The Neutrophil-to-Lymphocyte Ratio as a Biomarker in Cutaneous Oncology: A Systematic Review of Evidence beyond Malignant Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
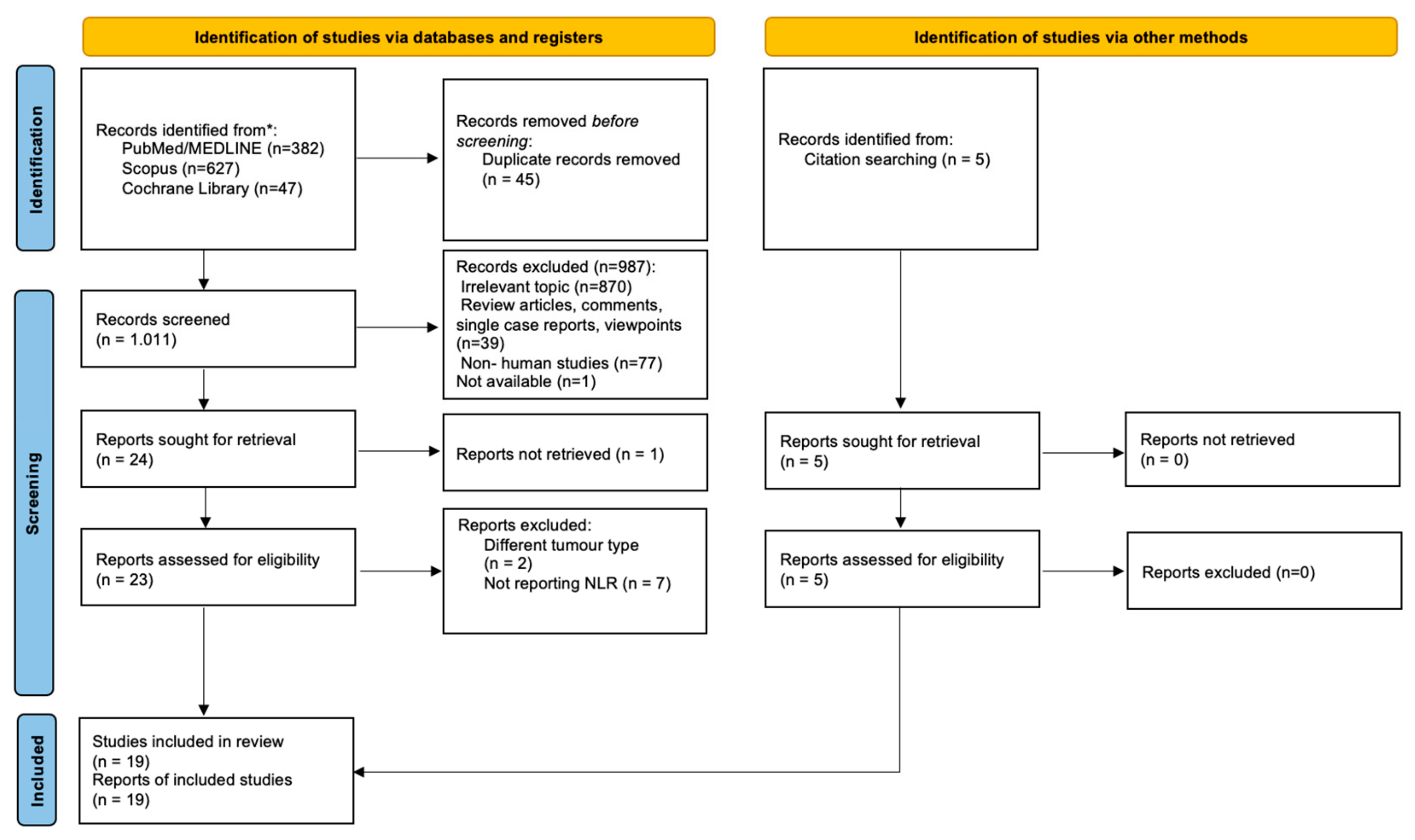
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility of Relevant Studies
2.3. Study Selection
2.4. Data Collection and Risk of Bias Assessment
2.5. Data Synthesis and Analysis
3. Results
3.1. Cancer of Epithelial Cell Origin
3.1.1. Keratinocyte Skin Cancer
Basal Cell Carcinoma (BCC)
Cutaneous Squamous Cell Carcinoma (cSCC)
3.1.2. Cutaneous Adenocarcinoma
3.2. Merkel Cell Carcinoma
3.3. Cutaneous Sarcomas
3.4. Primary Cutaneous Lymphomas
| Skin Cancer | Study | Entity/Patients (n) | Results | |||
|---|---|---|---|---|---|---|
| Author/Year * | Country | Type | End Point | Outcome | ||
| KSC | Di Raimondo/2022 [43] | Italy | RS | cSCC/51 | Tumor stage | NLR > 3.07 at diagnosis was associated with a higher than stage I |
| Strippoli/2021 [42] | Italy | RS | advanced-metastatic cSCC/30 | Response to cemiplimab | Low baseline NLR correlated with a better treatment response | |
| Seddon/2016 [40] | New Zealand | RS | cSCC/168 | Patient’s immune state | NLR was higher in immunosuppressed compared to immunocompetent patients (NLR = 3.0, range: 0.7–23.0; p < 0.001) | |
| Derebaşınlıoğlu/2022 [38] | Turkey | RS | BCC/144 cSCC/84 | Differential diagnosis | NLR was higher in cSCC compared to BCC patients | |
| Tumor location | No statistical differences in NLR according to location | |||||
| LN metastases | Higher PLR levels (>180.7) and PltxNLR (>747) were correlated with higher risk of LN metastasis at the time of initial diagnosis | |||||
| Maeda/2022 [41] | Japan | RS | cSCC/222 | Disease-specific survival | An elevated NLR was an independent prognostic factor for adverse DSM | |
| SLN metastasis | An elevated NLR was a predictive factor for SLN positivity | |||||
| Cutaneous adeno-carcinoma | Maeda/2022 [46] | Japan | RS | EMPD/109 | Metastases | Higher NLR in patients with metastatic EMPD (mean: 3.39 vs. 2.53; p < 0.005) |
| OS | NLR was an independent prognostic factors for OS (p = 0.019) | |||||
| Maeda/2023 [47] | Japan | RS | EMPD/85 | SLN metastasis | NLR was independent predictor of SLN positivity | |
| Ebata/2021 [48] | Japan | RS | EMPD/137 | LN metastases | NLR > 3.0 was a predictor of LN metastasis (OR = 3.311, 95%CI: 1.117–9.804; p = 0.0380) | |
| MCC | Zaragoza/2016 [51] | France | RS | MCC/75 | Disease specific mortality | Baseline NLR ≥ 4 is independently associated with DSM (multivariate analysis; HR = 3.30, 95%CI: 1.21–9.01, p = 0.020) |
| Recurrence | NSA | |||||
| Torchio/2020 [52] | Italy | RS | MCC/95 | DFS | Baseline NLR was strongly associated with worse DFS (multivariate analysis: HR = 1.63, 95%CI: 1.34–1.99; p < 0.001) | |
| OS | Baseline NLR was strongly associated with worse OS (multivariate analysis: HR = 1.44, 95%CI: 1.10–1.90; p = 0.008) | |||||
| Nghiem/2021 [53] | USA | PS | Advanced unresectable MCC treated with pembruli-zumab/50 | Treatment response | NLR across the first 3 months of therapy (but not any isolated value) correlated with improved objective response (p = 0.043) | |
| OS | NLR across the first 3 months of therapy (but not any isolated value) correlated with 30 months OS (p = 0.028) | |||||
| Gambichler/2022 [54] | Germany | RS | Stage I-III MCC/49 | Tumor stage | PIV was higher in MCC stage II and III in comparison to MCC stage I | |
| Recurrence | PIV >372 was significantly associated with MCC recurrence (p < 0.0001) | |||||
| Primary cutaneous sarcomas | Awaji/2021 [59] | Japan | RS | PCA/26 | OS | NLR > 2.4 was an independent prognostic factor for shorter OS (hazard ratio = 5.04, 95%CI: 1.26–20.1; p = 0.022) |
| Suzuki/2017 [60] | Japan | RS | ASFS/17 | Diagnosis | Higher NLR in patients (2.63 ± 0.94 in patient vs. 1.93 ± 0.81 in the control group; p = 0.0063) | |
| Healthy controls/56 | ||||||
| Primary cutaneous lymphomas | Eren/2016 [63] | Turkey | RS | MF/117 | Treatment indication | NSA |
| Progression in stage | NSA | |||||
| Time to progression | NSA | |||||
| Gambichler/2022 [64] | Germany | RS | MF/29 | Treatment outcome | NSA | |
| Patients’ survival | NSA | |||||
| Vonderheid/2019 [65] | USA | RS | MF/98 Other/17 | Prognosis | NSA | |
| Cengiz/2017 [66] | Turkey | RS | MF/119 | Diagnosis | Higher NLR in MF patients compared to controls (2.07 ± 1.17 vs. 1.76 ± 0.53; p < 0.05) | |
| Disease stage and progression in stage | NLR > 2.85 at diagnosis positively correlates with advanced disease stage and progression in stage | |||||
| Di Raimondo/2023 [67] | Italy | RS | MF/302 | Disease stage and progression in stage | NLR > 2.3 is associated with higher disease stage (>IIB), however, not with the risk of patients’ progression in stage (p = 0.077). | |
3.5. Evaluation of Different Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faria, S.S.; Fernandes, P.C., Jr.; Silva, M.J.; Lima, V.C.; Fontes, W.; Freitas-Junior, R.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Jiang, F.; Hu, L.; Chen, J.; Wang, Y. Distribution and reference interval establishment of neutral-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in Chinese healthy adults. J. Clin. Lab. Anal. 2021, 35, e23935. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.-P.; Latinne, D.; Van Pel, M.-C.; De Kock, M. What is the normal value of the neutrophil-to lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; Van Eijck, C.H.J.; Stricker, B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Valero, C.; Zanoni, D.K.; McGill, M.R.; Ganly, I.; Morris, L.G.T.; Quer, M.; Shah, J.P.; Wong, R.J.; León, X.; Patel, S.G. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer 2020, 126, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kalemaki, D.; Tzagkarakis, E.; Lydakis, C. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect. Dis. 2018, 50, 163–174. [Google Scholar] [CrossRef]
- Fouad, Y.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Schmidt, H.; Bastholt, L.; Geertsen, P.; Christensen, I.J.; Larsen, S.; Gehl, J.; von der Maase, H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: A prognostic model. Br. J. Cancer 2005, 93, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef]
- Kast, R.E. High Neutrophil-to-Lymphocyte Ratio Facilitates Cancer Growth-Currently Marketed Drugs Tadalafil, Isotretinoin, Colchicine, and Omega-3 to Reduce It: The TICO Regimen. Cancers 2022, 14, 4965. [Google Scholar] [CrossRef]
- Ravindranathan, D.; Master, V.A.; Bilen, M.A. Inflammatory Markers in Cancer Immunotherapy. Biology 2021, 10, 325. [Google Scholar] [CrossRef]
- Ocana, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.T.; Miner, T.J.; Vezeridis, M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020, 7, MMT47. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Ferrer, M.; Schuijs, M.J.; Kleeman, S.O.; Mourikis, N.; Hall, Z.; Perera, D.; Raghunathan, S.; Vacca, M.; Gaude, E.; et al. Early neutrophilia marked by aerobic glycolysis sustains host metabolism and delays cancer cachexia. Cancers 2022, 14, 963. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, S.; Qiao, J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: Evidence from a PRISMA-compliant meta-analysis. Medicine 2018, 97, e11446. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Ma, J.Y.; Jian, Q.C. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in melanoma patients: A meta-analysis. Clin. Chim. Acta 2018, 484, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, E.; Rau, R.; Lorenzi, M.; Shui, I.; Townson, S.; Larkin, J. Systematic literature review for the association of biomarkers with efficacy of anti-PD-1 inhibitors in advanced melanoma. Future Oncol. 2021, 17, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Kotenko, S.; Li, W. Prognostic value of neutrophil-lymphocyte ratio and lactate dehydrogenase in melanoma patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Medicine 2022, 101, e29536. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Y.; Sun, H.; Ye, L.; Zeng, F.; Chen, X.; Deng, G. The prognostic significance of baseline neutrophil-to-lymphocyte ratio in melanoma patients receiving immunotherapy. J. Immunother. 2022, 45, 43–50. [Google Scholar] [CrossRef]
- Hu, H.-P.; Archer, C.; Yip, D.; Peters, G. Clinical predictors of survival in real world practice in stage IV melanoma. Cancer Rep. 2023, 6, e1691. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Zappalà, A.R.; Passarelli, F.; Ricci, F.; Abeni, D.; Michelozzi, P. Early inflammatory biomarkers and melanoma survival. Int. J. Dermatol. 2023, 62, 752–758. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Li, X.; Cui, K.; Zhang, W.; Du, Y. Baseline neutrophil-to- ratio combined with the change during treatment provides risk stratification for metastatic malignant melanoma patients treated with PD-1 inhibitors in a Chinese population. Front. Oncol. 2023, 13, 1118301. [Google Scholar] [CrossRef] [PubMed]
- Kudura, K.; Nussbaumer, L.; Foerster, R.; Basler, L. Inflammatory blood parameters as biomarkers for response to immune checkpoint inhibition in metastatic melanoma patients. Biomedicines 2022, 10, 2135. [Google Scholar] [CrossRef]
- Cumpston, M.; Chandler, J. Chapter IV: Updating a Review. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 1–9. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease 2019 Cancer Collaboration. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Derebaşinlioğlu, H.; Demir, H.; Nemmezi Karaca, S. The role of inflammatory markers in the differential diagnosis of skin cancers. J. Contemp. Med. 2022, 12, 761–769. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Seddon, A.; Hock, B.; Miller, A.; Frei, L.; Pearson, J.; McKenzie, J.; Simcock, J.; Currie, M. Cutaneous squamous cell carcinomas with markers of increased metastatic risk are associated with elevated numbers of neutrophils and/or granulocytic myeloid derived suppressor cells. J. Dermatol. Sci. 2016, 83, 124–130. [Google Scholar] [CrossRef]
- Maeda, T.; Hiura, A.; Uehara, J.; Toyoshima, R.; Nakagawa, T.; Yoshino, K. Neutrophil-to-lymphocyte ratio is associated with survival and sentinel lymph node positivity in invasive cutaneous squamous cell carcinoma: A retrospective study. J. Am. Acad. Dermatol. 2022, 86, 615–620. [Google Scholar] [CrossRef]
- Strippoli, S.; Fanizzi, A.; Quaresmini, D.; Nardone, A.; Armenio, A.; Figliuolo, F.; Filotico, R.; Fucci, L.; Mele, F.; Traversa, M.; et al. Cemiplimab in an elderly frail population of patients with locally advanced or metastatic cutaneous squamous cell carcinoma: A single-center real-life experience from Italy. Front. Oncol. 2021, 11, 686308. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, C.; Caposiena Caro, R.D.; Spallone, D.; Silvaggio, D.; Lombardo, P.; Del Duca, E.; Campione, E.; Spallone, G.; Bianchi, L. Baseline neutrophil/lymphocyte ratio (NLR) and red blood cell distribution width (RDW) correlate with advanced stages in cutaneous squamous cell carcinoma. Int. J. Dermatol. 2022, 61, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ishizuki, S.; Nakamura, Y. Extramammary Paget’s Disease: Diagnosis, Pathogenesis, and Treatment with Focus on Recent Developments. Curr. Oncol. 2021, 28, 2969–2986. [Google Scholar] [CrossRef] [PubMed]
- Simonds, R.M.; Segal, R.J.; Sharma, A. Extramammary Paget’s disease: A review of the literature. Int. J. Dermatol. 2019, 58, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Uehara, J.; Toyoshima, R.; Nakagawa, T.; Yoshino, K. Neutrophil-to-lymphocyte ratio is a potential prognostic biomarker for extramammary Paget disease: A retrospective study. J. Dermatol. 2022, 49, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Nagai, K.; Uehara, J.; Toyoshima, R.; Nakagawa, T.; Yoshino, K. Sentinel lymph node biopsy in extramammary Paget disease: A 13-year institutional experience. J. Dermatol. 2023, 50, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ebata, A.; Taki, T.; Mori, S.; Murakami, Y.; Urata, T.; Okumura, M.; Akanabe, H.; Imai, S.; Yokota, K.; Akiyama, M. Neutrophil/lymphocyte ratio as a predictor of lymph node metastasis in extramammary Paget disease: A retrospective study. J. Am. Acad. Dermatol. 2021, 85, 1023–1025. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Mohsin, N.; Yaghi, M.; Frech, F.S.; Dreyfuss, I.; Nouri, K. Merkel cell carcinoma: An updated review of pathogenesis, diagnosis, and treatment options. Dermatol. Ther. 2022, 35, e15292. [Google Scholar] [CrossRef]
- Zaggana, E.; Konstantinou, M.P.; Krasagakis, G.H.; de Bree, E.; Kalpakis, K.; Mavroudis, D.; Krasagakis, K. Merkel cell carcinoma-Update on diagnosis, management and future perspectives. Cancers 2022, 15, 103. [Google Scholar] [CrossRef]
- Zaragoza, J.; Kervarrec, T.; Touzé, A.; Avenel-Audran, M.; Beneton, N.; Esteve, E.; Wierzbicka Hainaut, E.; Aubin, F.; Machet, L.; Samimi, M. A high neutrophil-to-lymphocyte ratio as a potential marker of mortality in patients with Merkel cell carcinoma: A retrospective study. J. Am. Acad. Dermatol. 2016, 75, 712–721.e1. [Google Scholar] [CrossRef]
- Torchio, M.; Corti, F.; Manca, P.; Prinzi, N.; Platania, M.; Maurichi, A.; Mattavelli, I.; Patuzzo, R.; Bedini, N.; Milione, M.; et al. 1182P Baseline neutrophil-lymphocyte ratio and its variations after adjuvant radiotherapy predict clinical survival outcomes in locally advanced Merkel cell carcinoma (MCC). Ann. Oncol. 2020, 31, s781. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J. Immunother. Cancer 2021, 9, e002478. [Google Scholar] [CrossRef]
- Gambichler, T.; Said, S.; Abu Rached, N.; Scheel, C.H.; Susok, L.; Stranzenbach, R.; Becker, J.C. Pan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2022, 148, 3183–3189. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, J.; Steimle-Grauer, S.A.; Hein, R. Cutaneous sarcomas. J. Dtsch. Dermatol. Ges. 2017, 15, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.N.; Liu, J.C. Cutaneous sarcomas. Otolaryngol. Clin. N. Am. 2021, 54, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Stoneham, S.; Hunter, A.; Raahimi, M.; Livesey, A.; Mitchell, C.D.; Keohane, S. Cutaneous sarcoma: A review and practical approach to management. Clin. Exp. Dermatol. 2023, 48, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef]
- Awaji, K.; Miyagawa, T.; Omatsu, J.; Numajiri, H.; Kawai, T.; Funamizu, K.; Saigusa, R.; Yamada, D.; Asano, Y.; Sato, S. Prognostic relevance of pretreatment peripheral neutrophil count and neutrophil-to-lymphocyte ratio in primary cutaneous angiosarcoma. Acta Derm. Venereol. 2021, 101, adv00527. [Google Scholar] [CrossRef]
- Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Sasaki, N.; Shimizu, D.; Kimoto, T.; Asai, J.; Wada, M.; Komori, S.; et al. Clinical usefulness of the platelet-to lymphocyte ratio in patients with angiosarcoma of the face and scalp. Int. J. Mol. Sci. 2017, 18, 2402. [Google Scholar] [CrossRef]
- Hague, C.; Farquharson, N.; Menasce, L.; Parry, E.; Cowan, R. Cutaneous T-cell lymphoma: Diagnosing subtypes and the challenges. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Dulmage, B.; Geskin, L.; Guitart, J.; Akilov, O.E. The biomarker landscape in mycosis fungoides and Sézary syndrome. Exp. Dermatol. 2017, 26, 668–676. [Google Scholar] [CrossRef]
- Eren, R.; Nizam, N.; Doğu, M.H.; Mercan, S.; Erdemir, A.V.; Suyanı, E. Evaluation of neutrophil-lymphocyte ratio in patients with early-stage mycosis fungoides. Ann. Hematol. 2016, 95, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Späth, J.; Said, S.; Scheel, C.H.; Susok, L.; Stranzenbach, R. Outcome of extracorporeal photopheresis in mycosis fungoides patients is not predicted by quotients of systemic immune-inflammatory biomarkers. J. Clin. Apher. 2022, 37, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Vonderheid, E.C.; Martinez, A.R. Prognostic significance of serum copper in patients with cutaneous T-cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 228–238.e4. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, F.P.; Emiroglu, N.; Ozkaya, D.B.; Bahali, A.G.; Su, O.; Onsun, N. Prognostic evaluation of neutrophil/lymphocyte ratio in patients with mycosis fungoides. Ann. Clin. Lab. Sci. 2017, 47, 25–28. [Google Scholar] [PubMed]
- Di Raimondo, C.; Lombardo, P.; Tesei, C.; Esposito, F.; Meconi, F.; Secchi, R.; Lozzi, F.; Monopoli, A.; Narducci, M.G.; Scala, E.; et al. Role of neutrophil-to-lymphocyte ratio (NLR) in patients with mycosis fungoides. Diagnostics 2023, 13, 1979. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.M.; Hidayatullah, F.; Kloping, Y.P.; Renaldo, J.; Chung, E.; Hakim, L. Prognostic value of neutrophil-to-lymphocyte ratio (NLR) in penile cancer: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 81, 104335. [Google Scholar] [CrossRef]
- Díaz de Mera-Sánchez Migallón, I.; Carrión-López, P.; Jesús Fernández-Anguita, P.; Herraiz-Raya, L.; Legido-Gómez, Ó.; Salinas-Sánchez, A.S. Prognostic value of the neutrophil/lymphocyte ratio in squamous cell carcinoma of the penis. Arch. Esp. Urol. 2022, 75, 706–713. [Google Scholar] [CrossRef]
- Ertas, I.E.; Gungorduk, K.; Akman, L.; Ozdemir, A.; Terek, M.C.; Ozsaran, A.; Sanci, M.; Dikmen, Y. Can preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios be used as predictive markers for lymph node metastasis in squamous cell carcinoma of the vulva? Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 138–142. [Google Scholar] [CrossRef]
- Makita, K.; Hamamoto, Y.; Takata, N.; Ishikawa, H.; Tsuruoka, S.; Uwatsu, K.; Hato, N.; Kido, T. Prognostic significance of inflammatory response markers for locally advanced squamous cell carcinoma of the external auditory canal and middle ear. J. Radiat. Res. 2021, 62, 662–668. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Sánchez-Canteli, M.; Triantafyllou, A.; de Bree, R.; Mäkitie, A.A.; Franchi, A.; Hellquist, H.; Saba, N.F.; Stenman, G.; Takes, R.P.; et al. Neutrophil to lymphocyte ratio in oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis. Cancers 2023, 15, 802. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck. 2022, 44, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Dell’Aversana Orabona, G.; Abbate, V.; Vaira, L.A.; Committeri, U.; Bonavolontà, P.; Piombino, P.; Maglitto, F.; Russo, C.; Russo, D.; et al. The prognostic role of the pre-treatment neutrophil to lymphocyte ratio (NLR) and tumor depth of invasion (DOI) in early-stage squamous cell carcinomas of the oral tongue. Oral. Maxillofac. Surg. 2022, 26, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Russo, D.; Maisto, M.; Troiano, G.; Caponio, V.C.A.; Annunziata, M.; Laino, L. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J. Oral. Pathol. Med. 2022, 51, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Nida-E-Zehra; Shoaib, D.; Soomar, S.M.; Afzal, M.; Sidhu, S.M.; Tariq, M.; Zahir, M.N.; Moosajee, M.; Jabbar, A.A.; et al. Mean level of pretreatment neutrophil to lymphocyte ratio in patients with squamous cell carcinoma of the head and neck-Cross-sectional study. Heliyon 2023, 9, e15894. [Google Scholar] [CrossRef] [PubMed]
- Tachinami, H.; Tomihara, K.; Yamada, S.I.; Ikeda, A.; Imaue, S.; Hirai, H.; Nakai, H.; Sonoda, T.; Kurohara, K.; Yoshioka, Y.; et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with recurrent oral squamous cell carcinoma treated with nivolumab. Br. J. Oral. Maxillofac. Surg. 2023, 61, 320–326. [Google Scholar] [CrossRef]
- Qi, W.X.; Wang, X.; Li, C.; Li, S.; Li, H.; Xu, F.; Chen, J.; Zhao, S.; Li, H. Pretreatment absolute lymphocyte count is an independent predictor for survival outcomes for esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiotherapy and pembrolizumab: An analysis from a prospective cohort. Thorac. Cancer 2023, 14, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Hamai, Y.; Emi, M.; Ibuki, Y.; Kurokawa, T.; Yoshikawa, T.; Ohsawa, M.; Hirohata, R.; Kitasaki, N.; Okada, M. Ability of blood cell parameters to predict clinical outcomes of nivolumab monotherapy in advanced esophageal squamous cell carcinoma. Onco Targets Ther. 2023, 16, 263–273. [Google Scholar] [CrossRef]
- Yamada, M.; Tanaka, K.; Yamasaki, M.; Yamashita, K.; Makino, T.; Saito, T.; Takahashi, T.; Kurokawa, Y.; Motoori, M.; Kimura, Y.; et al. Neutrophil-to-lymphocyte ratio after neoadjuvant chemotherapy as an independent prognostic factor in patients with esophageal squamous cell carcinoma. Oncol. Lett. 2022, 25, 58. [Google Scholar] [CrossRef]
- De, B.; Ludmir, E.B.; Messick, C.A.; Cagley, M.C.; Morris, V.K.; Das, P.; Minsky, B.D.; Taniguchi, C.M.; Smith, G.L.; Koay, E.J.; et al. Prognostic impact of lymphopenia and neutrophil-lymphocyte ratio for patients with anal squamous cell carcinoma. J. Gastrointest. Oncol. 2021, 12, 2412–2422. [Google Scholar] [CrossRef]
- Knight, K.; Choong, J.X.; McKee, R.F.; Anderson, J.H.; Horgan, P.G.; McMillan, D.C.; McDonald, A.; Roxburgh, C.S. The influence of systemic inflammation on treatment response and survival in anal squamous cell cancer. Clin. Oncol. (R Coll Radiol.) 2021, 33, e22–e30. [Google Scholar] [CrossRef] [PubMed]
- Bassukas, I.D.; Tsilidis, K.K.; Spyridonos, P. Advanced keratinocyte skin cancer is a tumor with considerable disease burden and aggressiveness. Arch. Dermatol. Res. 2021, 313, 707–709. [Google Scholar] [CrossRef]
- Li, L.Q.; Bai, Z.H.; Zhang, L.H.; Zhang, Y.; Lu, X.C.; Zhang, Y.; Liu, Y.K.; Wen, J.; Li, J.Z. Meta-analysis of hematological biomarkers as reliable indicators of soft tissue sarcoma prognosis. Front. Oncol. 2020, 10, 30. [Google Scholar] [CrossRef]
- Que, Y.; Qiu, H.; Li, Y.; Chen, Y.; Xiao, W.; Zhou, Z.; Zhang, X. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 2015, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; McClure, E.M.; Akaike, T.; Park, S.Y.; Huynh, E.T.; Goff, P.H.; Nghiem, P. The evolving treatment landscape of Merkel cell carcinoma. Curr. Treat. Options Oncol. 2023, 24, 1231–1258. [Google Scholar] [CrossRef]
- Bonini, F.; de Sousa, L.G.; Ferrarotto, R. New and emerging drugs for the treatment of advanced cutaneous squamous cell carcinoma. Expert Opin. Emerg. Drugs 2023, 28, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou, P.; Vornicova, O.; Genta, S.; Spreafico, A. Shaping the future of immunotherapy targets and biomarkers in melanoma and non-melanoma cutaneous cancers. Int. J. Mol. Sci. 2023, 24, 1294. [Google Scholar] [CrossRef] [PubMed]
- Stonesifer, C.J.; Djavid, A.R.; Grimes, J.M.; Khaleel, A.E.; Soliman, Y.S.; Maisel-Campbell, A.; Garcia-Saleem, T.J.; Geskin, L.J.; Carvajal, R.D. Immune checkpoint inhibition in non-melanoma skin cancer: A review of current evidence. Front. Oncol. 2021, 11, 734354. [Google Scholar] [CrossRef]
- Kartolo, A.; Holstead, R.; Khalid, S.; Emack, J.; Hopman, W.; Robinson, A.; Baetz, T. Serum neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in prognosticating immunotherapy efficacy. Immunotherapy 2020, 12, 785–798. [Google Scholar] [CrossRef]
- Jiang, T.; Qiao, M.; Zhao, C.; Li, X.; Gao, G.; Su, C.; Ren, S.; Zhou, C. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: A meta-analysis. Cancer Immunol. Immunother. 2018, 67, 713–727. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef] [PubMed]
| Neutrophil-to-Lymphocyte Ratio (NLR) | Platelet-to-Lymphocyte Ratio (PLR) | Pan-Immune Inflammation Value (PIV) | |
|---|---|---|---|
| cutaneous Squamous Cell Carcinoma (cSCC) |
|
| ND |
| Extramammary Paget’s Disease (EMPD) |
| ND | ND |
| Merkel Cell Carcinoma (MCC) |
| ND |
|
| Cutaneous Angiosarcoma |
|
| ND |
| Mycosis fungoides (MF) |
| ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seretis, K.; Sfaelos, K.; Boptsi, E.; Gaitanis, G.; Bassukas, I.D. The Neutrophil-to-Lymphocyte Ratio as a Biomarker in Cutaneous Oncology: A Systematic Review of Evidence beyond Malignant Melanoma. Cancers 2024, 16, 1044. https://doi.org/10.3390/cancers16051044
Seretis K, Sfaelos K, Boptsi E, Gaitanis G, Bassukas ID. The Neutrophil-to-Lymphocyte Ratio as a Biomarker in Cutaneous Oncology: A Systematic Review of Evidence beyond Malignant Melanoma. Cancers. 2024; 16(5):1044. https://doi.org/10.3390/cancers16051044
Chicago/Turabian StyleSeretis, Konstantinos, Konstantinos Sfaelos, Elena Boptsi, Georgios Gaitanis, and Ioannis D. Bassukas. 2024. "The Neutrophil-to-Lymphocyte Ratio as a Biomarker in Cutaneous Oncology: A Systematic Review of Evidence beyond Malignant Melanoma" Cancers 16, no. 5: 1044. https://doi.org/10.3390/cancers16051044
APA StyleSeretis, K., Sfaelos, K., Boptsi, E., Gaitanis, G., & Bassukas, I. D. (2024). The Neutrophil-to-Lymphocyte Ratio as a Biomarker in Cutaneous Oncology: A Systematic Review of Evidence beyond Malignant Melanoma. Cancers, 16(5), 1044. https://doi.org/10.3390/cancers16051044







