The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics
Abstract
Simple Summary
Abstract
1. Introduction
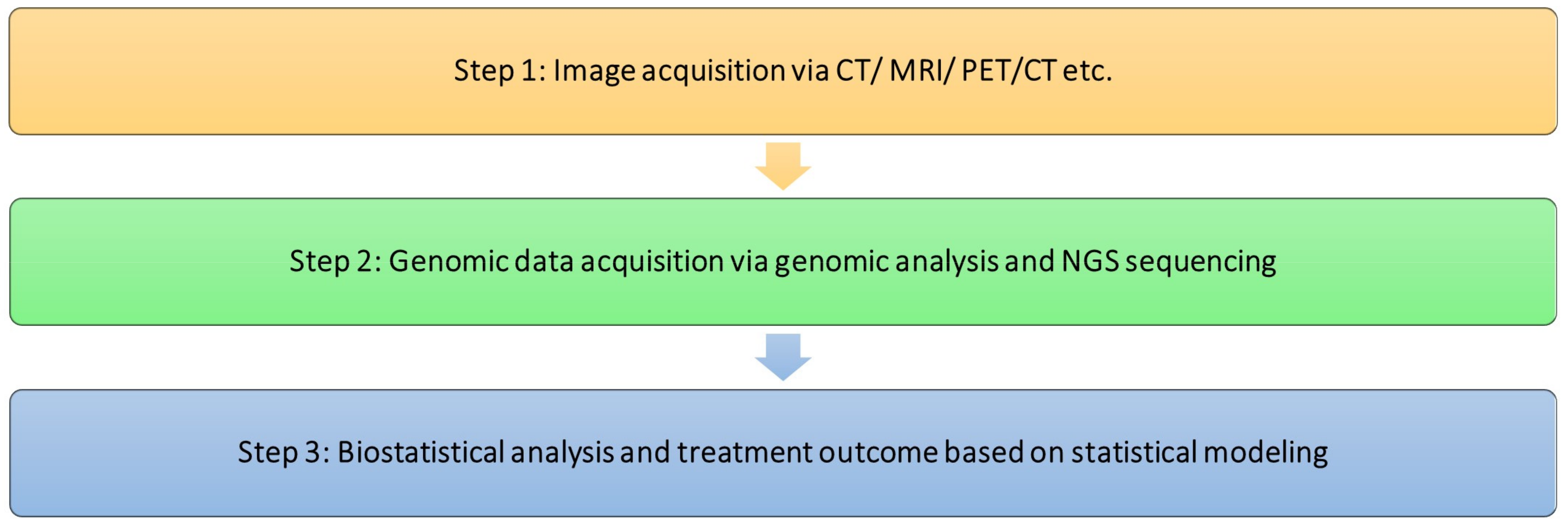
2. Triple Approach to Breast Imaging and Medical Imaging Techniques
3. Genomics
Sequencing
4. Radiogenomics and Its Use in Precision Medicine
4.1. Acquisition of Raw Images
4.2. Pre-Processing of Information
4.3. Extraction of Features
4.4. Data Analysis
5. Current Application of Radiogenomics in Oncology
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shui, L.; Ren, H.; Yang, X.; Li, J.; Chen, Z.; Yi, C.; Zhu, H.; Shui, P. The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 2021, 10, 570465. [Google Scholar] [CrossRef]
- Tan, D.S.; Mok, T.S.; Rebbeck, T.R. Cancer genomics: Diversity and disparity across ethnicity and geography. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 91–101. [Google Scholar] [CrossRef]
- Huo, D.; Hu, H.; Rhie, S.K.; Gamazon, E.R.; Cherniack, A.D.; Liu, J.; Yoshimatsu, T.F.; Pitt, J.J.; Hoadley, K.A.; Troester, M. Comparison of breast cancer molecular features and survival by African and European ancestry in The Cancer Genome Atlas. JAMA Oncol. 2017, 3, 1654–1662. [Google Scholar] [CrossRef]
- Mersha, T.B.; Abebe, T. Self-reported race/ethnicity in the age of genomic research: Its potential impact on understanding health disparities. Hum. Genom. 2015, 9, 1. [Google Scholar] [CrossRef]
- Cooper, R.S. Race and genomics. N. Engl. J. Med. 2003, 348, 1166. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Abdellateif, M.S.; Zekri, A.-R.N. Cancer in Africa: Is It a Genetic or Environmental Health Problem? Front. Oncol. 2020, 10, 604214. [Google Scholar] [CrossRef]
- Pinheiro, P.; Callahan, K.; Koru-Sengul, T.; Ransdell, J.; Bouzoubaa, L.; Brown, C.; Kobetz, E. Risk of Cancer Death Among White, Black, and Hispanic Populations in South Florida. Prev. Chronic Dis. 2019, 16, E83. [Google Scholar] [CrossRef]
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 29 August 2023).
- Metzger-Filho, O.; Tutt, A.; de Azambuja, E.; Saini, K.S.; Viale, G.; Loi, S.; Bradbury, I.; Bliss, J.M.; Azim, H.A., Jr.; Ellis, P.; et al. Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Metzger-Filho, O.; Michiels, S.; Bertucci, F.; Catteau, A.; Salgado, R.; Galant, C.; Fumagalli, D.; Singhal, S.K.; Desmedt, C.; Ignatiadis, M.; et al. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann. Oncol. 2013, 24, 377–384. [Google Scholar] [CrossRef]
- Saini, K.S.; Loi, S.; de Azambuja, E.; Metzger-Filho, O.; Saini, M.L.; Ignatiadis, M.; Dancey, J.E.; Piccart-Gebhart, M.J. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat. Rev. 2013, 39, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Franco, M.M.; Leon-Rodriguez, E. Delays in Breast Cancer Detection and Treatment in Developing Countries. Breast Cancer 2018, 12, 1178223417752677. [Google Scholar] [CrossRef] [PubMed]
- Espina, C.; McKenzie, F.; Dos-Santos-Silva, I. Delayed presentation and diagnosis of breast cancer in African women: A systematic review. Ann. Epidemiol. 2017, 27, 659–671.e657. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112, S92–S107. [Google Scholar] [CrossRef] [PubMed]
- Assi, H.A.; Khoury, K.E.; Dbouk, H.; Khalil, L.E.; Mouhieddine, T.H.; El Saghir, N.S. Epidemiology and prognosis of breast cancer in young women. J. Thorac. Dis. 2013, 5 (Suppl. S1), S2–S8. [Google Scholar]
- Jan, M.; Mattoo, J.A.; Salroo, N.A.; Ahangar, S. Triple assessment in the diagnosis of breast cancer in Kashmir. Indian J. Surg. 2010, 72, 97–103. [Google Scholar] [CrossRef][Green Version]
- Maha, R.; Alison, J.; Michael, S.; Manvydas, V. Triple assessment breast clinics: The value of clinical core biopsies. Ir. J. Med. Sci. 2023, 1–6. [Google Scholar] [CrossRef]
- Katti, G.; Ara, S.A.; Shireen, A. Magnetic resonance imaging (MRI)–A review. Int. J. Dent. Clin. 2011, 3, 65–70. [Google Scholar]
- Caldemeyer, K.S.; Buckwalter, K.A. The basic principles of computed tomography and magnetic resonance imaging. J. Am. Acad. Dermatol. 1999, 41, 768–771. [Google Scholar] [CrossRef]
- Boellaard, R.; O’Doherty, M.J.; Weber, W.A.; Mottaghy, F.M.; Lonsdale, M.N.; Stroobants, S.G.; Oyen, W.J.G.; Kotzerke, J.; Hoekstra, O.S.; Pruim, J. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.-J.; Wienke, A.; Surov, A. Associations between GLUT expression and SUV values derived from FDG-PET in different tumors—A systematic review and meta analysis. PLoS ONE 2019, 14, e0217781. [Google Scholar] [CrossRef] [PubMed]
- Peck, R.W. The right dose for every patient: A key step for precision medicine. Nat. Rev. Drug Discov. 2016, 15, 145–146. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. Precision Medicine Vision Statement: A Product of the World Economic Forum Global Precision Medicine Council. Available online: https://www.weforum.org/reports/precision-medicine-vision-statement-a-product-of-the-world-economic-forum-global-precision-medicine-council/ (accessed on 2 December 2023).
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef] [PubMed]
- Strande, N.T.; Riggs, E.R.; Buchanan, A.H.; Ceyhan-Birsoy, O.; DiStefano, M.; Dwight, S.S.; Goldstein, J.; Ghosh, R.; Seifert, B.A.; Sneddon, T.P. Evaluating the clinical validity of gene-disease associations: An evidence-based framework developed by the clinical genome resource. Am. J. Hum. Genet. 2017, 100, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef] [PubMed]
- Illumina. What Is NGS? Available online: https://www.illumina.com/science/technology/next-generation-sequencing.html (accessed on 6 June 2023).
- Muzzey, D.; Evans, E.A.; Lieber, C. Understanding the basics of NGS: From mechanism to variant calling. Curr. Genet. Med. Rep. 2015, 3, 158–165. [Google Scholar] [CrossRef]
- Visvikis, D.; Cheze Le Rest, C.; Jaouen, V.; Hatt, M. Artificial intelligence, machine (deep) learning and radio(geno)mics: Definitions and nuclear medicine imaging applications. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2630–2637. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, P. Extendable and explainable deep learning for pan-cancer radiogenomics research. Curr. Opin. Chem. Biol. 2022, 66, 102111. [Google Scholar] [CrossRef]
- Pinker, K.; Shitano, F.; Sala, E.; Do, R.K.; Young, R.J.; Wibmer, A.G.; Hricak, H.; Sutton, E.J.; Morris, E.A. Background, current role, and potential applications of radiogenomics. J. Magn. Reson. Imaging 2018, 47, 604–620. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Tixier, F.; Cheze-Le-Rest, C.; Schick, U.; Simon, B.; Dufour, X.; Key, S.; Pradier, O.; Aubry, M.; Hatt, M.; Corcos, L. Transcriptomics in cancer revealed by Positron Emission Tomography radiomics. Sci. Rep. 2020, 10, 5660. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Mu, W.; Tunali, I.; Gray, J.E.; Qi, J.; Schabath, M.B.; Gillies, R.J. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1168–1182. [Google Scholar] [CrossRef]
- Anderson, A.W.; Xie, J.; Pizzonia, J.; Bronen, R.A.; Spencer, D.D.; Gore, J.C. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn. Reson. Imaging 2000, 18, 689–695. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, D.; Yu, F.; Heidari, A.A.; Ru, J.; Chen, H.; Mafarja, M.; Turabieh, H.; Pan, Z. Performance optimization of differential evolution with slime mould algorithm for multilevel breast cancer image segmentation. Comput. Biol. Med. 2021, 138, 104910. [Google Scholar] [CrossRef]
- Yu, H.; Fan, Y.; Ma, H.; Zhang, H.; Cao, C.; Yu, X.; Sun, J.; Cao, Y.; Liu, Y. Segmentation of the cervical lesion region in colposcopic images based on deep learning. Front. Oncol. 2022, 12, 952847. [Google Scholar] [CrossRef]
- Ren, K.; Chang, L.; Wan, M.; Gu, G.; Chen, Q. An improved U-net based retinal vessel image segmentation method. Heliyon 2022, 8, e11187. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Lu, B.; Chen, B.; Shi, J.; Chen, G.; Wang, M.; Pan, Z.; Lin, Y.; Gao, Z.; Zhou, J.; et al. Attention guided neural ODE network for breast tumor segmentation in medical images. Comput. Biol. Med. 2023, 159, 106884. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Rios Velazquez, E.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Mitra, S.; Shankar, B.U.; Kikinis, R.; Haibe-Kains, B. Robust radiomics feature quantification using semiautomatic volumetric segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Parmar, C.; Blezek, D.; Estepar, R.S.J.; Pieper, S.; Kim, J.; Aerts, H.J.W.L. Application of the 3D slicer chest imaging platform segmentation algorithm for large lung nodule delineation. PLoS ONE 2017, 12, e0178944. [Google Scholar] [CrossRef]
- Dorador, J.; Rodríguez-Tovar, F.J. CroSSED sequence, a new tool for 3D processing in geosciences using the free software 3DSlicer. Sci. Data 2020, 7, 270. [Google Scholar] [CrossRef]
- Mouawad, M.; Biernaski, H.; Brackstone, M.; Lock, M.; Kornecki, A.; Shmuilovich, O.; Ben-Nachum, I.; Prato, F.S.; Thompson, R.T.; Gaede, S. The effect of registration on voxel-wise Tofts model parameters and uncertainties from DCE-MRI of early-stage breast cancer patients using 3DSlicer. J. Digit. Imaging 2020, 33, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.R.; Parmar, C.; Jermoumi, M.; Mak, R.H.; Van Baardwijk, A.; Fennessy, F.M.; Lewis, J.H.; De Ruysscher, D.; Kikinis, R.; Lambin, P. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci. Rep. 2013, 3, 3529. [Google Scholar] [CrossRef] [PubMed]
- Sensakovic, W.F.; Armato Iii, S.G.; Straus, C.; Roberts, R.Y.; Caligiuri, P.; Starkey, A.; Kindler, H.L. Computerized segmentation and measurement of malignant pleural mesothelioma. Med. Phys. 2011, 38, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Um, H.; Tixier, F.; Bermudez, D.; Deasy, J.O.; Young, R.J.; Veeraraghavan, H. Impact of image preprocessing on the scanner dependence of multi-parametric MRI radiomic features and covariate shift in multi-institutional glioblastoma datasets. Phys. Med. Biol. 2019, 64, 165011. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, H.; Dashevsky, B.Z.; Onishi, N.; Sadinski, M.; Morris, E.; Deasy, J.O.; Sutton, E.J. Appearance Constrained Semi-Automatic Segmentation from DCE-MRI is Reproducible and Feasible for Breast Cancer Radiomics: A Feasibility Study. Sci. Rep. 2018, 8, 4838. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wen, C.; Qin, S.; He, M. Extracting automata from neural networks using active learning. PeerJ Comput. Sci. 2021, 7, e436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, P.; Jiang, Y.; Ge, H.; Li, S.; Jin, H.; Li, Y. Prediction of Aneurysm Stability Using a Machine Learning Model Based on PyRadiomics-Derived Morphological Features. Stroke 2019, 50, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, W.; Ren, L.; Chen, J.; Tang, W.; Liu, T.; Jian, M.; Liu, Y.; Wei, Y.; Xu, J. Comprehensive Evaluation of Relapse Risk (CERR) Score for Colorectal Liver Metastases: Development and Validation. Oncologist 2020, 25, e1031–e1041. [Google Scholar] [CrossRef]
- Apte, A.P.; Iyer, A.; Crispin-Ortuzar, M.; Pandya, R.; van Dijk, L.V.; Spezi, E.; Thor, M.; Um, H.; Veeraraghavan, H.; Oh, J.H.; et al. Technical Note: Extension of CERR for computational radiomics: A comprehensive MATLAB platform for reproducible radiomics research. Med. Phys. 2018, 45, 3713–3720. [Google Scholar] [CrossRef]
- Bettinelli, A.; Branchini, M.; De Monte, F.; Scaggion, A.; Paiusco, M. Technical Note: An IBEX adaption toward image biomarker standardization. Med. Phys. 2020, 47, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Ger, R.B.; Cardenas, C.E.; Anderson, B.M.; Yang, Z.; Mackin, D.S.; Zhang, L.; Court, L.E. Guidelines and Experience Using Imaging Biomarker Explorer (IBEX) for Radiomics. JoVE 2018, 131, e57132. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z.; Huang, X.; Chen, M.; Hu, Z.T.; Tang, S.; Chou, I.M.; Pan, Z.; Wang, Q.; Wang, J. Catalytic supercritical water oxidation of o-chloroaniline over Ru/rGO: Reaction variables, conversion pathways and nitrogen distribution. Chemosphere 2023, 333, 138907. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, H.; Heidari, A.A.; Gui, W.; Ye, X.; Chen, Y.; Chen, H.; Pan, Z. MFeature: Towards high performance evolutionary tools for feature selection. Expert Syst. Appl. 2021, 186, 11565. [Google Scholar] [CrossRef]
- Nie, K.; Al-Hallaq, H.; Li, X.A.; Benedict, S.H.; Sohn, J.W.; Moran, J.M.; Fan, Y.; Huang, M.; Knopp, M.V.; Michalski, J.M. NCTN assessment on current applications of radiomics in oncology. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Goh, V.; Mandeville, H.C.; Ng, Q.S.; Hoskin, P.J.; Miles, K.A. Non–Small Cell Lung Cancer: Histopathologic Correlates for Texture Parameters at CT. Radiology 2013, 266, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Sibille, L.; Seifert, R.; Avramovic, N.; Vehren, T.; Spottiswoode, B.; Zuehlsdorff, S.; Schäfers, M. 18F-FDG PET/CT uptake classification in lymphoma and lung cancer by using deep convolutional neural networks. Radiology 2020, 294, 445–452. [Google Scholar] [CrossRef]
- Roffo, G. Feature selection library (MATLAB toolbox). arXiv 2016, arXiv:1607.01327. [Google Scholar]
- Gao, J.; Jiang, Q.; Zhou, B.; Chen, D. Convolutional neural networks for computer-aided detection or diagnosis in medical image analysis: An overview. Math. Biosci. Eng. 2019, 16, 6536–6561. [Google Scholar] [CrossRef]
- Liu, B.; Chi, W.; Li, X.; Li, P.; Liang, W.; Liu, H.; Wang, W.; He, J. Evolving the pulmonary nodules diagnosis from classical approaches to deep learning-aided decision support: Three decades’ development course and future prospect. J. Cancer Res. Clin. Oncol. 2020, 146, 153–185. [Google Scholar] [CrossRef]
- Xu, Y.; Hosny, A.; Zeleznik, R.; Parmar, C.; Coroller, T.; Franco, I.; Mak, R.H.; Aerts, H.J.W.L. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clin. Cancer Res. 2019, 25, 3266–3275. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Do, R.K.G.; Gultekin, D.H.; Gönen, M.; Schwartz, L.H.; Fong, Y.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Klimstra, D.S. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: A potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann. Surg. Oncol. 2014, 21, 2675–2683. [Google Scholar] [CrossRef]
- Gürsoy Çoruh, A.; Yenigün, B.; Uzun, Ç.; Kahya, Y.; Büyükceran, E.U.; Elhan, A.; Orhan, K.; Kayı Cangır, A. A comparison of the fusion model of deep learning neural networks with human observation for lung nodule detection and classification. Br. J. Radiol. 2021, 94, 20210222. [Google Scholar] [CrossRef]
- Dlamini, Z.; Skepu, A.; Kim, N.; Mkhabele, M.; Khanyile, R.; Molefi, T.; Mbatha, S.; Setlai, B.; Mulaudzi, T.; Mabongo, M. AI and precision oncology in clinical cancer genomics: From prevention to targeted cancer therapies-an outcomes based patient care. Inform. Med. Unlocked 2022, 31, 100965. [Google Scholar] [CrossRef]
- Rutman, A.M.; Kuo, M.D. Radiogenomics: Creating a link between molecular diagnostics and diagnostic imaging. Eur. J. Radiol. 2009, 70, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, M.; Aiello, M.; Infante, T.; Cavaliere, C.; Grimaldi, A.M.; Mirabelli, P.; Monti, S.; Salvatore, M. Radiogenomic Analysis of Oncological Data: A Technical Survey. Int. J. Mol. Sci. 2017, 18, 805. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, O.; Echegaray, S.; Khuong, A.; Hoang, C.D.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Guo, H.H.; Lau, C.; Plevritis, S.K. Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci. Rep. 2017, 7, 41674. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Lakhman, Y.; Gönen, M.; Goldman, D.A.; Miccò, M.; D’anastasi, M.; Johnson, S.A.; Juluru, K.; Arnold, A.G.; Sosa, R.E. High-grade serous ovarian cancer: Associations between BRCA mutation status, CT imaging phenotypes, and clinical outcomes. Radiology 2017, 285, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Veeraraghavan, H.; Micco, M.; Nougaret, S.; Lakhman, Y.; Meier, A.A.; Sosa, R.; Soslow, R.A.; Levine, D.A.; Weigelt, B. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur. Radiol. 2017, 27, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Lakhman, Y.; Molinari, N.; Feier, D.; Scelzo, C.; Vargas, H.A.; Sosa, R.E.; Hricak, H.; Soslow, R.A.; Grisham, R.N. CT features of ovarian tumors: Defining key differences between serous borderline tumors and low-grade serous carcinomas. AJR. Am. J. Roentgenol. 2018, 210, 918. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.; Xu, K.; Wang, K.; Fan, X.; Li, S.; Jiang, T.; Liu, X.; Wang, Y. MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. NeuroImage Clin. 2018, 17, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mangla, R.; Tian, W.; Qiu, X.; Li, D.; Walter, K.A.; Ekholm, S.; Johnson, M.D. The preliminary radiogenomics association between MR perfusion imaging parameters and genomic biomarkers, and their predictive performance of overall survival in patients with glioblastoma. J. Neuro-Oncol. 2017, 135, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Mazurowski, M.A.; Clark, K.; Czarnek, N.M.; Shamsesfandabadi, P.; Peters, K.B.; Saha, A. Radiogenomics of lower-grade glioma: Algorithmically-assessed tumor shape is associated with tumor genomic subtypes and patient outcomes in a multi-institutional study with The Cancer Genome Atlas data. J. Neuro-Oncol. 2017, 133, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.K.; Choi, S.H.; Shin, D.J.; Jo, S.W.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H. Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur. Radiol. 2018, 28, 4350–4361. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.-P.; Radbruch, A. Radiogenomics of glioblastoma: Machine learning–based classification of molecular characteristics by using multiparametric and multiregional MR imaging features. Radiology 2016, 281, 907–918. [Google Scholar] [CrossRef]
- Cui, Y.; Ren, S.; Tha, K.K.; Wu, J.; Shirato, H.; Li, R. Volume of high-risk intratumoral subregions at multi-parametric MR imaging predicts overall survival and complements molecular analysis of glioblastoma. Eur. Radiol. 2017, 27, 3583–3592. [Google Scholar] [CrossRef]
- Hu, L.S.; Ning, S.; Eschbacher, J.M.; Baxter, L.C.; Gaw, N.; Ranjbar, S.; Plasencia, J.; Dueck, A.C.; Peng, S.; Smith, K.A. Radiogenomics to characterize regional genetic heterogeneity in glioblastoma. Neuro-Oncology 2017, 19, 128–137. [Google Scholar] [CrossRef]
- Jamshidi, N.; Diehn, M.; Bredel, M.; Kuo, M.D. Illuminating Radiogenomic Characteristics of Glioblastoma Multiforme through Integration of MR Imaging, Messenger RNA Expression, and DNA Copy Number Variation. Radiology 2013, 270, 1–2. [Google Scholar] [CrossRef]
- Li, H.; Giger, M.L.; Lan, L.; Janardanan, J.; Sennett, C.A. Special Section on Pioneers in Medical Imaging: Honoring the Memory of Robert F. Wagner: Comparative analysis of image-based phenotypes of mammographic density and parenchymal patterns in distinguishing between BRCA1/2 cases, unilateral cancer cases, and controls. J. Med. Imaging 2014, 1, 031009. [Google Scholar]
- Grimm, L.J.; Zhang, J.; Mazurowski, M.A. Computational approach to radiogenomics of breast cancer: Luminal A and luminal B molecular subtypes are associated with imaging features on routine breast MRI extracted using computer vision algorithms. J. Magn. Reson. Imaging 2015, 42, 902–907. [Google Scholar] [CrossRef]
- Mazurowski, M.A.; Zhang, J.; Grimm, L.J.; Yoon, S.C.; Silber, J.I. Radiogenomic Analysis of Breast Cancer: Luminal B Molecular Subtype Is Associated with Enhancement Dynamics at MR Imaging. Radiology 2014, 273, 365–372. [Google Scholar] [CrossRef]
- Zhu, Z.; Albadawy, E.; Saha, A.; Zhang, J.; Harowicz, M.R.; Mazurowski, M.A. Deep learning for identifying radiogenomic associations in breast cancer. Comput. Biol. Med. 2019, 109, 85–90. [Google Scholar] [CrossRef]
- Yamamoto, S.; Han, W.; Kim, Y.; Du, L.; Jamshidi, N.; Huang, D.; Kim, J.H.; Kuo, M.D. Breast Cancer: Radiogenomic Biomarker Reveals Associations among Dynamic Contrast-enhanced MR Imaging, Long Noncoding RNA, and Metastasis. Radiology 2015, 275, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Karlo, C.A.; Di Paolo, P.L.; Chaim, J.; Hakimi, A.A.; Ostrovnaya, I.; Russo, P.; Hricak, H.; Motzer, R.; Hsieh, J.J.; Akin, O. Radiogenomics of clear cell renal cell carcinoma: Associations between CT imaging features and mutations. Radiology 2014, 270, 464–471. [Google Scholar] [CrossRef]
- Li, Z.-C.; Zhai, G.; Zhang, J.; Wang, Z.; Liu, G.; Wu, G.-y.; Liang, D.; Zheng, H. Differentiation of clear cell and non-clear cell renal cell carcinomas by all-relevant radiomics features from multiphase CT: A VHL mutation perspective. Eur. Radiol. 2019, 29, 3996–4007. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J. Breast MRI radiogenomics: Current status and research implications. J. Magn. Reson. Imaging 2016, 43, 1269–1278. [Google Scholar] [CrossRef]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.; Bassett, L.; Feig, S. Breast imaging reporting and data system (BI-RADS). Radiol. Rest. 2018, 40, 409–430. [Google Scholar]
- Mann, R.M.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Colin, C.; Cornford, E.; Evans, A.; Fallenberg, E.; Forrai, G.; Fuchsjäger, M.H. Breast MRI: EUSOBI recommendations for women’s information. Eur. Radiol. 2015, 25, 3669–3678. [Google Scholar] [CrossRef]
- Yamamoto, S.; Maki, D.D.; Korn, R.L.; Kuo, M.D. Radiogenomic analysis of breast cancer using MRI: A preliminary study to define the landscape. Am. J. Roentgenol. 2012, 199, 654–663. [Google Scholar] [CrossRef]
- Baltrušaitis, T.; Ahuja, C.; Morency, L.P. Multimodal Machine Learning: A Survey and Taxonomy. IEEE Trans. Pattern Anal. Mach. Intell. 2019, 41, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, X.; Zhang, W.; Zhang, G. Radar and Rain Gauge Merging-Based Precipitation Estimation via Geographical–Temporal Attention Continuous Conditional Random Field. IEEE Trans. Geosci. Remote Sens. 2018, 56, 5558–5571. [Google Scholar] [CrossRef]
- Zou, B.; Ji, Z.; Zhu, C.; Dai, Y.; Zhang, W.; Kui, X. Multi-scale deformable transformer for multi-contrast knee MRI super-resolution. Biomed. Signal Process. Control. 2023, 79, 1041. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Wen, Y.; Zhang, Y.; Yuan, X. Discriminative extraction of features from time series. Neurocomputing 2018, 275, 2317–2328. [Google Scholar] [CrossRef]
- Li, D.; Yang, X.; Tang, Y.; Zhang, C.; Zhang, W.; Ma, L. Active learning with effective scoring functions for semi-supervised temporal action localization. Displays 2023, 78, 102434. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Guo, W.; Cai, X.; Yuan, X. Learning disentangled representation for multimodal cross-domain sentiment analysis. IEEE Trans. Neural Netw. Learn. Syst. 2022, 34, 7956–7966. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.T.; Wang, P.; Zhao, R.; Zhang, Q. Tt-tsvd: A multi-modal tensor train decomposition with its application in convolutional neural networks for smart healthcare. ACM Trans. Multimed. Comput. Commun. Appl. (TOMM) 2022, 18, 1–17. [Google Scholar] [CrossRef]
- Zhu, L.; He, Q.; Huang, Y.; Zhang, Z.; Zeng, J.; Lu, L.; Kong, W.; Zhou, F. DualMMP-GAN: Dual-scale multi-modality perceptual generative adversarial network for medical image segmentation. Comput. Biol. Med. 2022, 144, 105387. [Google Scholar] [CrossRef]
- Xi, J.; Wang, D.; Yang, X.; Zhang, W.; Huang, Q. Cancer omic data based explainable AI drug recommendation inference: A traceability perspective for explainability. Biomed. Signal Process. Control. 2023, 79, 104144. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, C.; Hu, F.; Zhou, T.; Feng, L.; Xu, G.; Yi, Z.; Zhang, X. Efficient two-step liver and tumour segmentation on abdominal CT via deep learning and a conditional random field. Comput. Biol. Med. 2022, 150, 106076. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, Y.; Gong, Y.-J.; Zhou, Y. Prior knowledge regularized multiview self-representation and its applications. IEEE Trans. Neural Netw. Learn. Syst. 2020, 32, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Miao, Y.; Chen, J.; Zhang, X.; Han, L.; Ran, D.; Huang, Z.; Pei, N.; Liu, H.; An, C. Twist-Net: A multi-modality transfer learning network with the hybrid bilateral encoder for hypopharyngeal cancer segmentation. Comput. Biol. Med. 2023, 154, 106555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, X.; Zou, Q. Effector-GAN: Prediction of fungal effector proteins based on pretrained deep representation learning methods and generative adversarial networks. Bioinformatics 2022, 38, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q.; Xi, J. Deepda-ace: A novel domain adaptation method for species-specific acetylation site prediction. Mathematics 2022, 10, 2364. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhuo, Q.; Zhao, C.; Deng, X.; Zhu, T.; Wang, T.; Jiang, W.; Lei, B. Self-supervised multi-modal fusion network for multi-modal thyroid ultrasound image diagnosis. Comput. Biol. Med. 2022, 150, 106164. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, Q. Prediction of protein solubility based on sequence physicochemical patterns and distributed representation information with DeepSoluE. BMC Biol. 2023, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Yang, H.; Wei, L.; Chen, S.; Zou, Q. A multi-label learning model for predicting drug-induced pathology in multi-organ based on toxicogenomics data. PLoS Comput. Biol. 2022, 18, e1010402. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, P. A novel integrative computational framework for breast cancer radiogenomic biomarker discovery. Comput. Struct. Biotechnol. J. 2022, 20, 2484–2494. [Google Scholar] [CrossRef]
- Fan, M.; Cui, Y.; You, C.; Liu, L.; Gu, Y.; Peng, W.; Bai, Q.; Gao, X.; Li, L. Radiogenomic Signatures of Oncotype DX Recurrence Score Enable Prediction of Survival in Estrogen Receptor-Positive Breast Cancer: A Multicohort Study. Radiology 2022, 302, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Jinguji, M.; Kajiya, Y.; Kamimura, K.; Nakajo, M.; Sagara, Y.; Takahama, T.; Ando, M.; Rai, Y.; Sagara, Y.; Ohi, Y.; et al. Rim Enhancement of Breast Cancers on Contrast-Enhanced MR Imaging: Relationship with Prognostic Factors. Breast Cancer 2006, 13, 64–73. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Nicholson, B.T. Rim-enhancing breast masses with smooth or spiculated margins on magnetic resonance imaging: Histopathology and clinical significance. Jpn. J. Radiol. 2011, 29, 609–614. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining molecular and imaging metrics in cancer: Radiogenomics. Insights Imaging 2020, 11, 1. [Google Scholar] [CrossRef]
- Tung, N.M.; Garber, J.E. BRCA 1/2 testing: Therapeutic implications for breast cancer management. Br. J. Cancer 2018, 119, 141–152. [Google Scholar] [CrossRef]
- Kang, J.; Rancati, T.; Lee, S.; Oh, J.H.; Kerns, S.L.; Scott, J.G.; Schwartz, R.; Kim, S.; Rosenstein, B.S. Machine learning and radiogenomics: Lessons learned and future directions. Front. Oncol. 2018, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Mazurowski, M.A. Radiogenomics: What it is and why it is important. J. Am. Coll. Radiol. 2015, 12, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
- Andreassen, C.N.; Schack, L.M.H.; Laursen, L.V.; Alsner, J. Radiogenomics–current status, challenges and future directions. Cancer Lett. 2016, 382, 127–136. [Google Scholar] [CrossRef]
- Gallivanone, F.; Bertoli, G.; Porro, D. Radiogenomics, Breast Cancer Diagnosis and Characterization: Current Status and Future Directions. Methods Protoc. 2022, 5, 78. [Google Scholar] [CrossRef]
- Demetriou, D.; Hull, R.; Kgoebane-Maseko, M.; Lockhat, Z.; Dlamini, Z. AI-Enhanced Digital Pathology and Radiogenomics in Precision Oncology. In Artificial Intelligence and Precision Oncology: Bridging Cancer Research and Clinical Decision Support; Dlamini, Z., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 93–113. [Google Scholar]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging imaging and genomics. Abdom. Radiol. 2019, 44, 1960–1984. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Savoldi, F.; Rossi, D.; Bellomi, M. Radiogenomics as association between non-invasive imaging features and molecular genomics of lung cancer. Ann. Transl. Med. 2018, 6, 447. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, B. A review of radiomics and genomics applications in cancers: The way towards precision medicine. Radiat. Oncol. 2022, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J.; Mazurowski, M.A. Breast Cancer Radiogenomics: Current Status and Future Directions. Acad. Radiol. 2020, 27, 39–46. [Google Scholar] [CrossRef]
- Saxena, S.; Jena, B.; Gupta, N.; Das, S.; Sarmah, D.; Bhattacharya, P.; Nath, T.; Paul, S.; Fouda, M.M.; Kalra, M.; et al. Role of Artificial Intelligence in Radiogenomics for Cancers in the Era of Precision Medicine. Cancers 2022, 14, 2860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demetriou, D.; Lockhat, Z.; Brzozowski, L.; Saini, K.S.; Dlamini, Z.; Hull, R. The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics. Cancers 2024, 16, 1076. https://doi.org/10.3390/cancers16051076
Demetriou D, Lockhat Z, Brzozowski L, Saini KS, Dlamini Z, Hull R. The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics. Cancers. 2024; 16(5):1076. https://doi.org/10.3390/cancers16051076
Chicago/Turabian StyleDemetriou, Demetra, Zarina Lockhat, Luke Brzozowski, Kamal S. Saini, Zodwa Dlamini, and Rodney Hull. 2024. "The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics" Cancers 16, no. 5: 1076. https://doi.org/10.3390/cancers16051076
APA StyleDemetriou, D., Lockhat, Z., Brzozowski, L., Saini, K. S., Dlamini, Z., & Hull, R. (2024). The Convergence of Radiology and Genomics: Advancing Breast Cancer Diagnosis with Radiogenomics. Cancers, 16(5), 1076. https://doi.org/10.3390/cancers16051076






