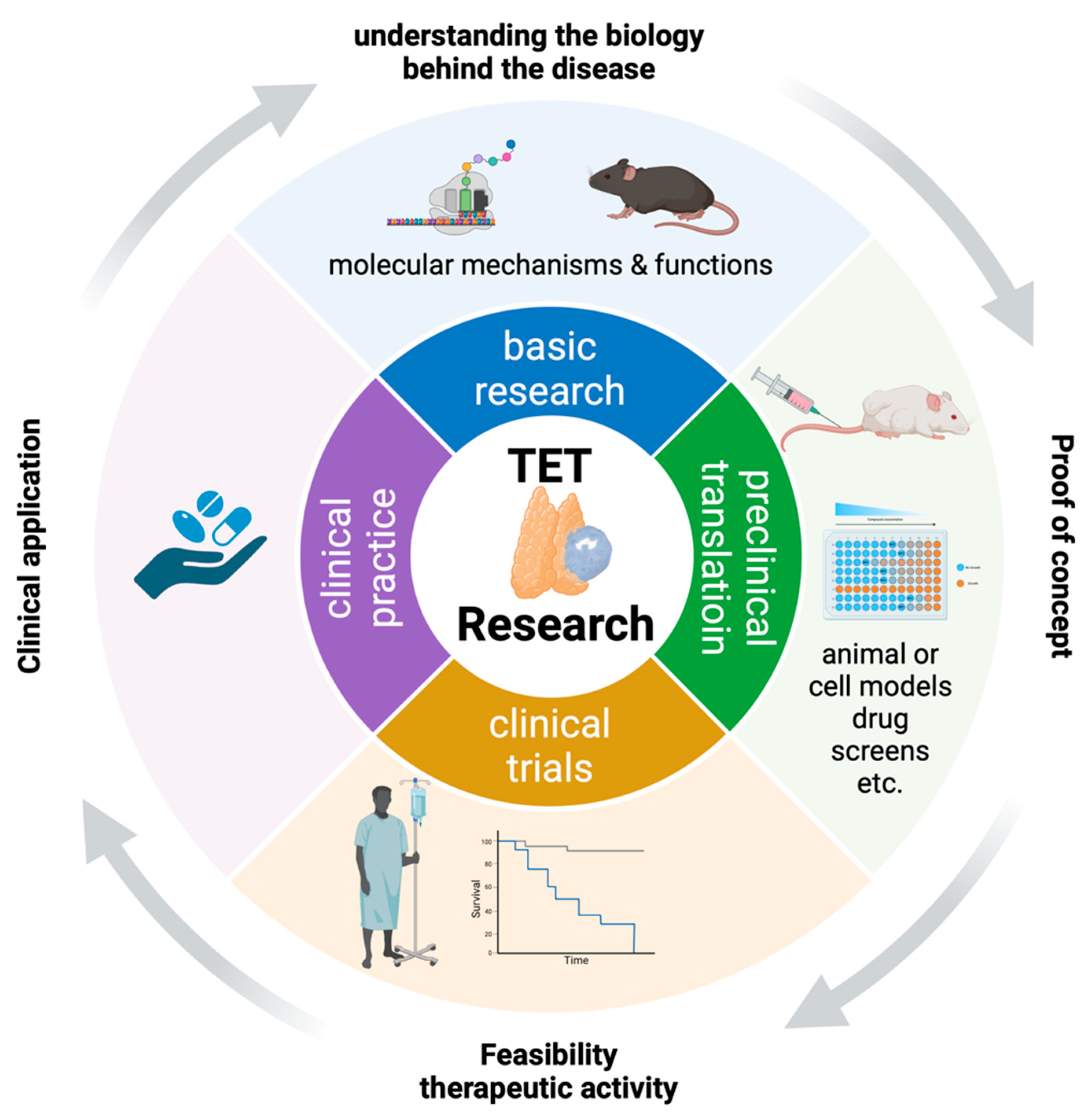
The Way Ahead: Lessons Learned from Decades of Cancer Research on Thymomas and Thymic Carcinomas
Conflicts of Interest
References
- Bell, E. Tumors or the thymus in myasthenia gravis. J. Nerv. Ment. Dis. 1917, 45, 130–143. [Google Scholar] [CrossRef][Green Version]
- Levine, G.D.; Rosai, J. Thymic hyperplasia and neoplasia: A review of current concepts. Hum. Pathol. 1978, 9, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Detterbeck, F.; Marom, E.M.; Ströbel, P.; Rajan, A. Tumors of the thymus: Introduction. In WHO Classification of Tumours. Thoracic Tumours, 5th ed.; Marx, A., Chan, J.K.C., Eds.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 5. [Google Scholar]
- Gokmen-Polar, Y.; Sanders, K.L.; Goswami, C.P.; Cano, O.D.; Zaheer, N.A.; Jain, R.K.; Kesler, K.A.; Nelson, R.P., Jr.; Vance, G.H.; Smith, D.; et al. Establishment and characterization of a novel cell line derived from human thymoma AB tumor. Lab. Investig. 2012, 92, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gu, Z.; Zhu, L.; Yang, X.; Zhao, W.; Guan, S.; Fang, W. Establishment and characterization of a novel cell line derived from type AB thymoma. Transl. Cancer Res. 2018, 7, 1634–1642. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Zhang, P.; Chen, Y.; Liu, Y.; Guo, F.; Zhang, H. Establishment and characterization of a novel cell line derived from thymoma with myasthenia gravis patients. Thorac. Cancer 2015, 6, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Liu, Y.; Wang, Y.; Wang, H.; Lu, C.; Zhang, P. Decreased Wnt4 expression inhibits thymoma development through downregulation of FoxN1. J. Thorac. Dis. 2017, 9, 1574–1583. [Google Scholar] [CrossRef]
- Ehemann, V.; Kern, M.A.; Breinig, M.; Schnabel, P.A.; Gunawan, B.; Schulten, H.J.; Schlaeger, C.; Radlwimmer, B.; Steger, C.M.; Dienemann, H.; et al. Establishment, characterization and drug sensitivity testing in primary cultures of human thymoma and thymic carcinoma. Int. J. Cancer 2008, 122, 2719–2725. [Google Scholar] [CrossRef]
- Alberobello, A.T.; Wang, Y.; Beerkens, F.J.; Conforti, F.; McCutcheon, J.N.; Rao, G.; Raffeld, M.; Liu, J.; Rahhal, R.; Zhang, Y.W.; et al. PI3K as a Potential Therapeutic Target in Thymic Epithelial Tumors. J. Thorac. Oncol. 2016, 11, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Inai, K.; Takagi, K.; Takimoto, N.; Okada, H.; Imamura, Y.; Ueda, T.; Naiki, H.; Noriki, S. Multiple inflammatory cytokine-productive ThyL-6 cell line established from a patient with thymic carcinoma. Cancer Sci. 2008, 99, 1778–1784. [Google Scholar] [CrossRef]
- Petrini, I.; Meltzer, P.S.; Kim, I.K.; Lucchi, M.; Park, K.S.; Fontanini, G.; Gao, J.; Zucali, P.A.; Calabrese, F.; Favaretto, A.; et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat. Genet. 2014, 46, 844–849. [Google Scholar] [CrossRef]
- Giorgetti, O.B.; Nusser, A.; Boehm, T. Human thymoma-associated mutation of the GTF2I transcription factor impairs thymic epithelial progenitor differentiation in mice. Commun. Biol. 2022, 5, 1037. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kim, I.K.; Bian, J.; Polyzos, A.; Di Giammartino, D.C.; Zhang, Y.W.; Luo, J.; Hernandez, M.O.; Kedei, N.; Cam, M.; et al. A Knock-In Mouse Model of Thymoma With the GTF2I L424H Mutation. J. Thorac. Oncol. 2022, 17, 1375–1386. [Google Scholar] [CrossRef]
- Radovich, M.; Pickering, C.R.; Felau, I.; Ha, G.; Zhang, H.; Jo, H.; Hoadley, K.A.; Anur, P.; Zhang, J.; McLellan, M.; et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018, 33, 244–258.e10. [Google Scholar] [CrossRef]
- Wang, X.D.; Lin, P.; Li, Y.X.; Chen, G.; Yang, H.; He, Y.; Li, Q.; Liu, R.C. Identification of potential agents for thymoma by integrated analyses of differentially expressed tumour-associated genes and molecular docking experiments. Exp. Ther. Med. 2019, 18, 2001–2014. [Google Scholar] [CrossRef]
- Yamada, Y.; Simon-Keller, K.; Belharazem-Vitacolonnna, D.; Bohnenberger, H.; Kriegsmann, M.; Kriegsmann, K.; Hamilton, G.; Graeter, T.; Preissler, G.; Ott, G.; et al. A Tuft Cell-Like Signature Is Highly Prevalent in Thymic Squamous Cell Carcinoma and Delineates New Molecular Subsets Among the Major Lung Cancer Histotypes. J. Thorac. Oncol. 2021, 16, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Jones, M.; Turley, H.; Gatter, K.C.; Nagvekar, N.; Newsom-Davis, J.; Willcox, N. Oncogene proteins and proliferation antigens in thymomas: Increased expression of epidermal growth factor receptor and Ki67 antigen. J. Clin. Pathol. 1995, 48, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.A.; Hsu, C.L.; Dzhagalov, I.L. Testing the Efficiency and Kinetics of Negative Selection Using Thymic Slices. Methods Mol. Biol. 2020, 2111, 205–219. [Google Scholar] [CrossRef]
- Montero, J.; Sarosiek, K.A.; DeAngelo, J.D.; Maertens, O.; Ryan, J.; Ercan, D.; Piao, H.; Horowitz, N.S.; Berkowitz, R.S.; Matulonis, U.; et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 2015, 160, 977–989. [Google Scholar] [CrossRef]
- Arjonen, A.; Makela, R.; Harma, V.; Rintanen, N.; Kuopio, T.; Kononen, J.; Rantala, J.K. Image-based ex vivo drug screen to assess targeted therapies in recurrent thymoma. Lung Cancer 2020, 145, 27–32. [Google Scholar] [CrossRef]
- Cavallo, F.; Troglio, F.; Faga, G.; Fancelli, D.; Shyti, R.; Trattaro, S.; Zanella, M.; D’Agostino, G.; Hughes, J.M.; Cera, M.R.; et al. High-throughput screening identifies histone deacetylase inhibitors that modulate GTF2I expression in 7q11.23 microduplication autism spectrum disorder patient-derived cortical neurons. Mol. Autism 2020, 11, 88. [Google Scholar] [CrossRef]
- Huang, J.; Ahmad, U.; Antonicelli, A.; Catlin, A.C.; Fang, W.; Gomez, D.; Loehrer, P.; Lucchi, M.; Marom, E.; Nicholson, A.; et al. Development of the international thymic malignancy interest group international database: An unprecedented resource for the study of a rare group of tumors. J. Thorac. Oncol. 2014, 9, 1573–1578. [Google Scholar] [CrossRef]
- Benveniste, M.F.; Korst, R.J.; Rajan, A.; Detterbeck, F.C.; Marom, E.M.; International Thymic Malignancy Interest, G. A practical guide from the International Thymic Malignancy Interest Group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J. Thorac. Oncol. 2014, 9, S119–S124. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; Frazier, A.A.; Giaccone, G.; Huang, J.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9, S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chalabreysse, L.; Thomas De Montpreville, V.; De Muret, A.; Hofman, V.; Lantuejoul, S.; Parrens, M.; Payan, M.J.; Rouquette, I.; Secq, V.; Girard, N.; et al. [Rythmic-pathology: The French national pathology network for thymic epithelial tumours]. Ann. Pathol. 2014, 34, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Basse, C.; Botticella, A.; Molina, T.J.; Falcoz, P.E.; Oulkhouir, Y.; Kerjouan, M.; Pichon, E.; Westeel, V.; Thiberville, L.; Quantin, X.; et al. RADIORYTHMIC: Phase III, Opened, Randomized Study of Postoperative Radiotherapy Versus Surveillance in Stage IIb/III of Masaoka Koga Thymoma after Complete Surgical Resection. Clin. Lung Cancer 2021, 22, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Yasumizu, Y.; Ohkura, N.; Murata, H.; Kinoshita, M.; Funaki, S.; Nojima, S.; Kido, K.; Kohara, M.; Motooka, D.; Okuzaki, D.; et al. Myasthenia gravis-specific aberrant neuromuscular gene expression by medullary thymic epithelial cells in thymoma. Nat. Commun. 2022, 13, 4230. [Google Scholar] [CrossRef]
| Cell Line | Initially Derived from | Accession No * | Reference |
|---|---|---|---|
| IU-TAB-1 | Type AB thymoma | RRID:CVCL_D551 | [4] |
| T68 | Type AB thymoma | RRID:CVCL_C6JV | [5] |
| Thy0517 | Type AB thymoma with MG | RRID:CVCL_2Z85 | [6] |
| Thy0517 | Type AB thymoma with MG | RRID:CVCL_2Z85 | [7] |
| T1682 | Type B1 thymoma | RRID:CVCL_D023 | [8] |
| T1889 | Thymic carcinoma | RRID:CVCL_D024 | [8] |
| MP57 | Thymic carcinoma | RRID:CVCL_VJ89 | [9] |
| ThyL-6 | Thymic carcinoma | RRID:CVCL_2Z86 | [10] |
| Hs 702.T | Thymic neoplasm (unspecified) | (RRID:CVCL_S306) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ströbel, P.; Marx, A. The Way Ahead: Lessons Learned from Decades of Cancer Research on Thymomas and Thymic Carcinomas. Cancers 2024, 16, 1040. https://doi.org/10.3390/cancers16051040
Ströbel P, Marx A. The Way Ahead: Lessons Learned from Decades of Cancer Research on Thymomas and Thymic Carcinomas. Cancers. 2024; 16(5):1040. https://doi.org/10.3390/cancers16051040
Chicago/Turabian StyleStröbel, Philipp, and Alexander Marx. 2024. "The Way Ahead: Lessons Learned from Decades of Cancer Research on Thymomas and Thymic Carcinomas" Cancers 16, no. 5: 1040. https://doi.org/10.3390/cancers16051040
APA StyleStröbel, P., & Marx, A. (2024). The Way Ahead: Lessons Learned from Decades of Cancer Research on Thymomas and Thymic Carcinomas. Cancers, 16(5), 1040. https://doi.org/10.3390/cancers16051040





