnet, V.; De Baère, T.; et al. Thermal Ablation Combined with Immune Checkpoint Blockers: A 10-Year Monocentric Experience. Cancers 2024, 16, 855. https://doi.org/10.3390/cancers16050855
Bonnet B, Tournier L, Deschamps F, Yevich S, Marabelle A, Robert C, Albiges L, Besse B, Bonnet V, De Baère T, et al. Thermal Ablation Combined with Immune Checkpoint Blockers: A 10-Year Monocentric Experience. Cancers. 2024; 16(5):855. https://doi.org/10.3390/cancers16050855
Chicago/Turabian StyleBonnet, Baptiste, Louis Tournier, Frédéric Deschamps, Steven Yevich, Aurélien Marabelle, Caroline Robert, Laurence Albiges, Benjamin Besse, Victoire Bonnet, Thierry De Baère, and et al. 2024. "Thermal Ablation Combined with Immune Checkpoint Blockers: A 10-Year Monocentric Experience" Cancers 16, no. 5: 855. https://doi.org/10.3390/cancers16050855
APA StyleBonnet, B., Tournier, L., Deschamps, F., Yevich, S., Marabelle, A., Robert, C., Albiges, L., Besse, B., Bonnet, V., De Baère, T., & Tselikas, L. (2024). Thermal Ablation Combined with Immune Checkpoint Blockers: A 10-Year Monocentric Experience. Cancers, 16(5), 855. https://doi.org/10.3390/cancers16050855