Cost–Utility Analysis of Tenofovir Alafenamide and Entecavir in Chronic Hepatitis B Patients: A Markov Decision Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
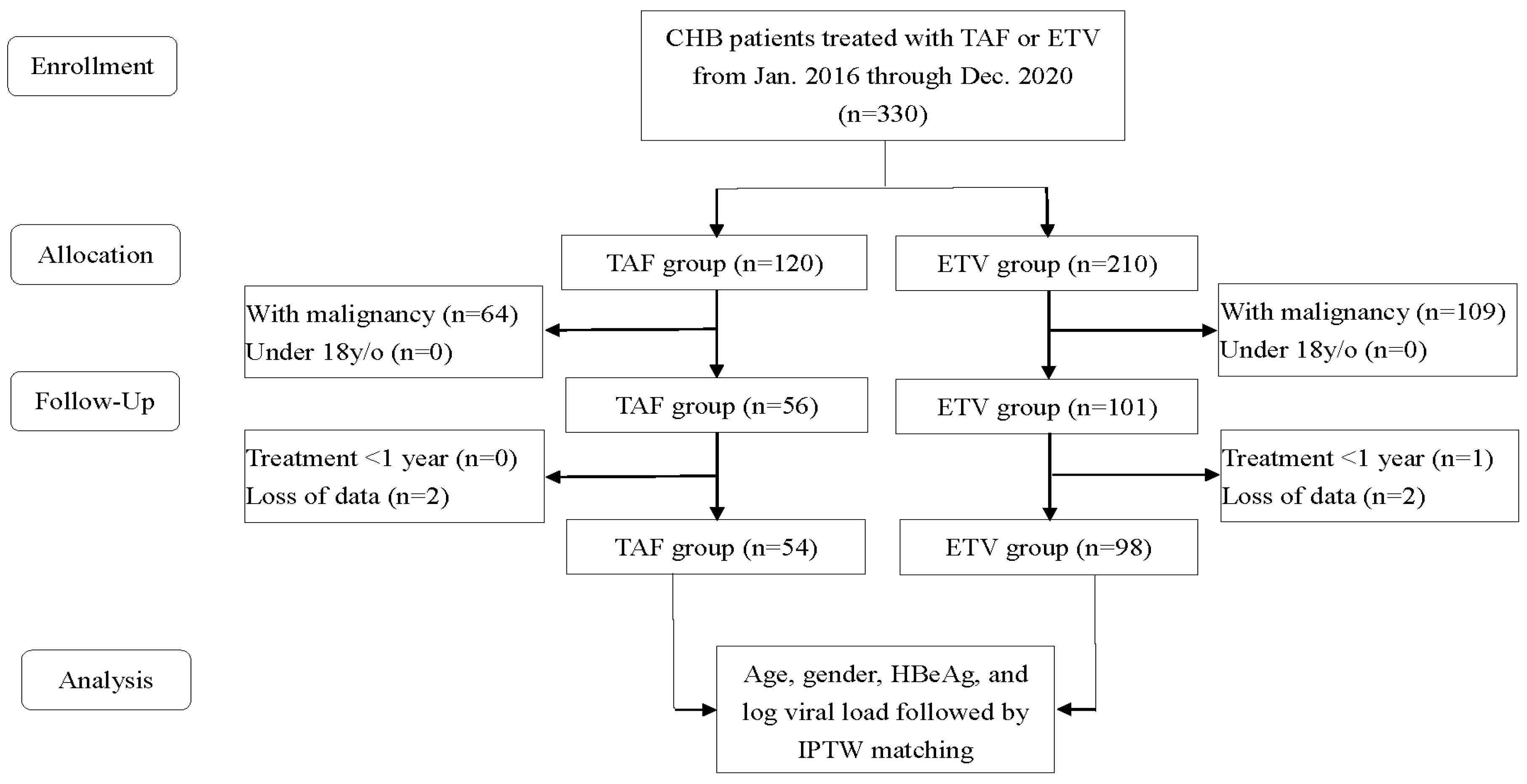
2.1. Research Samples and Study Design
2.2. Confounding Variables
2.3. Economic Evaluation
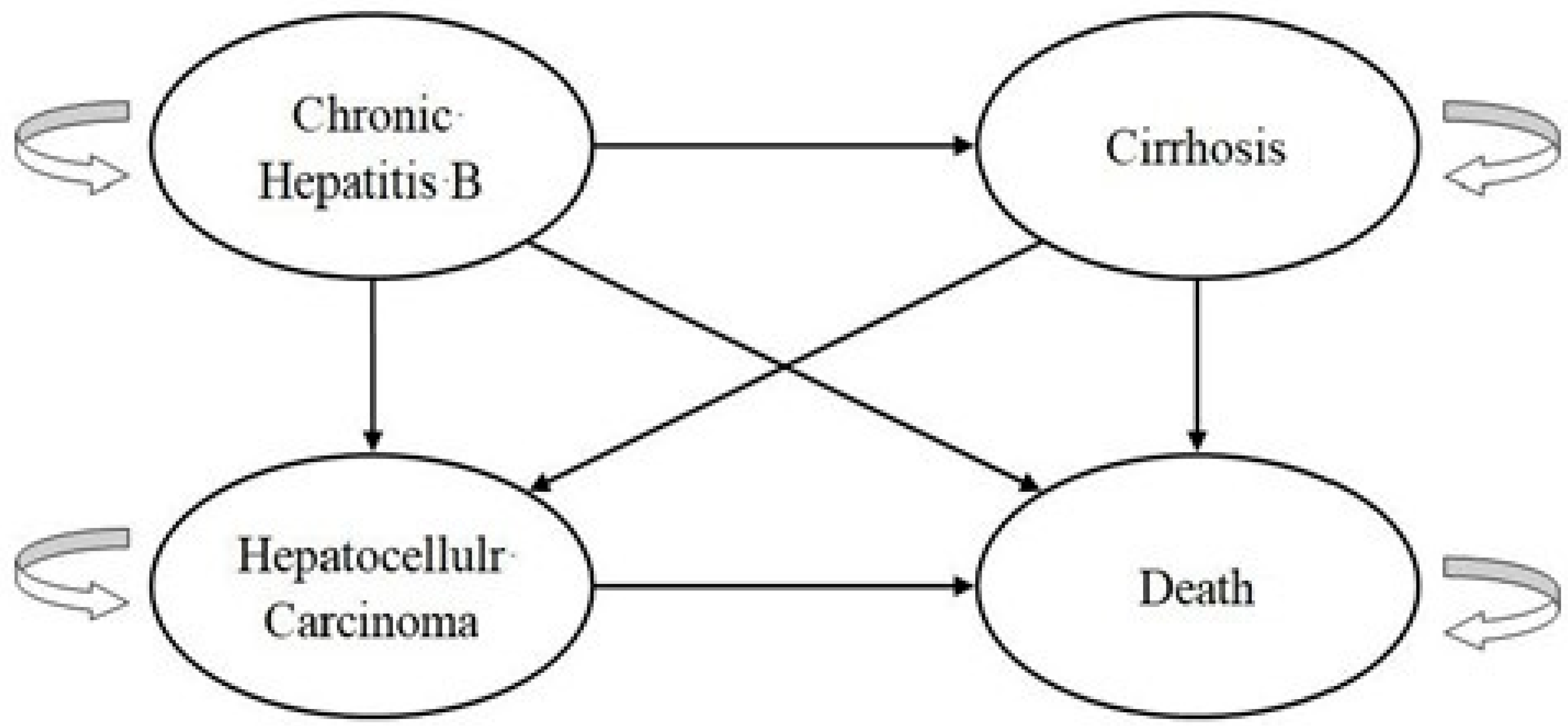
2.4. Cost–Utility Analysis
2.5. Statistical Analysis
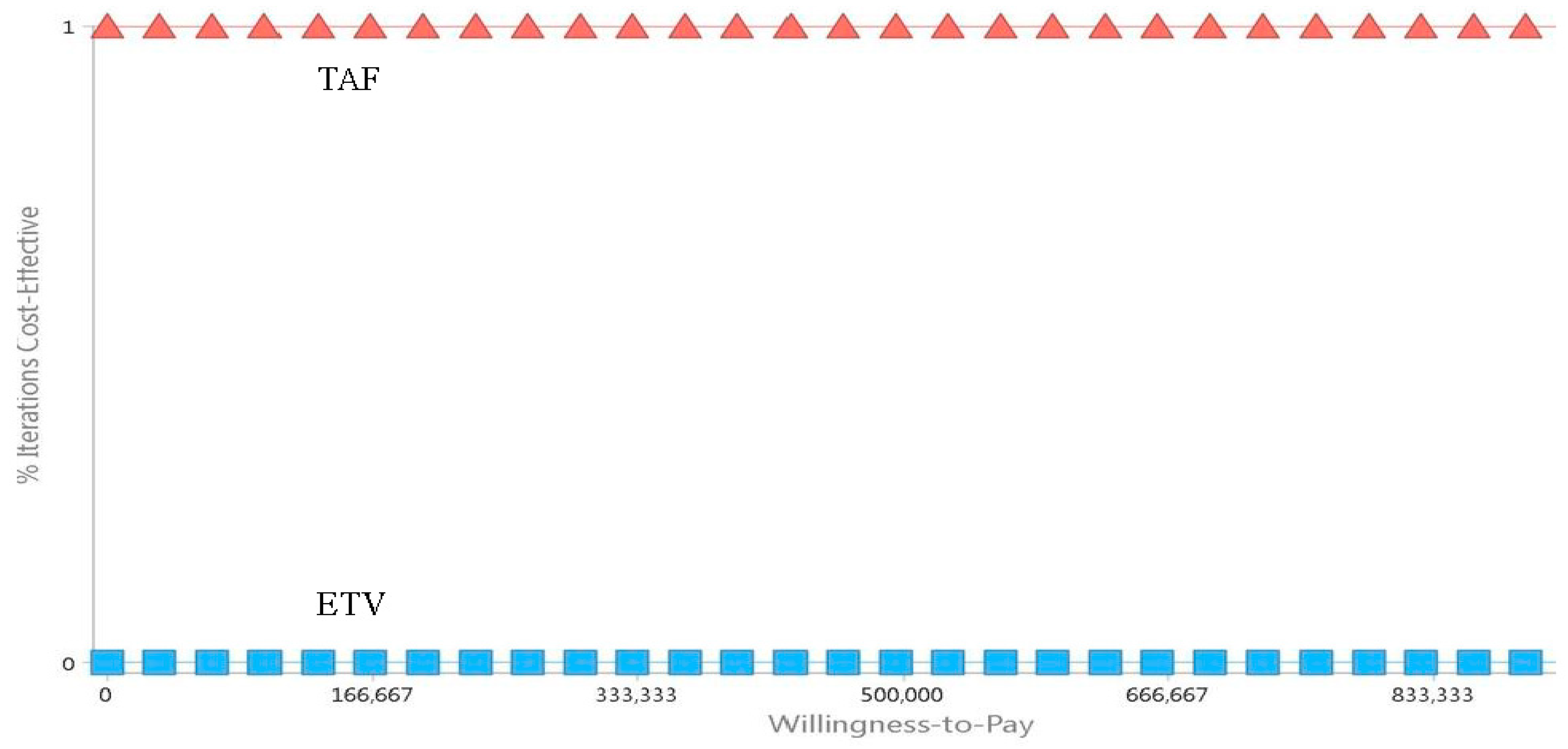
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 1 August 2022).
- Taiwan Ministry of Health and Welfare. Cause of Death Statistics in Taiwan. 2021. Available online: https://dep.mohw.gov.tw/DOS/cp-5069-70304-113.html. (accessed on 1 August 2022).
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, H.Y.; Kim, J.; Kim, Y.J. Systematic Review with Meta-Analysis: Comparison of the Risk of Hepatocellular Carcinoma in Antiviral-Naive Chronic Hepatitis B Patients Treated with Entecavir versus Tenofovir: The Devil in the Detail. Cancers 2022, 14, 2617. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Kowdley, K. Management of chronic hepatitis B infection. BMJ 2015, 351, h4263. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Buti, M.; Fung, S.; Ahn, S.H.; Chuang, W.-L.; Tak, W.Y.; Ramji, A.; Chen, C.-Y.; Tam, E.; Bae, H.; et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: A randomied, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol. Hepatol. 2020, 5, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Brunetto, M.; Seto, W.K.; Lim, Y.-S.; Fung, S.; Marcellin, P.; Ahn, S.H.; Izumi, N.; Chuang, W.-L.; Bae, H.; et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol. 2018, 68, 672–681. [Google Scholar] [CrossRef]
- Liang, L.Y.; Yip, T.C.-F.; Lai, J.C.-T.; Lam, A.S.-M.; Tse, Y.K.; Hui, V.W.-K.; Chan, H.L.-Y.; Wong, V.W.-S.; Wong, G.L.-H. Tenofovir alafenamide is associated with improved alanine aminotransferase and renal safety compared to tenofovir disoproxil fumarate. J. Med. Virol. 2022, 94, 4440–4448. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.W.; Lim, T.S.; Shin, H.J.; Lee, H.W.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, B.K. Novel Liver Stiffness-Based Nomogram for Predicting Hepatocellular Carcinoma Risk in Patients with Chronic Hepatitis B Virus Infection Initiating Antiviral Therapy. Cancers 2021, 13, 5892. [Google Scholar] [CrossRef]
- Liem, K.S.; Gehring, A.J.; Feld, J.J.; Janssen, H.L.A. Challenges with Stopping Long-term Nucleos(t)ide Analogue Therapy in Patients With Chronic Hepatitis B. Gastroenterology 2020, 158, 1185–1190. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Hung, M.-C.; Hu, F.-C.; Chang, Y.Y.; Hsieh, C.L.; Wang, J.-D. Estimating quality weights for EQ-5D (EuroQol-5 dimensions) health states with the time trade-off method in Taiwan. J. Formos. Med. Assoc. 2013, 112, 699–706. [Google Scholar] [CrossRef]
- Khan, Y.D.; Mahmood, M.K.; Ahmad, D.; Al-Zidi, N.M. Content-Based Image Retrieval Using Gamma Distribution and Mixture Model. J. Funct. Space 2022, 8674038, 8674038. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chan, S.K.; Lee, S.F.; Choi, H.C. First-Line Atezolizumab Plus Bevacizumab versus Sorafenib in Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Cancers 2021, 13, 931. [Google Scholar] [CrossRef]
- Archambeau, K.; Couto, J.; Van Maanen, L. Non-parametric mixture modeling of cognitive psychological data: A new method to disentangle hidden strategies. Behav. Res. Methods 2023, 55, 2232–2248. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.R.; Kowdley, K.V.; Iloeje, U.; Tafesse, E.; Mukherjee, J.; Gish, R.; Bzowej, N.; Briggs, A.H. The impact of chronic hepatitis B on quality of life: A multinational study of utilities from infected and uninfected persons. Value Health 2008, 11, 527–538. [Google Scholar] [CrossRef]
- Toy, M.; Salomon, J.A.; Jiang, H.; Gui, H.; Wang, H.; Wang, J.; Richardus, J.H.; Xie, Q. Population health impact and cost-effectiveness of monitoring inactive chronic hepatitis B and treating eligible patients in Shanghai, China. Hepatology 2014, 60, 46–55. [Google Scholar] [CrossRef]
- Chon, H.Y.; Ahn, S.H.; Kim, Y.J.; Yoon, J.-H.; Lee, J.-H.; Sinn, D.H.; Kim, S.U. Efficacy of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide in treatment-naive hepatitis B patients. Hepatol. Int. 2021, 15, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, C.Y.; Wang, J.H.; Lai, H.C.; Hung, C.H.; Lu, S.N.; Peng, C.Y. Comparison of incidence of hepatocellular carcinoma between chronic hepatitis B patients with cirrhosis treated with entecavir or tenofovir in Taiwan—A retrospective study. Am. J. Cancer Res. 2020, 10, 3882–3895. [Google Scholar]
- Wang, X.; Liu, X.; Wang, P.; Yu, L.; Yan, F.; Yan, H.; Zhou, D.; Yang, Z. Antiviral Therapy Reduces Mortality in Hepatocellular Carcinoma Patients with Low-Level Hepatitis B Viremia. J. Hepatocell. Carcinoma 2021, 8, 1253–1267. [Google Scholar] [CrossRef]
- Tian, F.; Houle, S.K.D.; Alsabbagh, M.W.; Wong, W.W.L. Cost-Effectiveness of Tenofovir Alafenamide for Treatment of Chronic Hepatitis B in Canada. Pharmacoeconomics 2020, 38, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wong, I.O.L.; Xie, C.; Xu, W.; Xiang, Y.; Peng, L.; Lau, E.H.Y. Cost-effectiveness analysis of first-line treatment for chronic hepatitis B in China. Clin. Microbiol. Infect. 2022, 28, 300.e1–300.e8. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Stel, V.S.; Tripepi, G.; Dekker, F.W.; Fu, E.L.; Zoccali, C.; Jager, K.J. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 2021, 15, 14–20. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z. Can remdesivir and its parent nucleoside GS-441524 be potential oral drugs? An in vitro and in vivo DMPK assessment. Acta. Pharm. Sin. B 2021, 11, 1607–1616. [Google Scholar] [CrossRef]
- Pan, C.Q.; Afdhal, N.H.; Ankoma-Sey, V.; Bae, H.; Curry, M.P.; Dieterich, D.; Frazier, L.; Frick, A.; Hann, H.-W.; Kim, W.R.; et al. First-line therapies for hepatitis B in the United States: A 3-year prospective and multicenter real-world study after approval of tenofovir alefenamide. Hepatol. Commun. 2022, 6, 1881–1894. [Google Scholar] [CrossRef]
- Kumada, T.; Toyoda, H.; Tada, T.; Yasuda, S.; Miyake, N.; Tanaka, J. Comparison of the impact of tenofovir alafenamide and entecavir on declines of hepatitis B surface antigen levels. Eur. J. Gastroenterol. Hepatol. 2021, 32, 255–260. [Google Scholar] [CrossRef]
- Li, J.; Hu, C.; Chen, Y.; Zhang, R.; Fu, S.; Zhou, M.; Gao, Z.; Fu, M.; Yan, T.; Yang, Y.; et al. Short-term and long-term safety and efficacy of tenofovir alafenamide, tenofovir disoproxil fumarate and entecavir treatment of acute-on-chronic liver failure associated with hepatitis B. BMC Infect. Dis. 2021, 21, 567. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Kim, H.W.; Ahn, S.H.; Kim, S.U.; Kim, B.S. Higher risk of kidney function decline with entecavir than tenofovir alafenamide in patients with chronic hepatitis B. Liver Int. 2022, 42, 1017–1026. [Google Scholar] [CrossRef]
- Bermingham, S.L.; Hughes, R.; Fenu, E.; Sawyer, L.M.; Boxall, E.; Kennedy, P.T.; Dusheiko, G.; Hill-Cawthorne, G.; Thomas, H. Cost-Effectiveness Analysis of Alternative Antiviral Strategies for the Treatment of HBeAg-Positive and HBeAg-Negative Chronic Hepatitis B in the United Kingdom. Value Health 2015, 18, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhang, C.; Liu, L.; Gao, Y.; Yao, Z.; Ye, Z.; Zhou, S.; Yang, Y. Cost-effectiveness analysis of tenofovir disoproxil fumarate for treatment of chronic hepatitis B in China. Hepatol. Int. 2016, 10, 924–936. [Google Scholar] [CrossRef]
- Yin, X.-R.; Liu, Z.-H.; Liu, J.; Liu, Y.Y.; Xie, L.; Tao, L.-B.; Jia, J.-D.; Cui, F.-Q.; Zhuang, G.-H.; Hou, J.-L. First line nucleos(t)ide analog monotherapy is more cost-effective than combination strategies in hepatitis B e antigen-positive chronic hepatitis B patients in China. Chin. Med. J. 2019, 132, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, S.; Wang, M.; Wang, Y.; Du, S.; Xin, Y.; Xuan, S. Tenofovir Alafenamide Fumarate, Tenofovir Disoproxil Fumarate and Entecavir: Which is the Most Effective Drug for Chronic Hepatitis B? A Systematic Review and Meta-analysis. J. Clin. Transl. Hepatol. 2021, 9, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Bhadoria, A.S.; Cui, F.; Tan, A.; Holten, J.V.; Easterbrook, P.; Ford, N.; Han, Q.; Lu, Y.; Bulterys, M.; et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Ministry of the Interior. Population of Taiwan. 2022. Available online: https://www.moi.gov.tw/english/cl.aspx?n=7872 (accessed on 1 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim(s) responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Variable | Total (n = 152) Mean ± Standard Deviation or Median (IQR) | Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|---|---|
| TAF (n = 54) | ETV (n = 98) | Standardized Difference (%) | TAF | ETV | Standardized Difference (%) | |||
| Age | 57.66 ± 13.29 57.5 [46–67.75] | 52.94 ± 11.02 50.0 [44.75–62.25] | 60.27 ± 13.76 61 [47.75–72.0] | −58.78 | 56.52 ± 11.35 56.0 [46.0–66.0] | 57.45 ± 13.78 58.0 [45.0–67.63] | −7.37 | |
| Gender | Male | 115 (75.7%) | 41 (75.9%) | 74 (75.5%) | 0.932 | 71.8% | 74.3% | −5.62 |
| Female | 37 (24.3) | 13 (24.1%) | 24 (24.5%) | 28.2% | 25.7% | |||
| HBeAg | Positive | 50 (32.9%) | 26 (48.1%) | 24 (24.5%) | 50.62 | 33.6% | 32.9% | 1.49 |
| Negative | 102 (67.1%) | 28 (51.9%) | 74(75.5%) | 66.4% | 67.1% | |||
| Log Viral Load | 5.97 ± 1.85 6.12 [4.53–7.81] | 6.27 ± 1.85 6.80 [4.97–7.89] | 5.81 ± 1.84 5.97 [4.24–7.63] | 25 | 5.89 ± 2.00 6.38 [4.45–7.80] | 5.96 ± 1.83 6.11 [4.30–7.66] | −3.65 | |
| TAF | ETV | Distribution | Reference | |
|---|---|---|---|---|
| Transition probability | ||||
| Chronic hepatitis B | ||||
| Chronic hepatitis B | 96.58% | 94.38% | Beta | [16] |
| Cirrhosis | 2.82% | 2.82% | Beta | [16] |
| Hepatocellular carcinoma | 0.30% | 0.70% | Beta | [16] |
| Death | 0.30% | 2.10% | Beta | [16] |
| Cirrhosis | ||||
| Cirrhosis | 96.56% | 95.16% | Beta | [17] |
| Hepatocellular carcinoma | 2.20% | 3.60% | Beta | [1] |
| Death | 1.24% | 1.24% | Beta | [17] |
| Hepatocellular carcinoma | ||||
| Hepatocellular carcinoma | 84.90% | 84.90% | Beta | [18] |
| Death | 15.10% | 15.10% | Beta | [18] |
| Cost (NT$) | ||||
| Outpatient (per cycle for 1 year) | ||||
| Medicine | 50,912 ± 6826 | 52,143 ± 12,492 | Gamma | This study |
| Physician | 5374 ± 4540 | 6653 ± 4322 | Gamma | This study |
| Laboratory | 2928 ± 460 | 3098 ± 724 | Gamma | This study |
| Pharmacist | 1008 ± 122 | 990 ± 198 | Gamma | This study |
| Total outpatient costs | 60,241 ± 8033 | 62,956 ± 13,867 | ||
| Hospitalization (per cycle for 1 year) | ||||
| Medicine | 10,730 ± 7181 | 12,876 ± 20,532 | Gamma | This study |
| Physician | 20,609 ± 11,598 | 15,037 ± 11,156 | Gamma | This study |
| Laboratory | 12,262 ± 4952 | 14,460 ± 13,337 | Gamma | This study |
| Ward | 25,645 ± 17,678 | 30,160 ± 32,093 | Gamma | This study |
| Radiology | 3748 ± 4069 | 7037 ± 14,222 | Gamma | This study |
| Therapy treatment | 7874 ± 4788 | 4954 ± 8452 | Gamma | This study |
| Total hospitalization costs | 80,868 ± 33,999 | 87,387 ± 101,745 | ||
| Total direct medical costs | 141,109 ± 120,398 | 150,343 ± 152,825 | ||
| Utility | ||||
| Chronic hepatitis B | 0.85 (0.68–0.89) | 0.85 (0.68–0.89) | Beta | [15] |
| Compensated cirrhosis | 0.69 (0.66–0.71) | 0.69 (0.66–0.71) | Beta | [2] |
| Decompensated cirrhosis | 0.35 (0.32–0.37) | 0.35 (0.32–0.37) | Beta | [2] |
| Hepatocellular carcinoma | 0.38 (0.36–0.41) | 0.38 (0.36–0.41) | Beta | [2] |
| Liver transplantation | 0.57 (0.54–0.60) | 0.57 (0.54–0.60) | Beta | [2] |
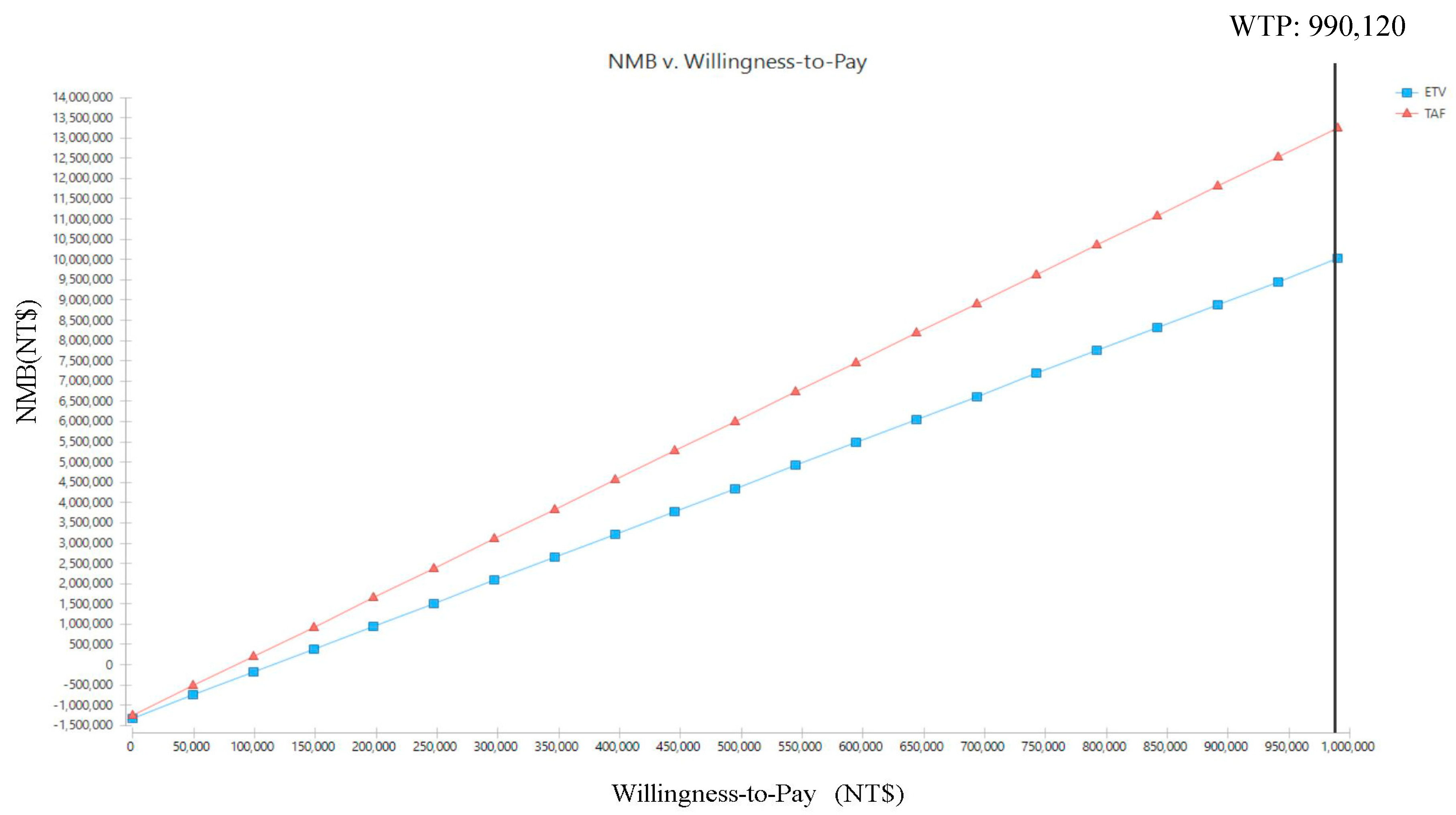
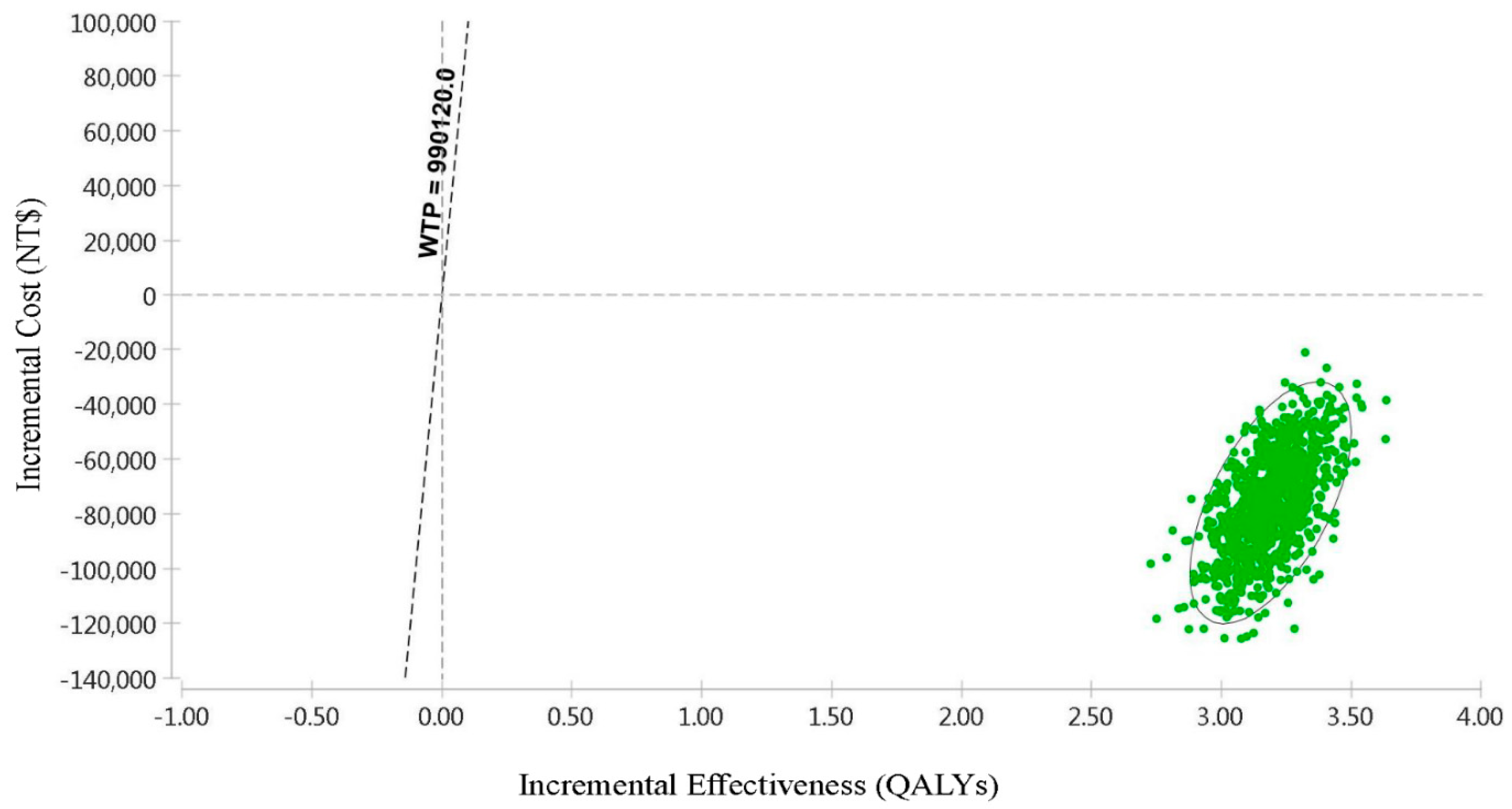
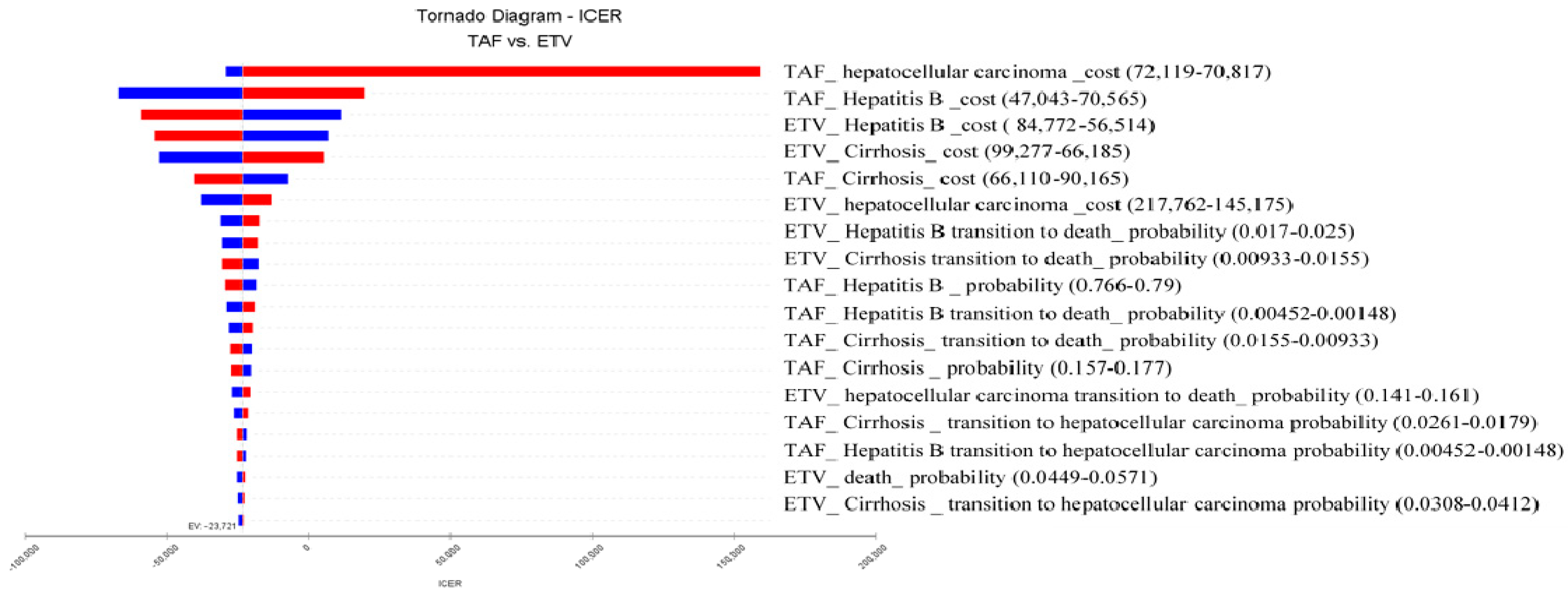
| Treatment | Cost (NT$) | Incremental Cost (NT$) | QALYs | Incremental QALYs | NMB (NT$) | CE | ICER (NT$/QALY) |
|---|---|---|---|---|---|---|---|
| TAF | 1,241,822 | −76,098 | 14.63 | 3.19 | 13,241,786 | 84,893 | −23,878 |
| ETV | 1,317,920 | 11.44 | 10,010,058 | 1,151,926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-H.; Shi, H.-Y.; Tsai, C.-E.; Yang, Y.-C.; Chiu, S.-U.F. Cost–Utility Analysis of Tenofovir Alafenamide and Entecavir in Chronic Hepatitis B Patients: A Markov Decision Model. Cancers 2024, 16, 813. https://doi.org/10.3390/cancers16040813
Lai C-H, Shi H-Y, Tsai C-E, Yang Y-C, Chiu S-UF. Cost–Utility Analysis of Tenofovir Alafenamide and Entecavir in Chronic Hepatitis B Patients: A Markov Decision Model. Cancers. 2024; 16(4):813. https://doi.org/10.3390/cancers16040813
Chicago/Turabian StyleLai, Chun-Huang, Hon-Yi Shi, Cheng-En Tsai, Yuan-Chieh Yang, and Si-Un Frank Chiu. 2024. "Cost–Utility Analysis of Tenofovir Alafenamide and Entecavir in Chronic Hepatitis B Patients: A Markov Decision Model" Cancers 16, no. 4: 813. https://doi.org/10.3390/cancers16040813
APA StyleLai, C.-H., Shi, H.-Y., Tsai, C.-E., Yang, Y.-C., & Chiu, S.-U. F. (2024). Cost–Utility Analysis of Tenofovir Alafenamide and Entecavir in Chronic Hepatitis B Patients: A Markov Decision Model. Cancers, 16(4), 813. https://doi.org/10.3390/cancers16040813







