Feasibility of Introducing a Prehabilitation Program into the Care of Gynecological Oncology Patients—A Single Institution Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Recruitment
2.3. Intervention
2.4. Data Collection
2.5. Study Outcome
- (1)
- Exercise intervention
- Supervised program: The percentage of completed training sessions out of possible training sessions in the optimal training period (the time between the first training session and surgery). The interval between patient inclusion and the first training session is excluded due to the logistical factor of referral to the physiotherapist and not patient adherence. The number of possible training sessions in the optimal training period entails three sessions per seven days.
- Low-intensity exercise advice: The percentage of completed low-intensity exercise sessions out of the number of instructed low-intensity exercise sessions per week, with the maximum being four, as participants were instructed to execute this on days without supervised training.
- (2)
- Nutritional intervention
- Protein supplementation: The percentage of days on which protein supplementation was completed out of the number of days that protein supplementation was prescribed. The maximum number is fixed at seven as participants were instructed to take daily supplementation.
- Vitamin supplementation: The percentage of completed doses of vitamin supplements out of prescribed doses (multivitamin supplementation was prescribed daily).
- (3)
- Psychological intervention: The psychological intervention comprised sessions with a medical psychologist to receive counseling to improve coping strategies and anxiety regarding their diagnosis and surgery. This component was designed as a consulting and supporting intervention.
- (4)
- Intoxication cessation
- Smoking: The number of participants successfully quitting smoking out of all active smokers.
- Alcohol: The number of participants successfully quitting alcohol consumption out of all participants consuming alcohol.
2.6. Statistical Analysis
3. Results
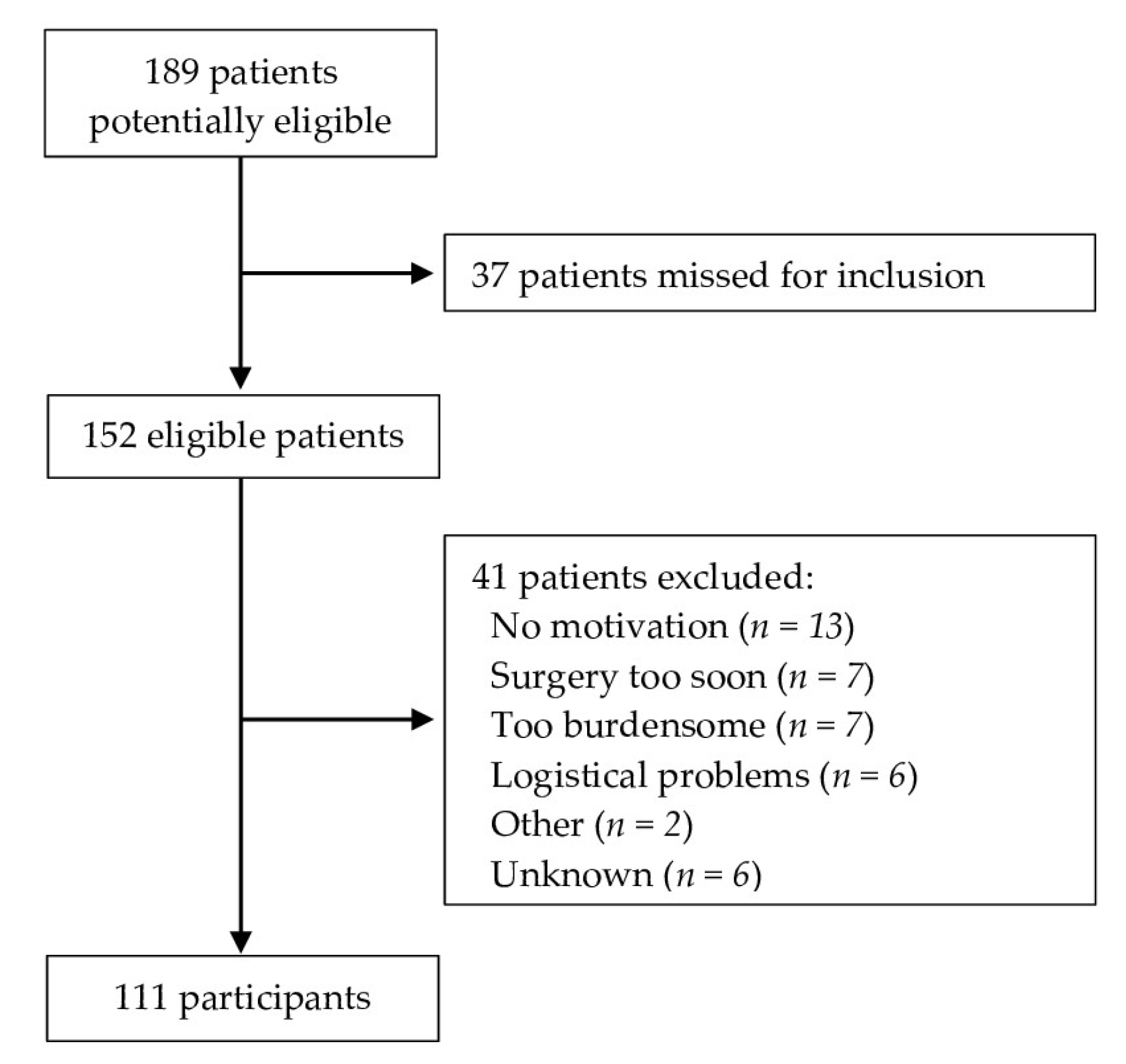
3.1. Recruitment
3.2. Patient Demographics and Clinical Characteristics
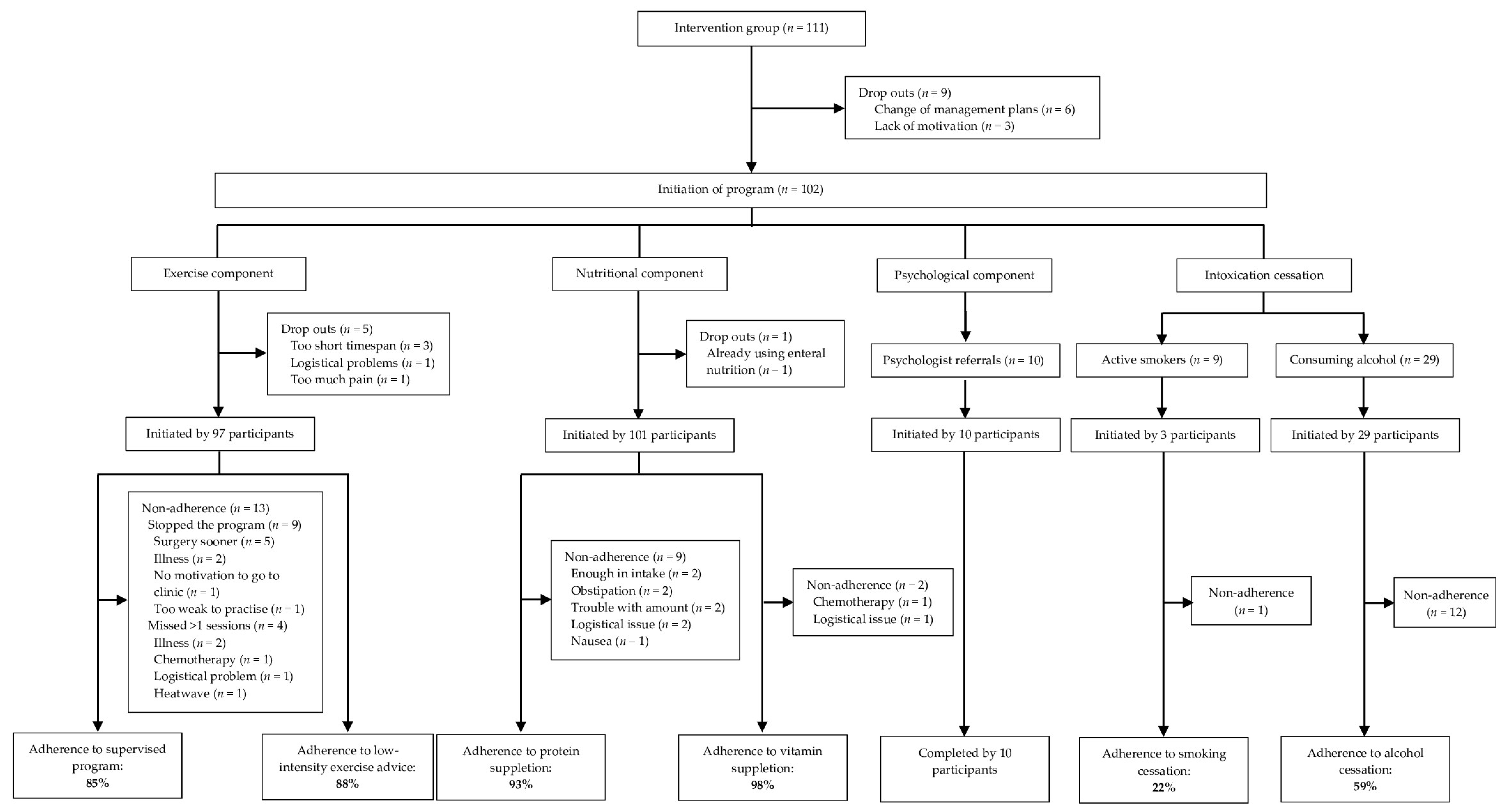
3.3. Adherence
3.3.1. Exercise Component
3.3.2. Nutritional Component
3.3.3. Psychological Component
3.3.4. Intoxication Cessation
3.4. Factors Associated with Participation and Adherence
3.5. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smits, A.; Smits, E.; Lopes, A.; Das, N.; Hughes, G.; Talaat, A.; Pollard, A.; Bouwman, F.; Massuger, L.; Bekkers, R.; et al. Body mass index, physical activity and quality of life of ovarian cancer survivors: Time to get moving? Gynecol. Oncol. 2015, 139, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, A.S.; Brookfield, K.F.; Schuman, S.I.; Lucci, J.A., 3rd. Malnutrition as a predictor of poor postoperative outcomes in gynecologic cancer patients. Arch. Gynecol. Obstet. 2011, 284, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Orekoya, O.; Samson, M.E.; Trivedi, T.; Vyas, S.; Steck, S.E. The Impact of Obesity on Surgical Outcome in Endometrial Cancer Patients: A Systematic Review. J. Gynecol. Surg. 2016, 32, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Hami, L.T.; Lampe, B.; Mallmann, P.; Forner, D.M. The Impact of Age on the Prognosis of Vulvar Cancer. Oncol. Res. Treat. 2018, 41, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Anthropometry, physical activity, and endometrial cancer risk: Results from The Netherlands cohort study. Int. J. Gynecol. Cancer 2006, 16 (Suppl. S2), 492. [Google Scholar] [CrossRef]
- Zhou, W.L.; Yue, Y.Y. Trends in the Incidence of Vulvar and Vaginal Cancers with Different Histology by Race, Age, and Region in the United States (2001–2018). Int. J. Public Health 2022, 67, 1605021. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Knobf, M.T.; Lanceley, A. Obesity, diet, physical activity, and health-related quality of life in endometrial cancer survivors. Nutr. Rev. 2015, 73, 399–408. [Google Scholar] [CrossRef]
- Bouwman, F.; Smits, A.; Lopes, A.; Das, N.; Pollard, A.; Massuger, L.; Bekkers, R.; Galaal, K. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery–An institutional study and systematic review of the literature. Gynecol. Oncol. 2015, 139, 369–376. [Google Scholar] [CrossRef]
- Lin, L.L.; Brown, J.C.; Segal, S.; Schmitz, K.H. Quality of life, body mass index, and physical activity among uterine cancer patients. Int. J. Gynecol. Cancer 2014, 24, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Gierach, G.L.; Schatzkin, A.; Matthews, C.E. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br. J. Cancer 2010, 103, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Laky, B.; Janda, M.; Cleghorn, G.; Obermair, A. Comparison of different nutritional assessments and body-composition measurements in detecting malnutrition among gynecologic cancer patients. Am. J. Clin. Nutr. 2008, 87, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Biller, V.S.; Leitzmann, M.F.; Sedlmeier, A.M.; Berger, F.F.; Ortmann, O.; Jochem, C. Sedentary behaviour in relation to ovarian cancer risk: A systematic review and meta-analysis. Eur. J. Epidemiol. 2021, 36, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Gentry-Maharaj, A.; Nordin, A.; Burnell, M.; Liston, R.; Manchanda, R.; Das, N.; Desai, R.; Gornall, R.; Beardmore-Gray, A.; et al. Predictors of complications in gynaecological oncological surgery: A prospective multicentre study (UKGOSOC-UK gynaecological oncology surgical outcomes and complications). Br. J. Cancer 2015, 112, 475–484. [Google Scholar] [CrossRef]
- Baldewpersad Tewarie, N.M.S.; van Driel, W.J.; van Ham, M.; Wouters, M.W.; Kruitwagen, R.; Participants of the Dutch Gynecological Oncology Collaborator Group. Postoperative outcomes of primary and interval cytoreductive surgery for advanced ovarian cancer registered in the Dutch Gynecological Oncology Audit (DGOA). Gynecol. Oncol. 2021, 162, 331–338. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.J.A.; Maessen, J.M.C.; Dejong, C.H.C.; Winkens, B.; Kruitwagen, R.; Slangen, B.F.M.; van der Weijden, T.; all the Members of the Study Group. Interdepartmental Spread of Innovations: A Multicentre Study of the Enhanced Recovery After Surgery Programme. World J. Surg. 2018, 42, 2348–2355. [Google Scholar] [CrossRef]
- Lucas, A.R.; Focht, B.C.; Cohn, D.E.; Klatt, M.D.; Buckworth, J. Recruiting Endometrial Cancer Survivors to Studies Examining Lifestyle Behaviors and Quality of Life: Challenges Faced and Lessons Learned. J. Cancer Educ. 2018, 33, 857–864. [Google Scholar] [CrossRef]
- Kokts-Porietis, R.L.; Elmrayed, S.; Brenner, D.R.; Friedenreich, C.M. Obesity and mortality among endometrial cancer survivors: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13337. [Google Scholar] [CrossRef]
- El-Sherif, A.; El-Sherif, S.; Taylor, A.H.; Ayakannu, T. Ovarian Cancer: Lifestyle, Diet and Nutrition. Nutr. Cancer 2021, 73, 1092–1107. [Google Scholar] [CrossRef]
- Johnston, E.A.; Ibiebele, T.I.; Friedlander, M.L.; Grant, P.T.; van der Pols, J.C.; Webb, P.M.; Ovarian cancer Prognosis and Lifestyle (OPAL) Study Group. Association of Protein Intake with Recurrence and Survival Following Primary Treatment of Ovarian Cancer. Am. J. Clin. Nutr. 2023, 118, 50–58. [Google Scholar] [CrossRef] [PubMed]
- van de Berg, N.J.; van Beurden, F.P.; Wendel-Vos, G.C.W.; Duijvestijn, M.; van Beekhuizen, H.J.; Maliepaard, M.; van Doorn, H.C. Patient-Reported Mobility, Physical Activity, and Bicycle Use after Vulvar Carcinoma Surgery. Cancers 2023, 15, 2324. [Google Scholar] [CrossRef] [PubMed]
- Banugo, P.; Amoako, D. Prehabilitation. BJA Educ. 2017, 17, 401–405. [Google Scholar] [CrossRef]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery with High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2022, 275, e299–e306. [Google Scholar] [CrossRef] [PubMed]
- Bruns, E.R.J.; van Rooijen, S.J.; Argillander, T.E.; van der Zaag, E.S.; van Grevenstein, W.M.U.; van Duijvendijk, P.; Buskens, C.J.; Bemelman, W.A.; van Munster, B.C.; Slooter, G.D.; et al. Improving Outcomes in Oncological Colorectal Surgery by Prehabilitation. Am. J. Phys. Med. Rehabil. 2019, 98, 231–238. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, M.; van Dalen, D.H.; Nahar-van Venrooij, L.M.W.; Meijerink, W.; Verdaasdonk, E.G.G. A multimodal prehabilitation program in high-risk patients undergoing elective resection for colorectal cancer: A retrospective cohort study. Eur. J. Surg. Oncol. 2021, 47, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Hayes, L.D.; Keegan, T.J.; Subar, D.A.; Gaffney, C.J. The Impact of Prehabilitation on Patient Outcomes in Hepatobiliary, Colorectal, and Upper Gastrointestinal Cancer Surgery: A PRISMA-Accordant Meta-analysis. Ann. Surg. 2021, 274, 70–77. [Google Scholar] [CrossRef]
- Waterland, J.L.; McCourt, O.; Edbrooke, L.; Granger, C.L.; Ismail, H.; Riedel, B.; Denehy, L. Efficacy of Prehabilitation Including Exercise on Postoperative Outcomes Following Abdominal Cancer Surgery: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 628848. [Google Scholar] [CrossRef]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martinez-Palli, G.; Lopez-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef]
- Bruns, E.R.; van den Heuvel, B.; Buskens, C.J.; van Duijvendijk, P.; Festen, S.; Wassenaar, E.B.; van der Zaag, E.S.; Bemelman, W.A.; van Munster, B.C. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: A systematic review. Colorectal Dis. 2016, 18, O267–O277. [Google Scholar] [CrossRef]
- West, M.A.; Jack, S.; Grocott, M.P.W. Prehabilitation before surgery: Is it for all patients? Best. Pract. Res. Clin. Anaesthesiol. 2021, 35, 507–516. [Google Scholar] [CrossRef]
- Dhanis, J.; Keidan, N.; Blake, D.; Rundle, S.; Strijker, D.; van Ham, M.; Pijnenborg, J.M.A.; Smits, A. Prehabilitation to Improve Outcomes of Patients with Gynaecological Cancer: A New Window of Opportunity? Cancers 2022, 14, 3448. [Google Scholar] [CrossRef] [PubMed]
- Weggemans, R.M.; Backx, F.J.G.; Borghouts, L.; Chinapaw, M.; Hopman, M.T.E.; Koster, A.; Kremers, S.; van Loon, L.J.C.; May, A.; Mosterd, A.; et al. The 2017 Dutch Physical Activity Guidelines. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- van Exter, S.H.; Drager, L.D.; van Asseldonk, M.; Strijker, D.; van der Schoot, N.D.; van den Heuvel, B.; Verlaan, S.; van den Berg, M.G.A. Adherence to and Efficacy of the Nutritional Intervention in Multimodal Prehabilitation in Colorectal and Esophageal Cancer Patients. Nutrients 2023, 15, 2133. [Google Scholar] [CrossRef] [PubMed]
- Strijker, D. Multimodal intensive prehabilitation in high impact surgery to reduce postoperative complications. Int. Clin. Trial Regist. Platf. 2020. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=NL8699 (accessed on 1 May 2023).
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Sauerwein, H.P.; Kondrup, J. Protein recommendations in the ICU: G protein/kg body weight—Which body weight for underweight and obese patients? Clin. Nutr. 2012, 31, 774–775. [Google Scholar] [CrossRef]
- Spinhoven, P.; Ormel, J.; Sloekers, P.P.; Kempen, G.I.; Speckens, A.E.; Van Hemert, A.M. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol. Med. 1997, 27, 363–370. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- IBM. SPSS: Version 25 Pen Drive IBM SPSS Statistics 25; IBM: Armonk, NY, USA, 2018. [Google Scholar]
- Diaz-Feijoo, B.; Agusti-Garcia, N.; Sebio, R.; Lopez-Hernandez, A.; Siso, M.; Glickman, A.; Carreras-Dieguez, N.; Fuste, P.; Marina, T.; Martinez-Egea, J.; et al. Feasibility of a Multimodal Prehabilitation Programme in Patients Undergoing Cytoreductive Surgery for Advanced Ovarian Cancer: A Pilot Study. Cancers 2022, 14, 1635. [Google Scholar] [CrossRef]
- Hertlein, L.; Zeder-Goss, C.; Furst, S.; Bayer, D.; Trillsch, F.; Czogalla, B.; Mahner, S.; Burges, A.; Rittler, P. Peri-operative oral immunonutrition in malnourished ovarian cancer patients assessed by the nutritional risk screening. Arch. Gynecol. Obstet. 2018, 297, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Vidrine, J.I.; Sutton, S.K.; Wetter, D.W.; Shih, Y.T.; Ramondetta, L.M.; Elting, L.S.; Walker, J.L.; Smith, K.M.; Frank-Pearce, S.G.; Li, Y.; et al. Efficacy of a Smoking Cessation Intervention for Survivors of Cervical Intraepithelial Neoplasia or Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Bohlin, K.S.; Lofgren, M.; Lindkvist, H.; Milsom, I. Smoking cessation prior to gynecological surgery-A registry-based randomized trial. Acta Obstet. Gynecol. Scand. 2020, 99, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, H.; Rosenberg, J.; Nielsen, H.J.; Rasmussen, V.; Hauge, C.; Pedersen, I.K.; Kehlet, H. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: Randomised controlled trial. BMJ 1999, 318, 1311–1316. [Google Scholar] [CrossRef]
- Chang, M.C.; Choo, Y.J.; Kim, S. Effect of prehabilitation on patients with frailty undergoing colorectal cancer surgery: A systematic review and meta-analysis. Ann. Surg. Treat. Res. 2023, 104, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.L.; Lee, M.J.; George, J.; Kerr, K.; Moug, S.; Wilson, T.R.; Brown, S.R.; Wyld, L. Prehabilitation in elective abdominal cancer surgery in older patients: Systematic review and meta-analysis. BJS Open 2020, 4, 1022–1041. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, L.; Miao, X.; Jiang, X.; Xu, T.; Xu, X.; Zhu, S.; Xu, Q.; Hu, J. Effects of prehabilitation on postoperative outcomes in frail cancer patients undergoing elective surgery: A systematic review and meta-analysis. Support. Care Cancer 2022, 31, 57. [Google Scholar] [CrossRef]
- Miralpeix, E.; Sole-Sedeno, J.M.; Rodriguez-Cosmen, C.; Taus, A.; Muns, M.D.; Fabrego, B.; Mancebo, G. Impact of prehabilitation during neoadjuvant chemotherapy and interval cytoreductive surgery on ovarian cancer patients: A pilot study. World J. Surg. Oncol. 2022, 20, 46. [Google Scholar] [CrossRef]
- Miralpeix, E.; Fabrego, B.; Rodriguez-Cosmen, C.; Sole-Sedeno, J.M.; Gayete, S.; Jara-Bogunya, D.; Corcoy, M.; Mancebo, G. Prehabilitation in an ERAS program for endometrial cancer patients: Impact on post-operative recovery. Int. J. Gynecol. Cancer 2023, 33, 528–533. [Google Scholar] [CrossRef]
- Bland, K.A.; Krishnasamy, M.; Parr, E.B.; Mulder, S.; Martin, P.; van Loon, L.J.C.; Cormie, P.; Michael, N.; Zopf, E.M. “I want to get myself as fit as I can and not die just yet”—Perceptions of exercise in people with advanced cancer and cachexia: A qualitative study. BMC Palliat. Care 2022, 21, 75. [Google Scholar] [CrossRef]
- Farrokhzadi, L.; Dhillon, H.M.; Goumas, C.; Young, J.M.; Cust, A.E. Physical Activity Correlates, Barriers, and Preferences for Women with Gynecological Cancer. Int. J. Gynecol. Cancer 2016, 26, 1530–1537. [Google Scholar] [CrossRef]
| Characteristics | Participants n = 111 (%) |
|---|---|
| Age [years], median (IQR; range) | 66 (59–75; 24–87) |
| Weight [kg], median (IQR; range) | 76.6 (64.1–87.8; 55–120.8) |
| BMI [kg/m2], n (%) | |
| 18.5–25 | 34 (30.6) |
| 25–30 | 37 (33.3) |
| >30 | 40 (36.0) |
| Comorbidities, n (%) | |
| None | 30 (27.0) |
| 1 | 35 (31.5) |
| 2 | 18 (16.2) |
| >2 | 28 (25.2) |
| Charlson Comorbidity Index, n (%) | |
| 0–1 | 85 (76.6) |
| >1 | 26 (23.4) |
| ECOG, n (%) | |
| 0 | 80 (72.1) |
| 1 | 23 (20.7) |
| 2 | 7 (6.3) |
| 3 | 1 (0.9) |
| Smoking, n (%) | |
| Yes | 9 (8.1) |
| No | 102 (91.7) |
| Alcohol consumption, n (%) | |
| Yes | 29 (26.1) |
| No | 52 (46.8) |
| Unknown | 30 (27.1) |
| ASA score, n (%) | |
| 1 | 5 (4.5) |
| 2 | 76 (67.5) |
| 3 | 30 (27.0) |
| Cancer diagnosis, n (%) | |
| Uterine | 48 (43.2) |
| FIGO I–II | 30 (62.6) |
| FIGO III–IV | 14 (29.2) |
| Benign | 4 (8.3) |
| Ovarian | 40 (36.0) |
| FIGO I–II | 14 (35.0) |
| FIGO III–IV | 23 (57.5) |
| Borderline/teratoma | 3 (7.5) |
| Vulvar | 22 (19.8) |
| FIGO I–II | 14 (63.63) |
| FIGO III–IV | 6 (27.2) |
| DVIN/melanoma | 2 (9.1) |
| Uterine and ovarian | 1 (0.9) |
| Surgical procedure, n (%) | |
| Ovarian cancer | |
| Debulking | 23 (57.5) |
| Laparoscopic staging | 5 (12.5) |
| Exploratory laparotomy | 10 (25.0) |
| Other | 2 (5.0) |
| Uterine cancer | |
| Uterus + Adnexa | 17 (35.4) |
| Uterus + Adnexa + SN + LND | 30 (62.5) |
| Other | 1 (2.1) |
| Vulvar cancer | |
| Vulvectomy | 4 (18.2) |
| Vulvectomy + lymph node surgery | 17 (77.3) |
| Exenterative surgery | 1 (4.5) |
| Participants (n = 97) | |
| Supervised training program | |
| Possible training sessions, median (IQR; range) a | 6 (3–8; 2–19) |
| Completed training sessions, median (IQR; range) b | 6 (3–7; 0–10) |
| Adherence rate, % c | 85% |
| Unsupervised training program | |
| Possible training sessions per week, fixed n | 4 |
| Completed training sessions per week, median (IQR; range) a | 4 (3–4; 0–4) |
| Adherence rate, % a | 88% |
| Participants (n = 101) | |
| Protein supplementation | |
| Possible servings, fixed n | 7 |
| Completed servings, median (IQR; range) | 7 (7–7; 0–7) |
| Adherence rate, % | 93% |
| Multivitamin supplementation | |
| Possible doses, fixed n | 7 |
| Completed doses, median (IQR; range) | 7 (7–7; 0–7) |
| Adherence rate, % | 98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanis, J.; Strijker, D.; Drager, L.D.; van Ham, M.; van Laarhoven, C.J.H.M.; Pijnenborg, J.M.A.; Smits, A.; van den Heuvel, B. Feasibility of Introducing a Prehabilitation Program into the Care of Gynecological Oncology Patients—A Single Institution Experience. Cancers 2024, 16, 1013. https://doi.org/10.3390/cancers16051013
Dhanis J, Strijker D, Drager LD, van Ham M, van Laarhoven CJHM, Pijnenborg JMA, Smits A, van den Heuvel B. Feasibility of Introducing a Prehabilitation Program into the Care of Gynecological Oncology Patients—A Single Institution Experience. Cancers. 2024; 16(5):1013. https://doi.org/10.3390/cancers16051013
Chicago/Turabian StyleDhanis, Joëlle, Dieuwke Strijker, Luuk D. Drager, Maaike van Ham, Cornelis J. H. M. van Laarhoven, Johanna M. A. Pijnenborg, Anke Smits, and Baukje van den Heuvel. 2024. "Feasibility of Introducing a Prehabilitation Program into the Care of Gynecological Oncology Patients—A Single Institution Experience" Cancers 16, no. 5: 1013. https://doi.org/10.3390/cancers16051013
APA StyleDhanis, J., Strijker, D., Drager, L. D., van Ham, M., van Laarhoven, C. J. H. M., Pijnenborg, J. M. A., Smits, A., & van den Heuvel, B. (2024). Feasibility of Introducing a Prehabilitation Program into the Care of Gynecological Oncology Patients—A Single Institution Experience. Cancers, 16(5), 1013. https://doi.org/10.3390/cancers16051013






