Preventing and Treating Pain and Anxiety during Needle-Based Procedures in Children with Cancer in Low- and Middle-Income Countries
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Context
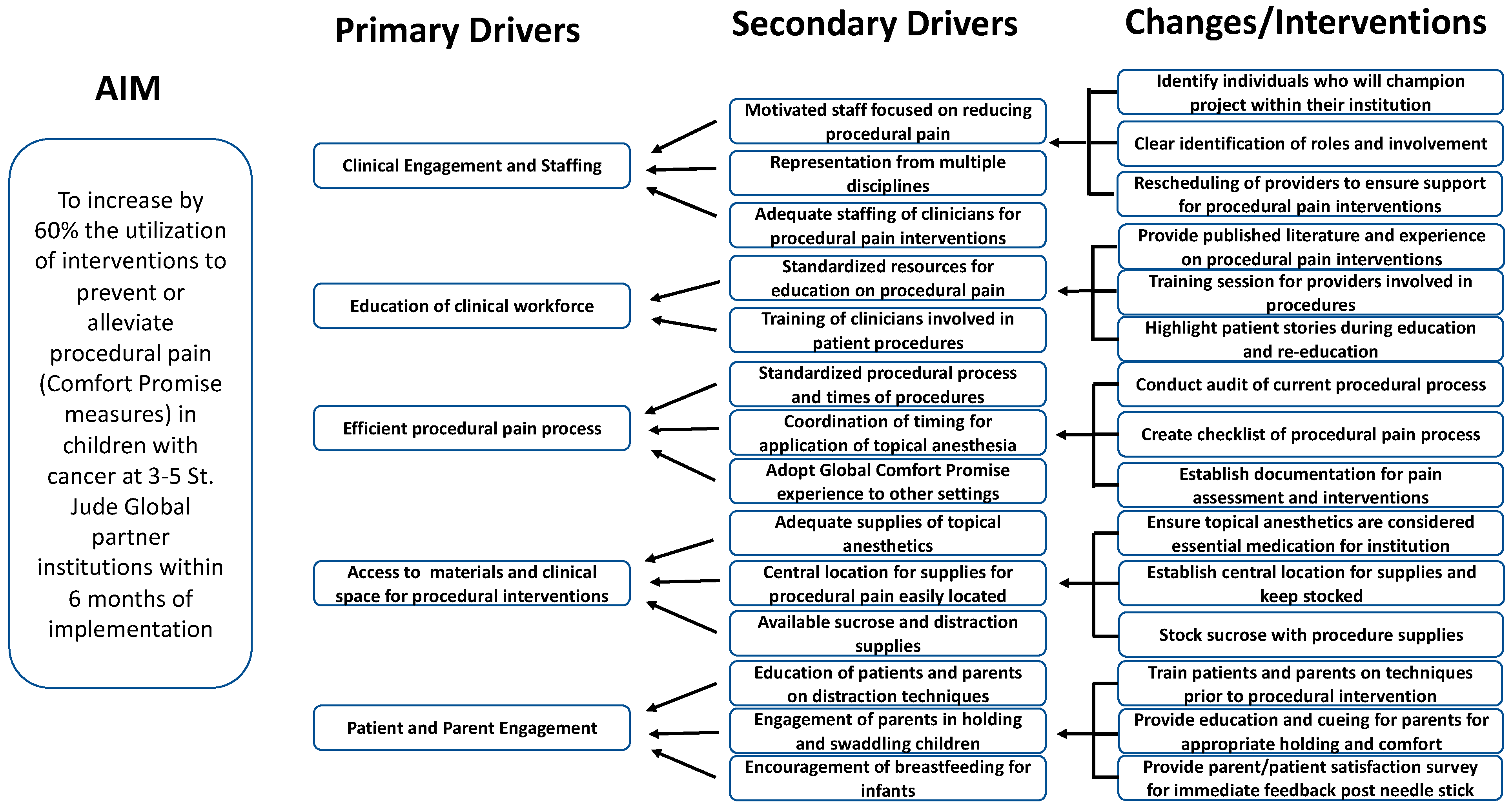
2.2. Interventions
2.3. Measures
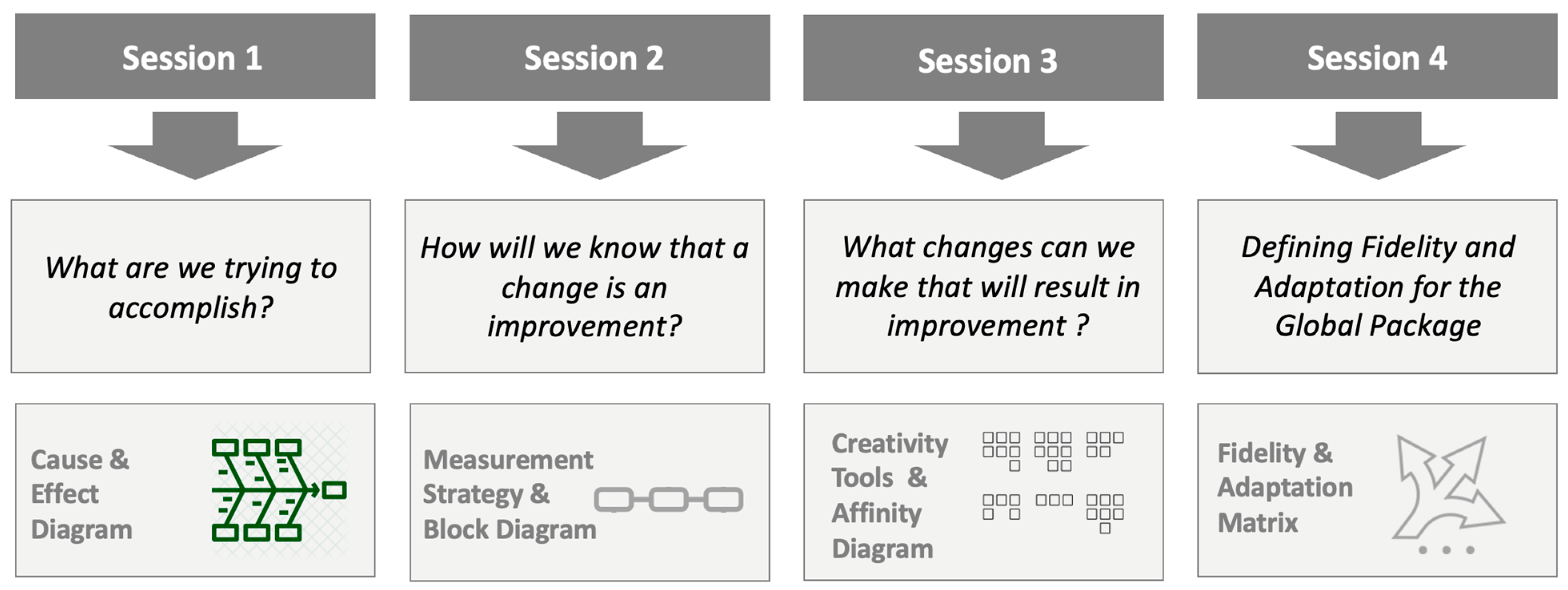
2.4. Study of the Intervention
2.5. Analysis
2.6. Ethical Considerations/IRB Approval
3. Results
3.1. Demographics
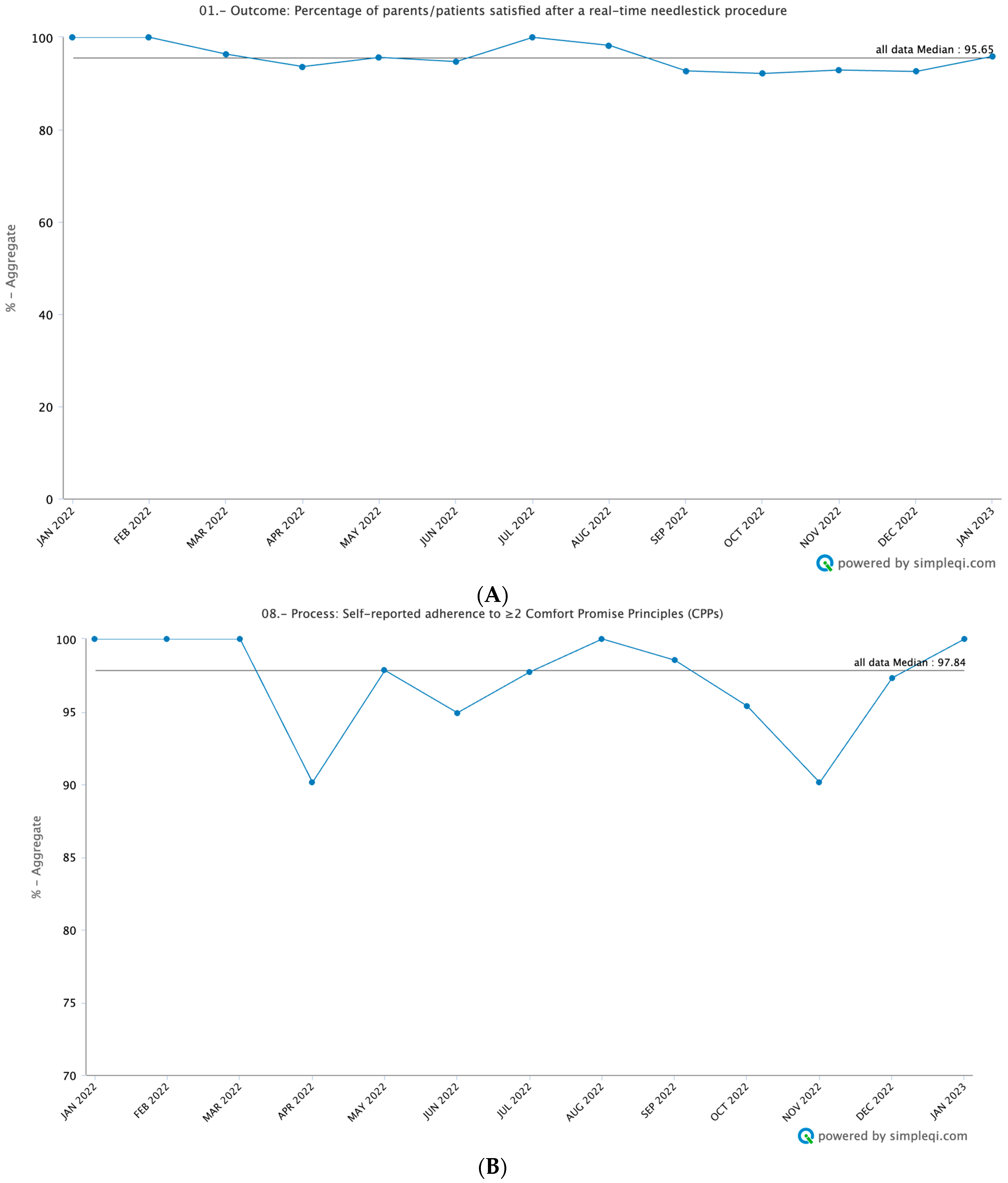
3.2. Outcome Measures
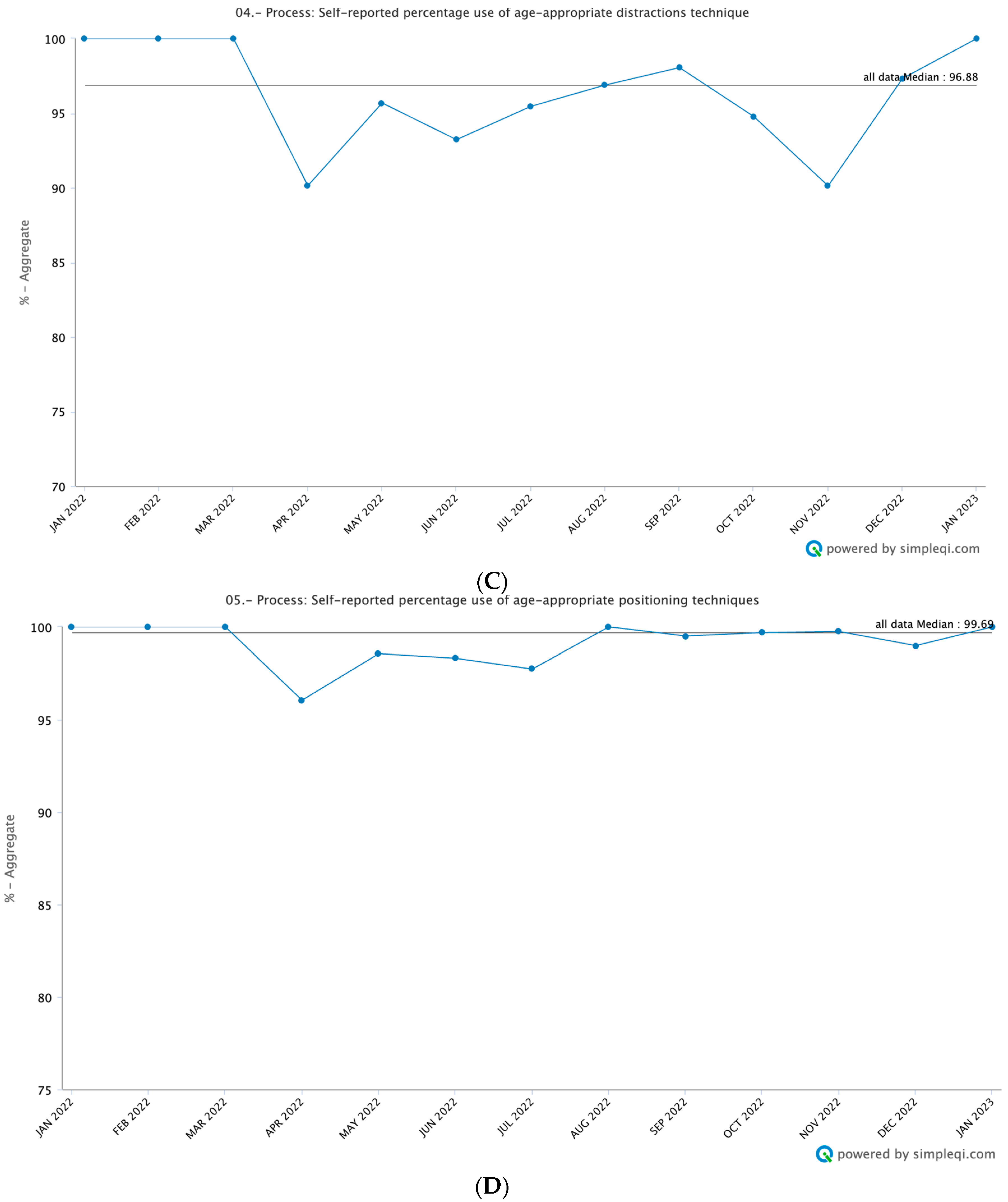
3.3. Process Measures
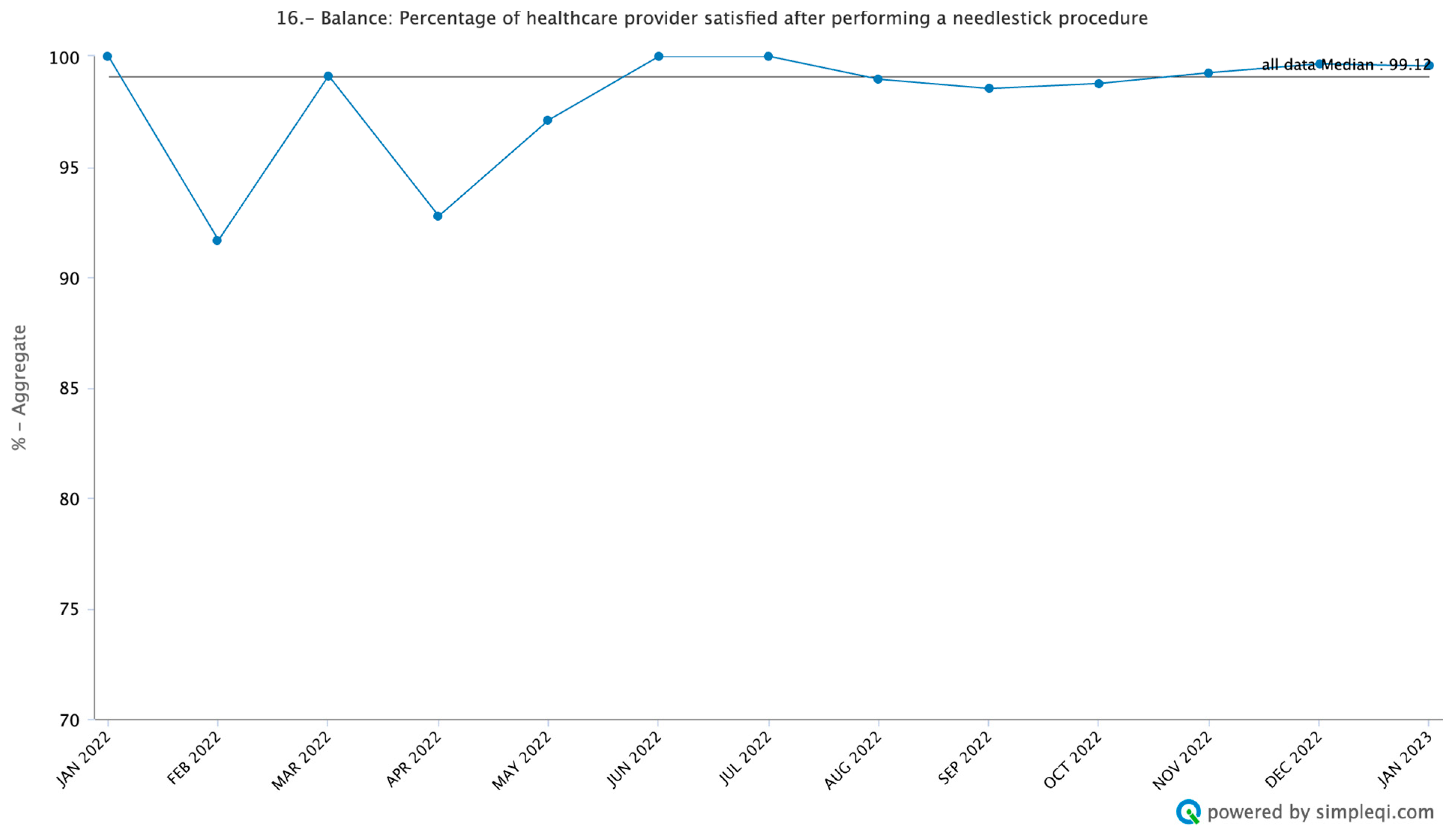
3.4. Balance Measures
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Association for the Study of Pain (IASP). Declaration of Montreal. 2010. Available online: http://www.iasppain.org/DeclarationofMontreal?navItemNumber5582 (accessed on 6 August 2023).
- Birnie, K.A.; Chambers, C.T.; Fernandez, C.V.; Forgeron, P.A.; Latimer, M.A.; McGrath, P.J.; Cummings, E.A.; Finley, G.A. Hospitalized children continue to report undertreated and preventable pain. Pain. Res. Manag. 2014, 19, 198–204. [Google Scholar] [CrossRef]
- Friedrichsdorf, S.J.; Postier, A.; Eull, D.; Weidner, C.; Foster, L.; Gilbert, M.; Campbell, F. Pain outcomes in a US Children’s hospital: A prospective cross-sectional survey. Hosp. Pediatr. 2015, 5, 18–26. [Google Scholar] [CrossRef]
- Walther-Larsen, S.; Pedersen, M.T.; Friis, S.M.; Aagaard, G.B.; Romsing, J.; Jeppesen, E.M.; Friedrichsdorf, S.J. Pain prevalence in hospitalized children: A prospective cross-sectional survey in four Danish university hospitals. Acta Anaesthesiol. Scand. 2017, 61, 328–337. [Google Scholar] [CrossRef]
- Ammentorp, J.; Mainz, J.; Sabroe, S. Parents’ priorities and satisfaction with acute pediatric care. Arch. Pediatr. Adolesc. Med. 2005, 159, 127–131. [Google Scholar] [CrossRef]
- Tiedeman, M.E. Anxiety responses of parents during and after the hospitalization of their 5- to 11-year-old children. J. Pediatr. Nurs. 1997, 12, 110–119. [Google Scholar] [CrossRef]
- Melnyk, B.M. Intervention studies involving parents of hospitalized young children: An analysis of the past and future recommendations. J. Pediatr. Nurs. 2000, 15, 4–13. [Google Scholar] [CrossRef]
- Anand, K.J.; Barton, B.A.; McIntosh, N.; Lagercrantz, H.; Pelausa, E.; Young, T.E.; Vasa, R. Analgesia and sedation in preterm neonates who require ventilatory support: Results from the NOPAIN trial. Neonatal outcome and prolonged analgesia in neonates. Arch. Pediatr. Adolesc. Med. 1999, 153, 331–338. [Google Scholar] [CrossRef]
- Taddio, A.; Katz, J.; Ilersich, A.L.; Koren, G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 1997, 349, 599–603. [Google Scholar] [CrossRef]
- Grunau, R.E.; Tu, M.T. Long-term consequences of pain in human neonates. In Pain in Neonates and Infants: Pain Research and Clinical Management; Anand, K.J.S., Stevens, B.J., McGrath, P.J., Eds.; Elsevier: Toronto, ON, Canada, 2007; pp. 45–55. [Google Scholar]
- Holsti, L.; Grunau, R.E.; Oberlander, T.F.; Whitfield, M.F. Specific Newborn Individualized Developmental Care and Assessment Program movements are associated with acute pain in preterm infants in the neonatal intensive care unit. Pediatrics 2004, 114, 65–72. [Google Scholar] [CrossRef]
- Velazquez Cardona, C.; Rajah, C.; Mzoneli, Y.N.; Friedrichsdorf, S.J.; Campbell, F.; Cairns, C.; Rodseth, R.N. An audit of paediatric pain prevalence, intensity, and treatment at a South African tertiary hospital. Pain Rep. 2019, 4, e789. [Google Scholar] [CrossRef]
- Friedrichsdorf, S.J.; Eull, D.; Weidner, C.; Postier, A. A hospital-wide initiative to eliminate or reduce needle pain in children using lean methodology. Pain Rep. 2018, 3 (Suppl. S1), e671. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Childhood Cancer Collaborators. The global burden of childhood and adolescent cancer in 2017: An analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019, 20, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Friedrich, P.; Alcasabas, P.; Antillon, F.; Banavali, S.; Castillo, L.; Israels, T.; Jeha, S.; Harif, M.; Sullivan, M.J.; et al. Toward the cure of all children with cancer through collaborative efforts: Pediatric oncology as a global challenge. J. Clin. Oncol. 2015, 33, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 contributors. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.C.; Metzger, M.L.; Wilimas, J.A.; Quintana, Y.; Pui, C.H.; Robison, L.L.; Ribeiro, R.C. Childhood cancer epidemiology in low-income countries. Cancer 2008, 112, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, P.; Lam, C.G.; Kaur, G.; Itriago, E.; Ribeiro, R.C.; Arora, R.S. Determinants of Treatment Abandonment in Childhood Cancer: Results from a Global Survey. PLoS ONE 2016, 11, e0163090. [Google Scholar] [CrossRef]
- Langley, G.L.; Moen, R.; Nolan, K.M.; Nolan, T.W.; Norman, C.L.; Provost, L.P. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance, 2nd ed.; Jossey-Bass Publishers: San Francisco, CA, USA, 2009. [Google Scholar]
- Ogrinc, G.; Davies, L.; Goodman, D.; Batalden, P.B.; Davidoff, F.; Stevens, D. SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual. Saf. 2016, 25, 986–992. [Google Scholar] [CrossRef]
- St. Jude Global: Taking St. Jude to the World. Memphis 2021. Available online: https://www.stjude.org/global.html (accessed on 4 August 2023).
- WHO: Global Initiative for Childhood Cancer. Geneva 2020. Available online: https://www.who.int/publications/m/item/global-initiative-for-childhood-cancer (accessed on 4 August 2023).
- St. Jude Global. Available online: https://global.stjude.org/en-us/ (accessed on 4 November 2023).
- St. Jude Global CARES. Available online: https://www.stjude.org/global/sjcares.html (accessed on 4 November 2023).
- Doyle, C.; Howe, C.; Woodcock, T.; Woodcock, T.; Myron, R.; Phekoo, K.; McNicholas, C.; Saffer, J.; Bell, D. Making change last: Applying the NHS institute for innovation and improvement sustainability model to healthcare improvement. Implement. Sci. 2013, 8, 127. [Google Scholar] [CrossRef]
- SimpleQI. Available online: https://www.simpleqi.com/ (accessed on 7 November 2023).
- National Research Cooperation. Picker Patient Experience Questionnaire; NRC Health: Lincoln, NE, USA, 2016; Available online: http://www.nationalresearch.com (accessed on 23 July 2023).
- Graetz, D.E.; Rivas, S.E.; Fuentes, A.L.; Caceres-Serrano, A.; Antillon-Klussmann, F.; Rodriguez-Galindo, C.; Mack, J.W. Development and Adaptation of a Patient-Centered Communication Survey for Parents of Children with Cancer in Guatemala. JCO Glob. Oncol. 2022, 8, e2200124. [Google Scholar] [CrossRef]
- Run Chart Tool|IHI-Institute for Healthcare Improvement. Available online: http://www.ihi.org/resources/Pages/Tools/RunChart.aspx (accessed on 12 August 2023).
- Lloyd, R. Chapter 8 Understanding Variation with Run Charts. In Quality Health Care: A Guide to Developing and Using Indicators; Jones and Bartlett Publishing: Burlington, MA, USA, 2019; pp. 187–209. [Google Scholar]
- McKenzie, D.; Madowo, L.; Alberti, M.; Dewan, A. Over 300 Killed after Flooding Washed away Roads, Destroyed Homes in South Africa. CNN. 14 April 2022. Available online: https://www.cnn.com/2022/04/13/africa/south-africa-rain-floods-climate-intl/index.html (accessed on 4 February 2023).
- Al Jazeera. Thousands Strike for Wage Increases in South Africa. 22 November 2022. Available online: https://www.aljazeera.com/news/2022/11/22/thousands-strike-for-wage-increases-in-south-africa (accessed on 4 February 2023).
- Rebaza, C.; John, T.; Pozzebon, S.; Atay Alam, H. Peru’s President Impeached after He Attempts to Dissolve Congress. CNN. 8 December 2022. Available online: https://www.cnn.com/2022/12/07/americas/peru-president-castillo-congress-dissolves-intl/index.html (accessed on 4 February 2023).
- Taj, M.; Turkewitz, J. Amid Deadly Protests, Peru Declares a National Sate of Emergency. New York Times. 14 December 2022. Available online: https://www.nytimes.com/2022/12/14/world/americas/peru-state-of-emergency-protests.html (accessed on 4 February 2023).
- World Health Organization. Infant and Young Child Feeding. 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 4 February 2023).
- Birnie, K.A.; Noel, M.; Chambers, C.T.; Uman, L.S.; Parker, J.A. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2018, 10, CD005179. [Google Scholar] [PubMed]
- Boerner, K.E.; Gillespie, J.M.; McLaughlin, E.N.; Kuttner, L.; Chambers, C.T. Implementation of evidence-based psychological interventions for pediatric needle pain. Clin. Pract. Pediatr. Psychol. 2014, 2, 224–235. [Google Scholar] [CrossRef]
- Karlsson, K.; Rydström, I.; Enskär, K.; Englund, A.C. Nurses’ perspectives on supporting children during needle-related medical procedures. Int J Qual Stud Health Well-being 2014, 9, 23063. [Google Scholar] [CrossRef] [PubMed]
- Strathern, M. ‘Improving ratings’: Audit in the British University system. Eur. Rev. 1997, 5, 305–321. [Google Scholar] [CrossRef]
- Goodhart, C. Papers in Monetary Economics; Monetary Relationships: A View from Threadneedle Street; Reserve Bank of Australia: Sydney, Australia, 1975. [Google Scholar]
- Elly, A.M. Patient satisfaction with pain management does not correlate with initial or discharge VAS pain score, verbal pain rating at discharge, or change in VAS score in the Emergency Department. J. Emerg. Med. 2000, 19, 113–116. [Google Scholar]
| Patient | 2185 |
|---|---|
| Provider Type | |
| Nurse | 1303 (59.6%) |
| Nursing technician | 678 (31.0%) |
| Physician | 204 (9.4%) |
| Gender | |
| Female | 944 (43.3%) |
| Male | 1241 (56.7%) |
| Age | |
| 0–12 months | 37 (1.7%) |
| 1–3 years | 386 (17.6%) |
| 4–6 years | 507 (23.2%) |
| 7–9 years | 395 (18.0%) |
| 10–12 years | 295 (13.5%) |
| 13–15 years | 315 (14.4%) |
| 16–18 years | 250 (11.4%) |
| Diagnosis | |
| Acute lymphoblastic leukemia | 769 (35.1%) |
| Acute myeloblastic leukemia | 104 (4.8%) |
| Central nervous system tumors | 212 (9.7%) |
| Hodgkin’s lymphoma | 120 (5.5%) |
| Non-Hodgkin’s lymphoma | 49 (2.2%) |
| Solid tumor | 829 (37.9%) |
| Other * JMML | 102 (4.7%) |
| Type of Needlestick | |
| Lab draw | 1223 (55.9%) |
| Peripheral IV placement | 962 (44.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNeil, M.J.; Garcia Quintero, X.; Gonzalez, M.; Zheng, Y.; Ugaz Olivares, C.; Morales, R.; Boldrini, E.; Rebollo de Campos, D.; Ferreira, D.; Coopasamy, K.; et al. Preventing and Treating Pain and Anxiety during Needle-Based Procedures in Children with Cancer in Low- and Middle-Income Countries. Cancers 2024, 16, 1025. https://doi.org/10.3390/cancers16051025
McNeil MJ, Garcia Quintero X, Gonzalez M, Zheng Y, Ugaz Olivares C, Morales R, Boldrini E, Rebollo de Campos D, Ferreira D, Coopasamy K, et al. Preventing and Treating Pain and Anxiety during Needle-Based Procedures in Children with Cancer in Low- and Middle-Income Countries. Cancers. 2024; 16(5):1025. https://doi.org/10.3390/cancers16051025
Chicago/Turabian StyleMcNeil, Michael J., Ximena Garcia Quintero, Miriam Gonzalez, Yawen Zheng, Cecilia Ugaz Olivares, Roxana Morales, Erica Boldrini, Débora Rebollo de Campos, Daiane Ferreira, Kamalina Coopasamy, and et al. 2024. "Preventing and Treating Pain and Anxiety during Needle-Based Procedures in Children with Cancer in Low- and Middle-Income Countries" Cancers 16, no. 5: 1025. https://doi.org/10.3390/cancers16051025
APA StyleMcNeil, M. J., Garcia Quintero, X., Gonzalez, M., Zheng, Y., Ugaz Olivares, C., Morales, R., Boldrini, E., Rebollo de Campos, D., Ferreira, D., Coopasamy, K., Caneba, J., Padernilla, M. L., Friedrichsdorf, S., Baker, J. N., & Friedrich, P. (2024). Preventing and Treating Pain and Anxiety during Needle-Based Procedures in Children with Cancer in Low- and Middle-Income Countries. Cancers, 16(5), 1025. https://doi.org/10.3390/cancers16051025







