The Combined Use of Inflammation Markers, Modified Glasgow Prognostic Score, and Sarculator Nomogram in Extremity Soft Tissue Sarcoma: A Multicenter Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Statistical Analyses
3. Results
3.1. Patients Characteristics
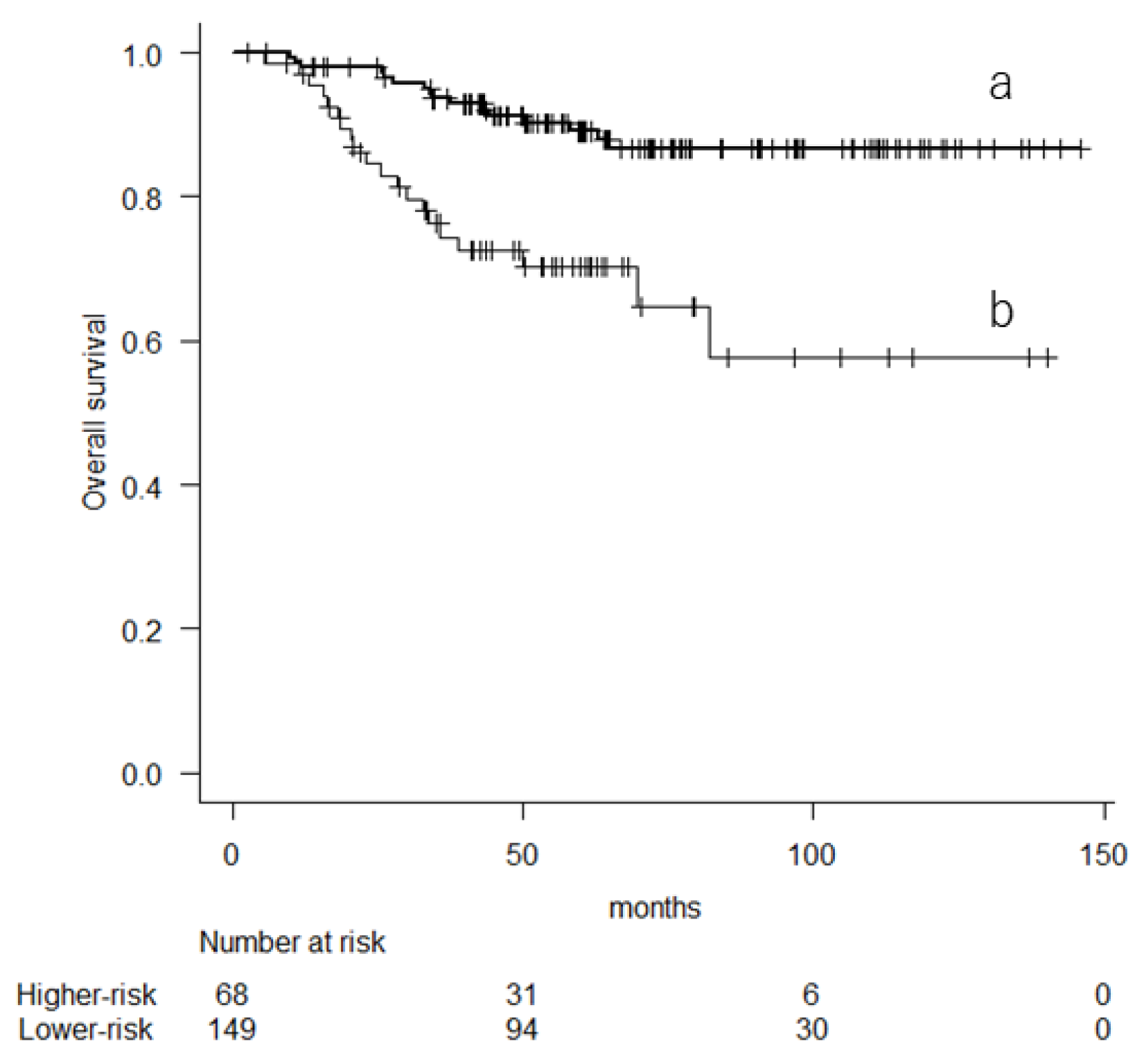
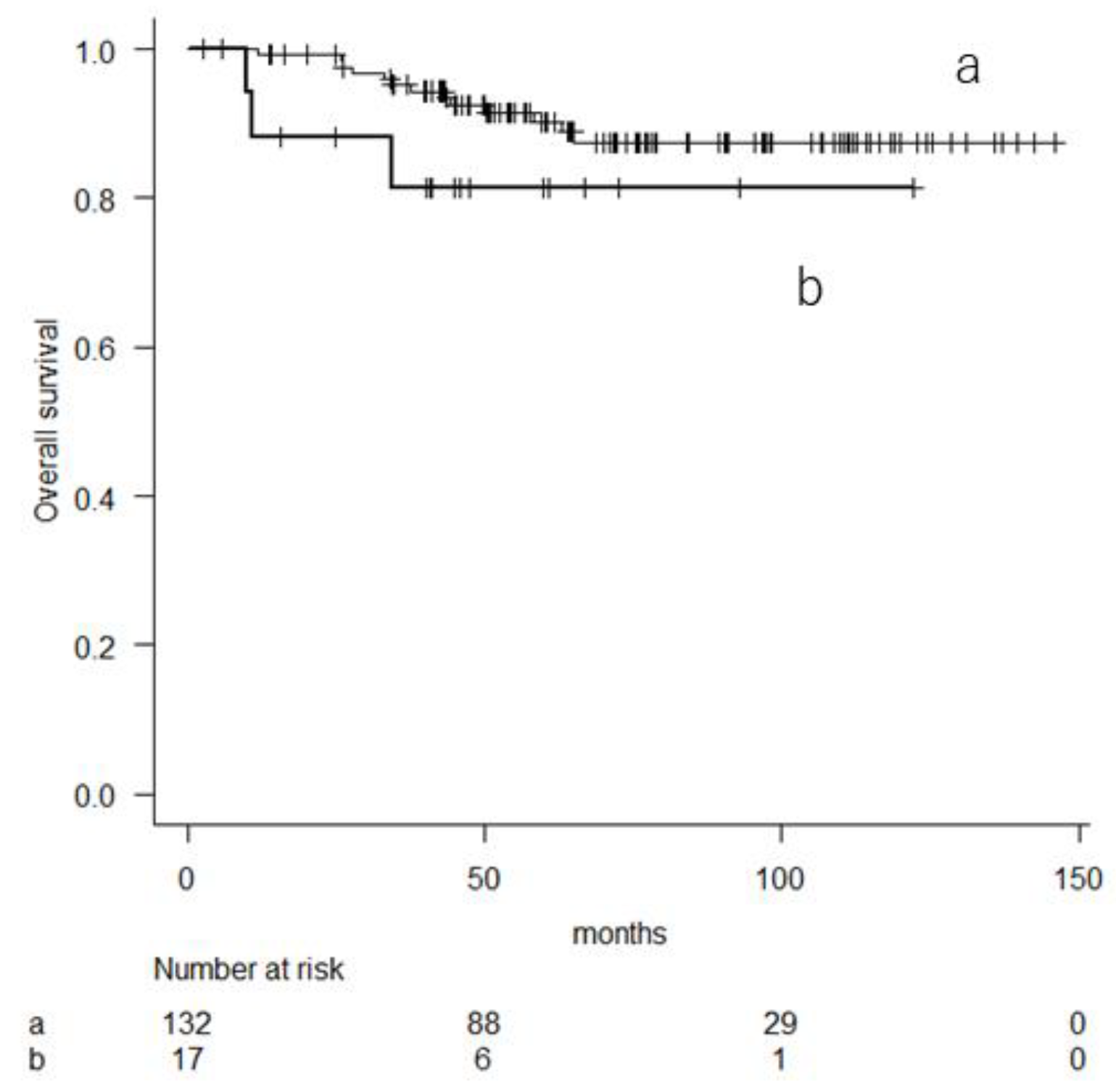
3.2. Prognostic Factor Analyses for Overall Survival
3.3. Metastatic-Free Survival and Prognostic Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-tissue sarcomas in adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [PubMed]
- WHO classification of Tumours. In Soft Tissue and Bone, 5th ed.; IARC Press: Lyon, France, 2020; pp. 2–3. ISBN 978-92-832-4502-5.
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014, 260, 416–421. [Google Scholar] [CrossRef]
- Hennon, M. Sarcoma pulmonary metastatic disease: Still a chance of cure. Surg. Clin. N. Am. 2021, 102, 615–624. [Google Scholar] [CrossRef]
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.; Strauss, D.C.; Levy, A.; Griffin, A.; Hayes, A.J.; Stacchiotti, S.; Pechoux, C.L.; et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: A retrospective analysis. Lancet Oncol. 2016, 17, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Acem, I.; van de Sande, M.A.J. Predictions tools for the personalized management of soft-tissue sarcomas of the extremity. Bone Jt. J. 2022, 104-B, 1011–1016. [Google Scholar] [CrossRef]
- Hu, Y.; Li, A.; Zhao, C.K.; Ye, X.H.; Peng, X.J.; Wang, P.P.; Shu, H.; Yao, Q.Y.; Liu, W.; Liu, Y.Y.; et al. A multiparametric clinic-ultrasonic nomogram for predicting extremity soft tissue tumor malignancy: A combined retrospective and prospective bicentric study. Radiol. Med. 2023, 128, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers. 2018, 4, 17105. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef]
- McMillan, D.C.; Crozier, J.E.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef]
- Spense, S.; Doonan, J.; Farhan-Alanie, O.M.; Chan, C.D.; Tong, D.; Cho, H.S.; Sahu, M.A.; Traub, F.; Gupta, S.; The mPGS Study Group. Does the modified Glasgow prognostic score aid in the management of patients undergoing surgery for soft-tissue sarcoma?: An international multicentre study. Bone Jt. J. 2022, 104-B, 168–176. [Google Scholar]
- Morhij, R.; Mahendra, A.; Jane, M.; McMillan, D.C. The modified Glasgow prognostic score in patients undergoing surgery for bone and soft tissue sarcoma. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Asanuma, K.; Matsubara, T.; Sudo, A. The value of the high-sensitivity modified Glasgow prognostic score in predicting the survival of patients with a soft-tissue sarcoma. Bone Jt. J. 2015, 97-B, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcoma: ESMO-EURACAN-GENTURIS Clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Mizusawa, J.; Fukuda, H.; Araki, N.; Chuman, H.; Takahashi, M.; Ozaki, T.; Hiruma, T.; Tsuchiya, H.; Morioka, H.; et al. Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcoma in the extremities (JCOG0304). Jpn. J. Clin. Oncol. 2015, 45, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Asanuma, K.; Hagi, T.; Sudo, A. Clinical outcome of systemic treatment for advanced soft tissue sarcoma: Real-life perspective in Japan. Drug. Des. Devel. Ther. 2020, 14, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A rondomised, double-blind, placebo-control phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Phillips, E.; Jones, R.L.; Huang, P.; Digklia, A. Efficacy of eribulin in soft tissue sarcomas. Front. Pharmacol. 2022, 13, 869754. [Google Scholar] [CrossRef]
- Grignani, G.; Le Cesne, A.; Martín-Broto, J. Trabectedin as second-line treatment in advanced soft tissue sarcoma: Quality of life and safe outcomes. Future Oncol. 2022, 18, 13–22. [Google Scholar] [CrossRef]
- Kawamoto, T.; Hara, H.; Morishita, M.; Fukase, N.; Kawakami, Y.; Takemori, T.; Fujiwara, S.; Kitayama, K.; Yahiro, S.; Miyamoto, T.; et al. Prognostic influence of the treatment approach for pulmonary metastasis in patients with soft tissue sarcoma. Clin. Exp. Metastasis 2020, 37, 509–517. [Google Scholar] [CrossRef]
- Vanni, S.; Fausti, V.; Fonzi, E.; Liverani, C.; Miserocchi, G.; Spadazzi, C.; Cocchi, C.; Calabrese, C.; Gurrieri, L.; Riva, N.; et al. Unveiling the genomic basis of chemosensitivity in sarcomas of the extremities: An integrated approach for an unmet clinical need. Int. J. Mol. Sci. 2023, 24, 6926. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Palmerini, E.; Quagliuolo, V.; Martin-Broto, J.; Lopez-Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.Y.; Tendero, O.; Diaz-Beveridge, R.; et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: A Sarculator-based risk stratification analysis of the ISG-STS 1001 randomized trial. Cancer 2022, 128, 85–93. [Google Scholar] [CrossRef]
- Pasquali, S.; Pizzamiglio, S.; Touati, N.; Litiere, S.; Marreaud, S.; Kasper, B.; Gelderblom, H.; Stacchiotti, S.; Judson, I.; Dei Tos, A.P.; et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: Revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur. J. Cancer 2019, 109, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.K.; Callegaro, D.; Chiang, Y.J.; Fiore, M.; Miceli, R.; Keung, E.Z.; Feig, B.W.; Torres, K.E.; Scally, C.P.; Hunt, K.K.; et al. Sarculator is a good model to predict survival in resected extremity and trunk sarcomas in US patients. Ann. Surg. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ke, L.C.; Sun, S.R. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in patients with soft tissue sarcoma: A meta-analysis. Medicine 2018, 97, e12176. [Google Scholar] [CrossRef]
- García-Ortega, D.Y.; Melendez-Fernandez, A.P.; Alvarez-Cano, A.; Clara-Altamirano, M.A.; Caro-Sanchez, C.; Alamilla-Garcia, G.; Luna-Ortiz, K. Neutrophil-to-lymphocyte ratio as a prognostic biomarker in extremities undifferentiated pleomorphic sarcoma. Surg. Oncol. 2022, 42, 101746. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hagi, T.; Asanuma, K.; Sudo, A. Is lymphocyte C-reactive protein ratio useful for predicting survival in patients with non-metastatic soft tissue sarcoma? Cancers 2022, 14, 5214. [Google Scholar] [CrossRef]
- Matsui, Y.; Matsuda, A.; Maejima, A.; Shinoda, Y.; Nakamura, E.; Komiyama, M.; Fujimoto, H. The clinical significance of perioperative inflammatory index as a prognostic factor for patients with retroperitoneal soft tissue sarcoma. Int. J. Clin. Oncol. 2022, 27, 1093–1100. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z.; et al. A novel systemic 369 inflammation response index (SIRI) for predicting the survival of patients with 370 pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, W.; Guan, Y.X.; Wang, W.; Chen, H.Y.; Fang, C.; Zhang, X.; Zhou, Z.W. Prognostic value of the C-reactive protein/albumin ratio (CAR) in patients with operable soft tissue sarcoma. Oncotarget 2017, 8, 98135–98147. [Google Scholar] [CrossRef]
- Liao, C.K.; Yu, Y.L.; Lin, Y.C.; Hsu, Y.J.; Chern, Y.J.; Chiang, J.M.; You, J.F. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: An update systematic review and meta-analysis. World J. Surg. Oncol. 2021, 19, 139. [Google Scholar] [CrossRef]


| Variables | N | |
|---|---|---|
| Age (years) | Mean (range) | 61 (20–93) |
| Sex | Male | 106 |
| Female | 111 | |
| Depth | Superficial | 42 |
| Deep | 175 | |
| Tumor size (cm) | Mean (range) | 9 (1–35) |
| Grade | 1 | 28 |
| 2 | 103 | |
| 3 | 86 | |
| NLR | Mean (range) | 3.0 (0.64–15.38) |
| mGPS | 0 | 181 |
| 1 | 24 | |
| 2 | 12 | |
| Surgery | R0 resection | 188 |
| R1 resection | 29 | |
| Adjuvant | Chemotherapy | 57 |
| Radiotherapy | 45 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex | Female | 1 | 1 | ||||
| Male | 2.628 | 1.293–5.344 | 0.008 | 2.473 | 1.211–5.052 | 0.013 | |
| Depth | Deep | 1 | 1 | ||||
| Superficial | 0.22 | 0.053–0.918 | 0.037 | 0.335 | 0.078–1.432 | 0.14 | |
| Sarculator | Lower risk | 1 | 1 | ||||
| Higher risk | 3.533 | 1.823–6.845 | <0.001 | 2.616 | 1.307–5.238 | 0.007 | |
| Albumin | ≤3.5 g/dL | 1 | |||||
| >3.5 g/dL | 1.442 | 0.442–4.708 | 0.544 | ||||
| NLR | 1.151 | 1.009–1.314 | 0.036 | 1.041 | 0.894–1.213 | 0.604 | |
| mGPS | 0 | 1 | 1 | ||||
| 1 or 2 | 3.516 | 1.749–7.068 | <0.001 | 2.109 | 0.968–4.595 | 0.06 | |
| Adjuvant Cx | No | 1 | |||||
| Yes | 1.095 | 0.528–2.273 | 0.808 | ||||
| Adjuvant Rx | No | 1 | |||||
| Yes | 0.892 | 0.391–2.037 | 0.786 | ||||
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex | Female | 1 | 1 | ||||
| Male | 1.738 | 1.016–2.976 | 0.043 | 1.621 | 0.94–2.793 | 0.082 | |
| Depth | Deep | 1 | |||||
| Superficial | 0.8 | 0.391–1.636 | 0.541 | ||||
| Sarculator | Lower risk | 1 | 1 | ||||
| Higher risk | 2.69 | 1.586–4.563 | <0.001 | 2.36 | 1.364–4.086 | 0.002 | |
| Albumin | ≤3.5 g/dL | 1 | |||||
| >3.5 g/dL | 1.542 | 0.614–3.874 | 0.357 | ||||
| NLR | 1.115 | 0.9998–1.243 | 0.05 | ||||
| mGPS | 0 | 1 | 1 | ||||
| 1 or 2 | 2.512 | 1.385–4.557 | 0.002 | 1.819 | 0.973–3.402 | 0.06 | |
| Adjuvant Cx | No | 1 | |||||
| Yes | 1.007 | 0.548–1.85 | 0.982 | ||||
| Adjuvant Rx | No | 1 | |||||
| Yes | 0.782 | 0.394–1.55 | 0.481 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T.; Takenaka, S.; Outani, H.; Hagi, T.; Tamiya, H.; Imura, Y.; Asanuma, K.; Sudo, A. The Combined Use of Inflammation Markers, Modified Glasgow Prognostic Score, and Sarculator Nomogram in Extremity Soft Tissue Sarcoma: A Multicenter Observational Study. Cancers 2024, 16, 1077. https://doi.org/10.3390/cancers16051077
Nakamura T, Takenaka S, Outani H, Hagi T, Tamiya H, Imura Y, Asanuma K, Sudo A. The Combined Use of Inflammation Markers, Modified Glasgow Prognostic Score, and Sarculator Nomogram in Extremity Soft Tissue Sarcoma: A Multicenter Observational Study. Cancers. 2024; 16(5):1077. https://doi.org/10.3390/cancers16051077
Chicago/Turabian StyleNakamura, Tomoki, Satoshi Takenaka, Hidetatsu Outani, Tomohito Hagi, Hironari Tamiya, Yoshinori Imura, Kunihiro Asanuma, and Akihiro Sudo. 2024. "The Combined Use of Inflammation Markers, Modified Glasgow Prognostic Score, and Sarculator Nomogram in Extremity Soft Tissue Sarcoma: A Multicenter Observational Study" Cancers 16, no. 5: 1077. https://doi.org/10.3390/cancers16051077
APA StyleNakamura, T., Takenaka, S., Outani, H., Hagi, T., Tamiya, H., Imura, Y., Asanuma, K., & Sudo, A. (2024). The Combined Use of Inflammation Markers, Modified Glasgow Prognostic Score, and Sarculator Nomogram in Extremity Soft Tissue Sarcoma: A Multicenter Observational Study. Cancers, 16(5), 1077. https://doi.org/10.3390/cancers16051077






