Short-Term Outcomes of Conventional Laparoscopic versus Robot-Assisted Distal Pancreatectomy for Malignancy: Evidence from US National Inpatient Sample, 2005–2018
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Data Source
2.2. Study Design and Approvals
2.3. Study Population
2.4. Main Outcomes and Study Variables
2.5. Statistical Analysis
3. Results
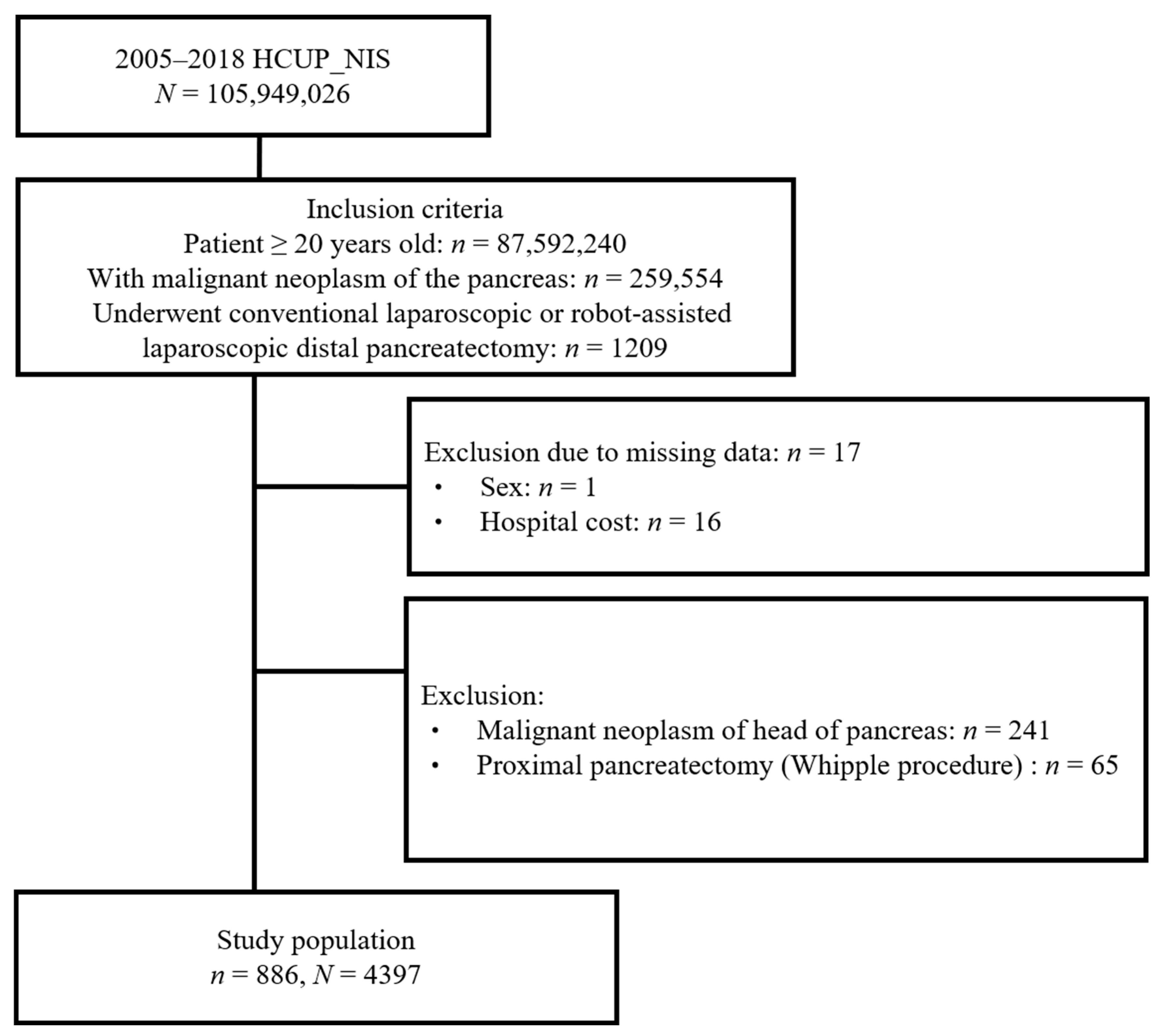
3.1. Patient Selection
3.2. Patient Characteristics
3.3. In-Hospital Outcomes
3.4. Associations between Type of Surgery, In-Hospital Outcomes, and Costs
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Partyka, O.; Pajewska, M.; Kwaśniewska, D.; Czerw, A.; Deptała, A.; Budzik, M.; Cipora, E.; Gąska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef]
- Buanes, T.A. Role of surgery in pancreatic cancer. World J. Gastroenterol. 2017, 23, 3765–3770. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; El Boghdady, M. Pancreatic cancer surgery. BMC Surg. 2023, 23, 196. [Google Scholar] [CrossRef]
- Jiang, L.; Ning, D.; Chen, X.P. Improvement in distal pancreatectomy for tumors in the body and tail of the pancreas. World J. Surg. Oncol. 2021, 19, 49. [Google Scholar] [CrossRef]
- Kleeff, J.; Diener, M.K.; Z’Graggen, K.; Hinz, U.; Wagner, M.; Bachmann, J.; Zehetner, J.; Müller, M.W.; Friess, H.; Büchler, M.W. Distal pancreatectomy: Risk factors for surgical failure in 302 consecutive cases. Ann. Surg. 2007, 245, 573–582. [Google Scholar] [CrossRef]
- Bausch, D.; Keck, T. Minimally Invasive Surgery of Pancreatic Cancer: Feasibility and Rationale. Visc. Med. 2018, 34, 440–443. [Google Scholar] [CrossRef]
- De Luca, L.; Repici, A.; Koçollari, A.; Auriemma, F.; Bianchetti, M.; Mangiavillano, B. Pancreatoscopy: An update. World J. Gastrointest. Endosc. 2019, 11, 22–30. [Google Scholar] [CrossRef]
- Fernández-Cruz, L. Distal pancreatic resection: Technical differences between open and laparoscopic approaches. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2006, 8, 49–56. [Google Scholar] [CrossRef]
- Yi, X.; Chen, S.; Wang, W.; Zou, L.; Diao, D.; Zheng, Y.; He, Y.; Li, H.; Luo, L.; Xiong, W.; et al. A Systematic Review and Meta-Analysis of Laparoscopic and Open Distal Pancreatectomy of Nonductal Adenocarcinomatous Pancreatic Tumor (NDACPT) in the Pancreatic Body and Tail. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 206–219. [Google Scholar] [CrossRef]
- Overview of the Nationwide Inpatient Sample (NIS). Available online: http://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 15 June 2023).
- Rivero-Moreno, Y.; Echevarria, S.; Vidal-Valderrama, C.; Pianetti, L.; Cordova-Guilarte, J.; Navarro-Gonzalez, J.; Acevedo-Rodríguez, J.; Dorado-Avila, G.; Osorio-Romero, L.; Chavez-Campos, C.; et al. Robotic Surgery: A Comprehensive Review of the Literature and Current Trends. Cureus 2023, 15, e42370. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Sikander, S.; Kulkarni, P. Recent advances in robot-assisted surgical systems. Biomed. Eng. Adv. 2023, 6, 100109. [Google Scholar] [CrossRef]
- Jien, H.; Xiaohua, L. Laparoscopic versus open surgery in the treatment of hepatic hemangioma: A meta-analysis. Medicine 2021, 100, e24155. [Google Scholar] [CrossRef] [PubMed]
- Shyr, B.U.; Chen, S.C.; Shyr, Y.M.; Wang, S.E. Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine 2018, 97, e13000. [Google Scholar] [CrossRef] [PubMed]
- van Ramshorst, T.M.E.; van Hilst, J.; Bannone, E.; Pulvirenti, A.; Asbun, H.J.; Boggi, U.; Busch, O.R.; Dokmak, S.; Edwin, B.; Hogg, M.; et al. International survey on opinions and use of robot-assisted and laparoscopic minimally invasive pancreatic surgery: 5-year follow up. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2024, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, T.; Zou, X.; Li, P.; Gao, R.; Dai, M.; Guo, J.; Zhang, T.; Liao, Q.; Liu, Z.; et al. The learning curve for robot-assisted distal pancreatectomy: A single-center experience of 301 cases. J. Pancreatol. 2022, 5, 118–124. [Google Scholar] [CrossRef]
- Chen, J.W.; van Ramshorst, T.M.E.; Lof, S.; Al-Sarireh, B.; Bjornsson, B.; Boggi, U.; Burdio, F.; Butturini, G.; Casadei, R.; Coratti, A.; et al. Robot-Assisted Versus Laparoscopic Distal Pancreatectomy in Patients with Resectable Pancreatic Cancer: An International, Retrospective, Cohort Study. Ann. Surg. Oncol. 2023, 30, 3023–3032. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, B.; Wang, T.; Hu, X.; Ye, Y.; Guo, W. Comparative Efficacy of Robot-Assisted and Laparoscopic Distal Pancreatectomy: A Single-Center Comparative Study. J. Healthc. Eng. 2022, 2022, 7302222. [Google Scholar] [CrossRef]
- Di Franco, G.; Peri, A.; Lorenzoni, V.; Palmeri, M.; Furbetta, N.; Guadagni, S.; Gianardi, D.; Bianchini, M.; Pollina, L.E.; Melfi, F.; et al. Minimally invasive distal pancreatectomy: A case-matched cost-analysis between robot-assisted surgery and direct manual laparoscopy. Surg. Endosc. 2022, 36, 651–662. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, K.; Zhang, S.; Shao, Z.; Cheng, P.; Zhang, Y.; Jin, G.; He, T. Robot-assisted distal pancreatectomy improves spleen preservation rate versus laparoscopic distal pancreatectomy for benign and low-grade malignant lesions of the pancreas. Transl. Cancer Res. 2020, 9, 5166–5172. [Google Scholar] [CrossRef] [PubMed]
- Lof, S.; van der Heijde, N.; Abuawwad, M.; Al-Sarireh, B.; Boggi, U.; Butturini, G.; Capretti, G.; Coratti, A.; Casadei, R.; D’Hondt, M.; et al. Robotic versus laparoscopic distal pancreatectomy: Multicentre analysis. Br. J. Surg. 2021, 108, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kwon, J.; Lee, J.H.; Park, S.Y.; Park, Y.; Lee, W.; Song, K.B.; Hwang, D.W.; Kim, S.C. Robotic versus laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: A propensity score-matched analysis. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2023, 22, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Souche, R.; Herrero, A.; Bourel, G.; Chauvat, J.; Pirlet, I.; Guillon, F.; Nocca, D.; Borie, F.; Mercier, G.; Fabre, J.M. Robotic versus laparoscopic distal pancreatectomy: A French prospective single-center experience and cost-effectiveness analysis. Surg. Endosc. 2018, 32, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Qin, Y.F.; Yu, D.D.; Li, X.; Zhao, Y.M.; Kong, D.J.; Jin, W.; Wang, H. Meta-analysis of short-term outcomes comparing robot-assisted and laparoscopic distal pancreatectomy. J. Comp. Eff. Res. 2020, 9, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Cheng, Y.; Wang, B.; Zhao, S.; Chen, L. Comparison of 3 Minimally Invasive Methods Versus Open Distal Pancreatectomy: A Systematic Review and Network Meta-Analysis. Surg. Laparosc. Endosc. Percutaneous Tech. 2020, 31, 104–112. [Google Scholar] [CrossRef]
- van Ramshorst, T.M.E.; van Bodegraven, E.A.; Zampedri, P.; Kasai, M.; Besselink, M.G.; Abu Hilal, M. Robot-assisted versus laparoscopic distal pancreatectomy: A systematic review and meta-analysis including patient subgroups. Surg. Endosc. 2023, 37, 4131–4143. [Google Scholar] [CrossRef]
- Zhou, J.; Lv, Z.; Zou, H.; Xiong, L.; Liu, Z.; Chen, W.; Wen, Y. Up-to-date comparison of robotic-assisted versus open distal pancreatectomy: A PRISMA-compliant meta-analysis. Medicine 2020, 99, e20435. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 886) | Laparoscopic Surgery | p-Value | |
|---|---|---|---|---|
| Conventional (n = 648) | Robot-Assisted (n = 238) | |||
| Age, years | 65.3 ± 0.4 | 64.7 ± 0.4 | 66.8 ± 0.6 | 0.040 |
| 20–29 | 17 (2.0) | 13 (2.1) | 4 (1.7) | 0.058 |
| 30–39 | 23 (2.6) | 16 (2.5) | 7 (3.0) | |
| 40–49 | 59 (6.7) | 47 (7.2) | 12 (5.1) | |
| 50–59 | 163 (18.4) | 129 (19.9) | 34 (14.3) | |
| 60–69 | 258 (29.2) | 191 (29.6) | 67 (28.0) | |
| 70–79 | 246 (27.7) | 172 (26.5) | 74 (31.0) | |
| ≥80 | 120 (13.5) | 80 (12.2) | 40 (16.9) | |
| Sex | 0.315 | |||
| Male | 421 (47.5) | 314 (48.4) | 107 (44.9) | |
| Female | 465 (52.5) | 334 (51.6) | 131 (55.1) | |
| Insurance status | 0.153 | |||
| Medicare/Medicaid | 509 (57.4) | 362 (55.8) | 147 (61.7) | |
| Private including HMO | 346 (39.3) | 263 (41.0) | 83 (34.9) | |
| Self-pay/no-charge/other | 29 (3.3) | 21 (3.2) | 8 (3.4) | |
| Missing | 2 | 2 | 0 | |
| Household income | 0.225 | |||
| Quartile 1 | 170 (19.6) | 125 (19.7) | 45 (19.3) | |
| Quartile 2 | 197 (22.5) | 153 (24.0) | 44 (18.6) | |
| Quartile 3 | 230 (26.6) | 168 (26.6) | 62 (26.6) | |
| Quartile 4 | 272 (31.3) | 189 (29.8) | 83 (35.5) | |
| Missing | 17 | 13 | 4 | |
| Smoking | 306 (34.6) | 211 (32.6) | 95 (39.9) | 0.018 |
| Weekend admission | 20 (2.2) | 16 (2.5) | 4 (1.7) | 0.489 |
| Hospital bed number | 0.644 | |||
| Small | 54 (6.1) | 39 (6.0) | 15 (6.3) | |
| Medium | 150 (17.1) | 106 (16.5) | 44 (18.7) | |
| Large | 681 (76.9) | 502 (77.5) | 179 (75.0) | |
| Missing | 1 | 1 | 0 | |
| Hospital location/teaching status | 0.021 | |||
| Rural | 8 (0.9) | 4 (0.6) | 4 (1.7) | |
| Urban nonteaching | 74 (8.2) | 51 (7.7) | 23 (9.6) | |
| Urban teaching | 803 (90.9) | 592 (91.7) | 211 (88.7) | |
| Missing | 1 | 1 | 0 | |
| Combined spleen removal | 776 (87.5) | 562 (86.7) | 214 (89.9) | 0.159 |
| Emergent admission (missing = 1) | 80 (9.1) | 66 (10.2) | 14 (6.0) | 0.016 |
| Comorbidities | ||||
| Coronary artery disease | 140 (15.7) | 93 (14.2) | 47 (19.6) | 0.019 |
| Congestive heart failure | 31 (3.4) | 23 (3.4) | 8 (3.4) | 0.954 |
| Persistent anemia | 113 (12.6) | 82 (12.5) | 31 (12.9) | 0.867 |
| Diabetes | 283 (31.8) | 202 (31.0) | 81 (34.0) | 0.377 |
| Hypertension | 462 (52.0) | 328 (50.5) | 134 (56.3) | 0.088 |
| Cerebrovascular disease | 14 (1.5) | 10 (1.5) | 4 (1.7) | 0.782 |
| Chronic pulmonary disease | 120 (13.5) | 87 (13.3) | 33 (13.9) | 0.823 |
| Hyperlipidemia | 343 (38.7) | 242 (37.4) | 101 (42.3) | 0.174 |
| Drug abuse | 74 (8.4) | 56 (8.7) | 18 (7.6) | 0.567 |
| Severe Liver disease | 17 (1.9) | 14 (2.2) | 3 (1.2) | 0.306 |
| Moderate or severe renal disease | 43 (4.8) | 33 (5.1) | 10 (4.2) | 0.552 |
| Rheumatic disease | 21 (2.4) | 13 (2.0) | 8 (3.4) | 0.199 |
| CCI | 0.289 | |||
| 0–1 | 702 (79.4) | 519 (80.3) | 183 (76.9) | |
| 2–3 | 138 (15.4) | 97 (14.8) | 41 (17.2) | |
| 4–5 | 37 (4.2) | 24 (3.7) | 13 (5.4) | |
| 6+ | 9 (1.0) | 8 (1.2) | 1 (0.4) | |
| Year of admission | 0.556 | |||
| 2005–2014 | 359 (40.1) | 267 (40.8) | 92 (38.3) | |
| 2015–2018 | 527 (59.9) | 381 (59.2) | 146 (61.7) | |
| In-Hospital Outcome | Total (n = 886) | Laparoscopic Surgery | p-Value | |
|---|---|---|---|---|
| Conventional (n = 648) | Robot-Assisted (n = 238) | |||
| In-hospital mortality | 9 (1.0) | 9 (1.4) | 0 (0.0) | - |
| Perioperative complication | 212 (23.8) | 168 (25.8) | 44 (18.4) | 0.004 |
| AMI | 1 (0.1) | 1 (0.2) | 0 (0.0) | - |
| CVA | 6 (0.7) | 4 (0.6) | 2 (0.8) | 0.708 |
| VTE | 33 (3.7) | 29 (4.5) | 4 (1.6) | 0.012 |
| Periprocedural shock, hypertension, or other cardiovascular complications | 15 (1.7) | 11 (1.7) | 4 (1.6) | 0.951 |
| Pneumonia | 27 (3.1) | 22 (3.4) | 5 (2.1) | 0.256 |
| Postprocedural pneumothorax | 4 (0.5) | 4 (0.6) | 0 (0.0) | - |
| Postprocedural air leak | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Acute respiratory failure | 28 (3.2) | 20 (3.1) | 8 (3.4) | 0.705 |
| Pulmonary collapse (atelectasis) | 64 (7.2) | 50 (7.6) | 14 (5.9) | 0.297 |
| Sepsis | 32 (3.6) | 25 (3.8) | 7 (3.0) | 0.435 |
| Infection | 32 (3.5) | 26 (3.9) | 6 (2.5) | 0.182 |
| Mechanical ventilation | 15 (1.7) | 11 (1.7) | 4 (1.7) | 0.989 |
| Postoperative blood transfusion | 61 (6.8) | 53 (8.0) | 8 (3.4) | <0.001 |
| Other complications of the respiratory system | 10 (1.1) | 7 (1.1) | 3 (1.3) | 0.709 |
| Perforations of organs or vessels | 8 (0.9) | 6 (1.0) | 2 (0.8) | 0.871 |
| LOS, days a | 6.6 ± 0.2 | 6.8 ± 0.2 | 6.0 ± 0.2 | 0.005 |
| Unfavorable discharge a | 57 (6.4) | 42 (6.5) | 15 (6.2) | 0.861 |
| Hospital cost, US dollars | 99,874 ± 3506 | 95,402 ± 3602 | 112,036 ± 4567 | 0.024 |
| Outcomes | Multivariable Analysis b | |
|---|---|---|
| aOR (95% CI) | p-Value | |
| In-hospital mortality | NA | - |
| Unfavorable discharge a | 0.73 (0.43–1.24) | 0.243 |
| Perioperative complication, any | 0.61 (0.45–0.83) | 0.002 |
| AMI | NA | - |
| CVA | 1.09 (0.22–5.35) | 0.912 |
| VTE | 0.35 (0.14–0.85) | 0.021 |
| Periprocedural shock, hypertension, or other cardiovascular complications | 0.94 (0.31–2.84) | 0.910 |
| Pneumonia | 0.56 (0.23–1.33) | 0.184 |
| Postprocedural pneumothorax | NA | - |
| Postprocedural air leak | NA | - |
| Acute respiratory failure | 1.08 (0.61–1.89) | 0.801 |
| Pulmonary collapse (atelectasis) | 0.70 (0.40–1.23) | 0.219 |
| Sepsis | 0.70 (0.36–1.37) | 0.298 |
| Infection | 0.62 (0.31–1.25) | 0.182 |
| Mechanical ventilation | 0.92 (0.33–2.52) | 0.864 |
| Postoperative blood transfusion | 0.37 (0.23–0.61) | <0.001 |
| Other complications of the respiratory system | 1.20 (0.50–2.86) | 0.680 |
| Perforations of organs or vessels | 0.84 (0.17–4.23) | 0.830 |
| Outcomes | Multivariable Analysis b | |
|---|---|---|
| aBeta (95% CI) | p-Value | |
| LOS a | −0.76 (−1.43, −0.09) | 0.026 |
| Total hospital costs | 18,284 (4369, 32,200) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-M.; Chen, S.-H.; Chen, T.-H. Short-Term Outcomes of Conventional Laparoscopic versus Robot-Assisted Distal Pancreatectomy for Malignancy: Evidence from US National Inpatient Sample, 2005–2018. Cancers 2024, 16, 1003. https://doi.org/10.3390/cancers16051003
Huang J-M, Chen S-H, Chen T-H. Short-Term Outcomes of Conventional Laparoscopic versus Robot-Assisted Distal Pancreatectomy for Malignancy: Evidence from US National Inpatient Sample, 2005–2018. Cancers. 2024; 16(5):1003. https://doi.org/10.3390/cancers16051003
Chicago/Turabian StyleHuang, Jyun-Ming, Sheng-Hsien Chen, and Te-Hung Chen. 2024. "Short-Term Outcomes of Conventional Laparoscopic versus Robot-Assisted Distal Pancreatectomy for Malignancy: Evidence from US National Inpatient Sample, 2005–2018" Cancers 16, no. 5: 1003. https://doi.org/10.3390/cancers16051003
APA StyleHuang, J.-M., Chen, S.-H., & Chen, T.-H. (2024). Short-Term Outcomes of Conventional Laparoscopic versus Robot-Assisted Distal Pancreatectomy for Malignancy: Evidence from US National Inpatient Sample, 2005–2018. Cancers, 16(5), 1003. https://doi.org/10.3390/cancers16051003






