Infectious Complications in Patients with Myelodysplastic Syndromes: A Report from the Düsseldorf MDS Registry
Abstract
Simple Summary
Abstract
1. Introduction
Infectious Complications in MDS
2. Methods
3. Results
3.1. Type of Infections and Most Common Sites
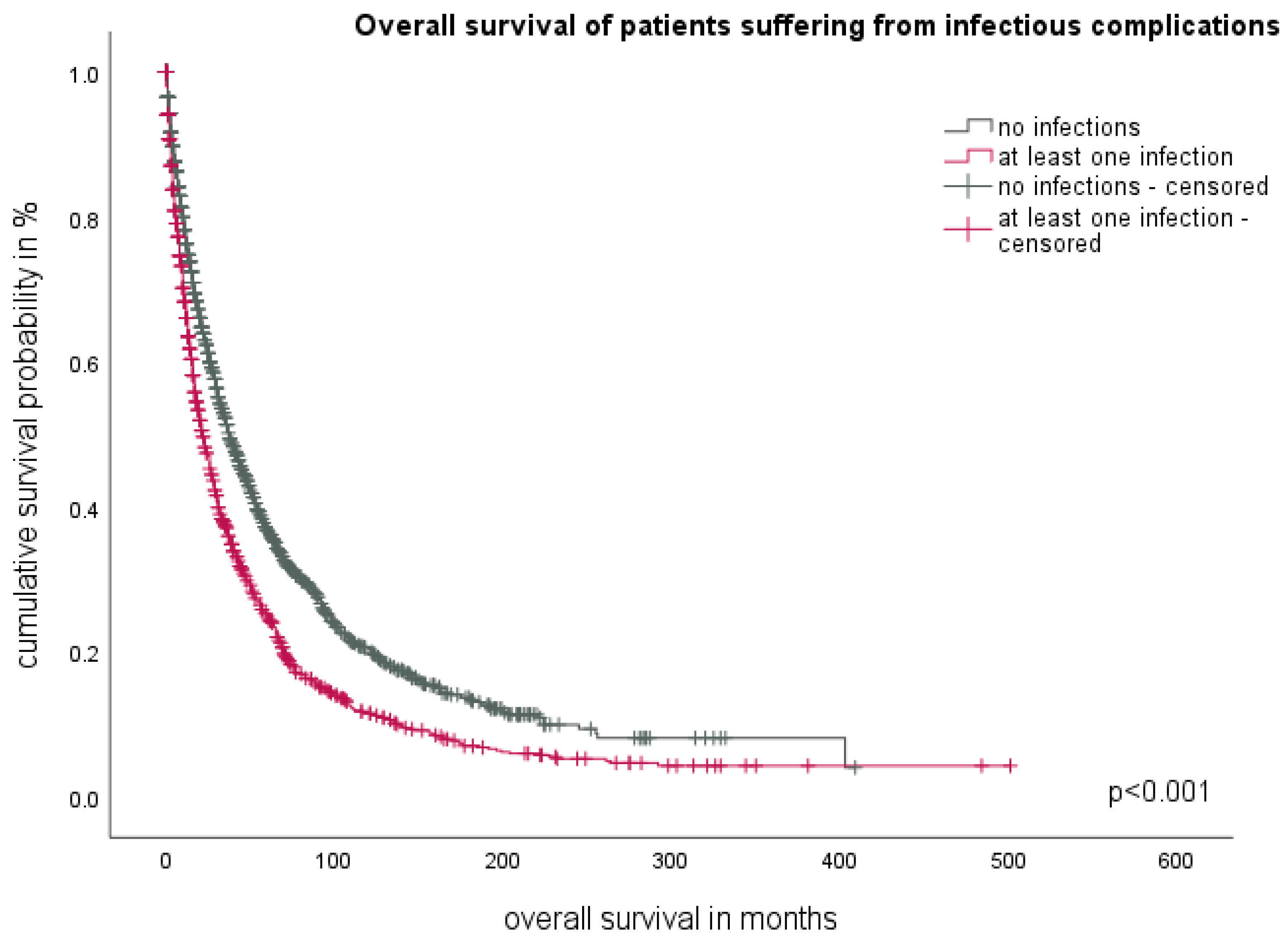
3.2. The Impact of Infections on Overall Survival
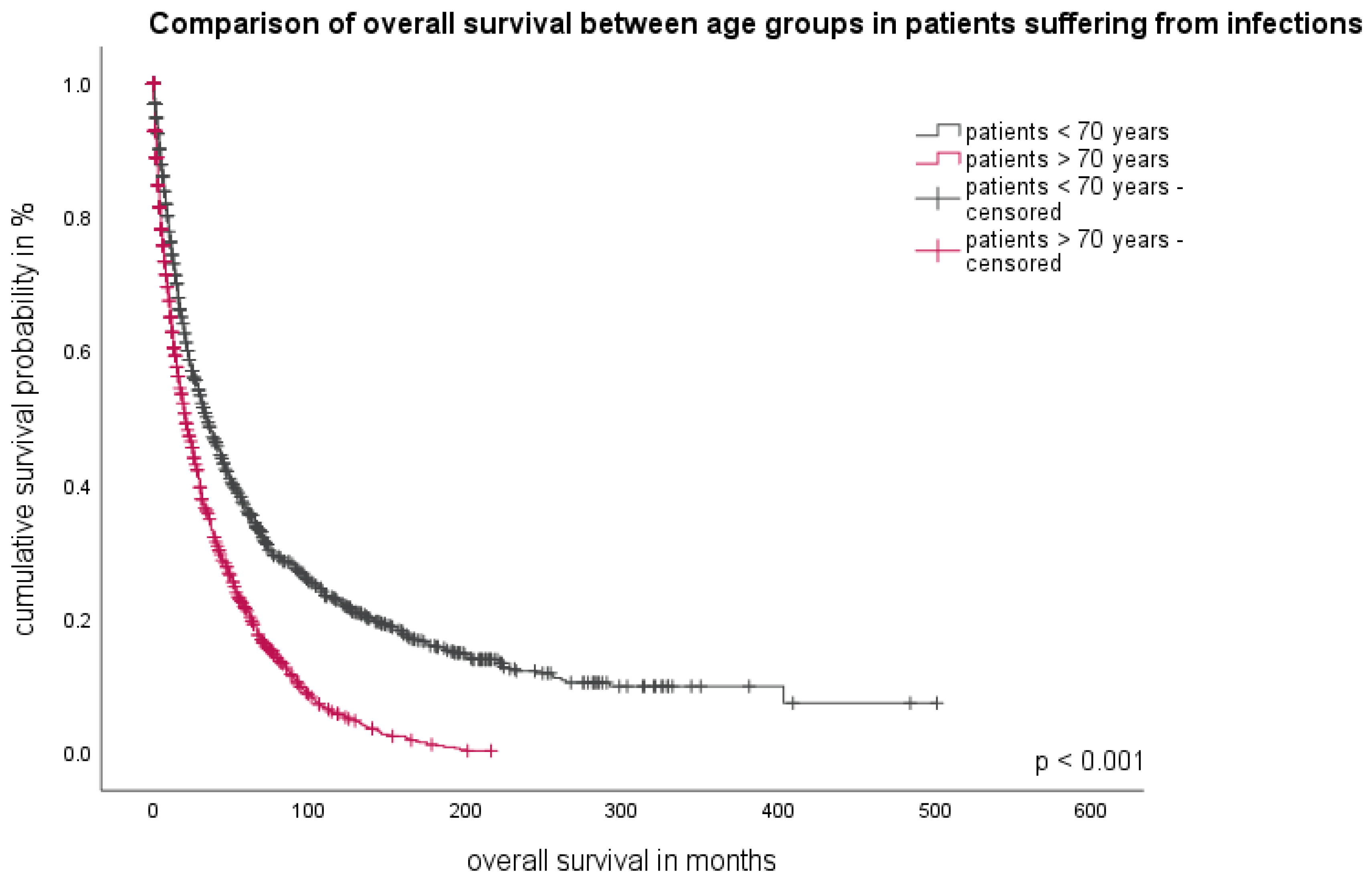
3.3. Patient-Related Parameter
3.4. Disease-Related Parameter
The Role of Peripheral Blood Counts
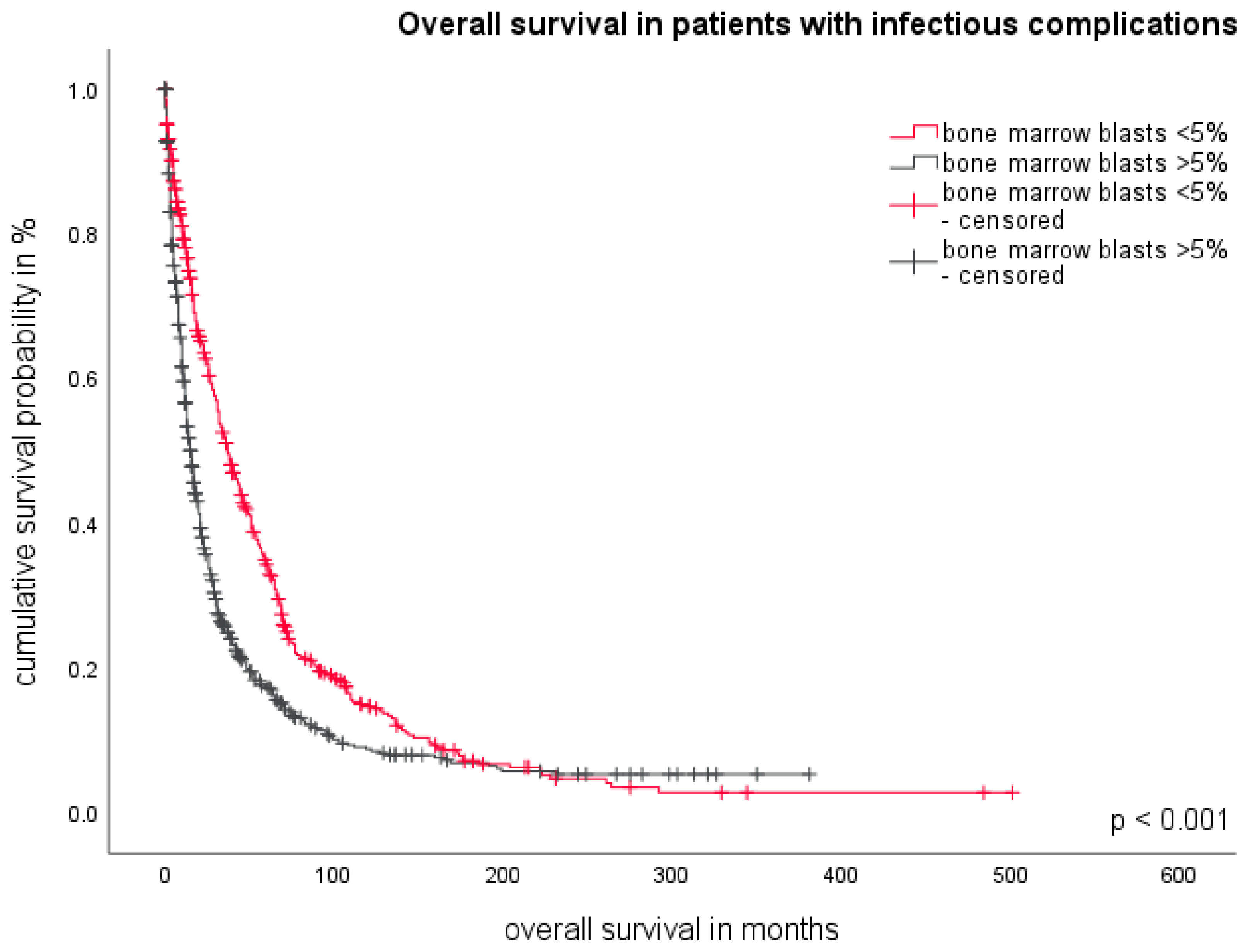
3.5. The Role of Bone Marrow Blasts
3.6. Multivariate Analysis for Disease-Related Factors
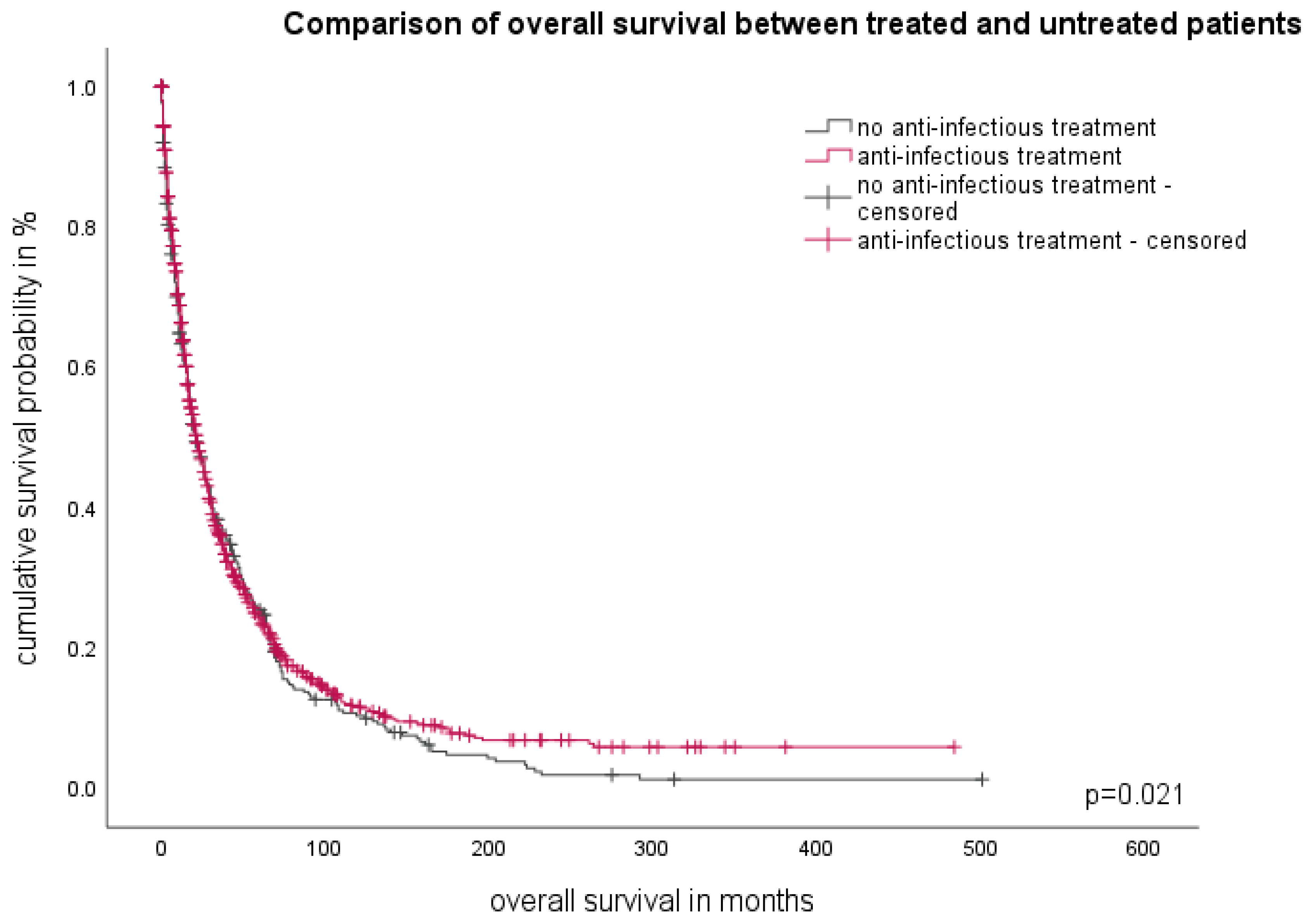
3.7. The Influence of Therapy on Infectious Susceptibility
3.8. Therapy of Infectious Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nachtkamp, K.; Stark, R.; Strupp, C.; Kündgen, A.; Giagounidis, A.; Aul, C.; Hildebrandt, B.; Haas, R.; Gattermann, N.; Germing, U. Causes of death in 2877 patients with myelodysplastic syndromes. Ann. Hematol. 2016, 95, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Mądry, K.; Lis, K.; Biecek, P.; Młynarczyk, M.; Rytel, J.; Górka, M.; Kacprzyk, P.; Dutka, M.; Rodzaj, M.; Bołkun, Ł.; et al. Predictive Model for Infection Risk in Myelodysplastic Syndromes, Acute Myeloid Leukemia, and Chronic Myelomonocytic Leukemia Patients Treated with Azacitidine; Azacitidine Infection Risk Model: The Polish Adult Leukemia Group Study. Clin. Lymphoma Myeloma Leuk. 2019, 19, 264–274.e4. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Shan, J.; Faderl, S.; Cortes, J.; Ravandi, F.; Borthakur, G.; Wierda, W.G.; Pierce, S.; Estey, E.; Liu, J.; et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia 2008, 22, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Mądry, K.; Lis, K.; Fenaux, P.; Bowen, D.; Symeonidis, A.; Mittelman, M.; Stauder, R.; Čermák, J.; Sanz, G.; Hellström-Lindberg, E.; et al. EUMDS Registry Participants. Cause of death and excess mortality in patients with lower-risk myelodysplastic syndromes (MDS): A report from the European MDS registry. Br. J. Haematol. 2023, 200, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and prevalence of myelodysplastic syndromes: Data from the Düsseldorf MDS-registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef]
- Schuster, M.; Moeller, M.; Bornemann, L.; Bessen, C.; Sobczak, C.; Schmitz, S.; Witjes, L.; Kruithoff, K.; Kohn, C.; Just, O.; et al. Surveillance of Myelodysplastic Syndrome via Migration Analyses of Blood Neutrophils: A Potential Prognostic Tool. J. Immunol. 2018, 201, 3546–3557. [Google Scholar] [CrossRef]
- Brings, C.; Fröbel, J.; Cadeddu, P.; Germing, U.; Haas, R.; Gattermann, N. Impaired formation of neutrophil extracellular traps in patients with MDS. Blood Adv. 2022, 6, 129–137. [Google Scholar] [CrossRef]
- Goldberg, S.L.; Chen, E.; Corral, M.; Guo, A.; Mody-Patel, N.; Pecora, A.L.; Laouri, M. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J. Clin. Oncol. 2010, 28, 2847–2852. [Google Scholar] [CrossRef]
- Cunningham, I.; MacCallum, S.J.; Nicholls, M.D.; Byth, K.; Hewson, J.W.; Arnold, B.; Motum, P.I.; Mulligan, S.P.; Crane, G.G. The myelodysplastic syndromes: An analysis of prognostic factors in 226 cases from a single institution. Br. J. Haematol. 1995, 90, 602–606. [Google Scholar] [CrossRef]
- Weber, S.; Parmon, A.; Kurrle, N.; Schnütgen, F.; Serve, H. The Clinical Significance of Iron Overload and Iron Metabolism in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Front. Immunol. 2021, 11, 627662. [Google Scholar] [CrossRef]
- Adès, L.; Itzykson, R.; Fenaux, P. Myelodysplastic syndromes. Lancet 2014, 383, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P. Myelodysplastic Syndromes: Diagnosis and Treatment. Mayo Clin. Proc. 2015, 90, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Malcovati, L.; Hellström-Lindberg, E.; Bowen, D.; Adès, L.; Cermak, J.; Del Cañizo, C.; Della Porta, M.G.; Fenaux, P.; Gattermann, N.; Germing, U.; et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood 2013, 122, 2943–2964. [Google Scholar] [CrossRef] [PubMed]
- Dayyani, F.; Conley, A.P.; Strom, S.S.; Stevenson, W.; Cortes, J.E.; Borthakur, G.; Faderl, S.; O’Brien, S.; Pierce, S.; Kantarjian, H.; et al. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer 2010, 116, 2174–2179. [Google Scholar] [CrossRef]
- Toma, A.; Fenaux, P.; Dreyfus, F.; Cordonnier, C. Infections in myelodysplastic syndromes. Haematologica 2012, 97, 1459–1470. [Google Scholar] [CrossRef]
- Winter, S.; Shoaie, S.; Kordasti, S.; Platzbecker, U. Integrating the “Immunome” in the Stratification of Myelodysplastic Syndromes and Future Clinical Trial Design. J. Clin. Oncol. 2020, 38, 1723–1735. [Google Scholar] [CrossRef]
- Bento, L.C.; Bacal, N.S.; Rocha, F.A.; Severino, P.; Marti, L.C. Bone Marrow Monocytes and Derived Dendritic Cells from Myelodysplastic Patients Have Functional Abnormalities Associated with Defective Response to Bacterial Infection. J. Immunol. 2020, 204, 2098–2109. [Google Scholar] [CrossRef]
- Germing, U.; Lauseker, M.; Hildebrandt, B.; Symeonidis, A.; Cermak, J.; Fenaux, P.; Kelaidi, C.; Pfeilstöcker, M.; Nösslinger, T.; Sekeres, M.; et al. Survival, prognostic factors and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): A multicenter study. Leukemia 2012, 26, 1286–1292. [Google Scholar] [CrossRef]
- Komrokji, R.; Swern, A.S.; Grinblatt, D.; Lyons, R.M.; Tobiasson, M.; Silverman, L.R.; Sayar, H.; Vij, R.; Fliss, A.; Tu, N.; et al. Azacitidine in Lower-Risk Myelodysplastic Syndromes: A Meta-Analysis of Data from Prospective Studies. Oncologist 2018, 23, 159–170. [Google Scholar] [CrossRef]
- Garcia, J.S.; Swords, R.T.; Roboz, G.J.; Jacoby, M.A.; Garcia-Manero, G.; Hong, W.J.; Yang, X.; Zhou, Y.; Platzbecker, U.; Steensma, D.P.; et al. A systematic review of higher-risk myelodysplastic syndromes clinical trials to determine the benchmark of azacitidine and explore alternative endpoints for overall survival. Leuk. Res. 2021, 104, 106555. [Google Scholar] [CrossRef]
- Itzykson, R.; Thépot, S.; Quesnel, B.; Dreyfus, F.; Beyne-Rauzy, O.; Turlure, P.; Vey, N.; Recher, C.; Dartigeas, C.; Legros, L.; et al. Groupe Francophone des Myelodysplasies(GFM). Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 2011, 117, 403–411. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; International Vidaza High-Risk MDS Survival Study Group; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Gore, S.D.; Fenaux, P.; Santini, V.; Bennett, J.M.; Silverman, L.R.; Seymour, J.F.; Hellström-Lindberg, E.; Swern, A.S.; Beach, C.L.; List, A.F. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica 2013, 98, 1067–1072. [Google Scholar] [CrossRef]
- Santini, V.; Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Silverman, L.R.; List, A.; Gore, S.D.; Seymour, J.F.; Backstrom, J.; Beach, C.L. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine*. Eur. J. Haematol. 2010, 85, 130–138. [Google Scholar] [CrossRef]
- Merkel, D.; Filanovsky, K.; Gafter-Gvili, A.; Vidal, L.; Aviv, A.; Gatt, M.E.; Silbershatz, I.; Herishanu, Y.; Arad, A.; Tadmor, T.; et al. Predicting infections in high-risk patients with myelodysplastic syndrome/acute myeloid leukemia treated with azacitidine: A retrospective multicenter study. Am. J. Hematol. 2013, 88, 130–134. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Adès, L. How we treat lower-risk myelodysplastic syndromes. Blood 2013, 121, 4280–4286. [Google Scholar] [CrossRef]
- Malcovati, L.; Porta, M.G.; Pascutto, C.; Invernizzi, R.; Boni, M.; Travaglino, E.; Passamonti, F.; Arcaini, L.; Maffioli, M.; Bernasconi, P.; et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision making. J. Clin. Oncol. 2005, 23, 7594–7603. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients with Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; Leibovici, L. Meta-analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Ann. Intern. Med. 2005, 142 Pt 1, 979–995, Erratum in Ann. Intern. Med. 2006, 144, 704. [Google Scholar] [CrossRef]
| MDS Subtype WHO 2016 | n | % |
|---|---|---|
| MDS-SLD | 54 | 3.4 |
| MDS-MLD | 446 | 28.0 |
| MDS-RS-SLD | 79 | 5.0 |
| MDS-RS-MLD | 9 | 0.6 |
| MDS del(5q) | 24 | 1.5 |
| MDS EB 1 | 212 | 13.3 |
| MDS EB 2 | 305 | 19.1 |
| CMML 1 | 148 | 9.3 |
| CMML 2 | 50 | 3.1 |
| RAEB-T | 220 | 13.8 |
| MDS/MPN U | 14 | 0.9 |
| RARS-t | 22 | 1.4 |
| unclassifiable | 10 | 0.6 |
| total | 1593 | 100 |
| Pathogen | Number of Patients with Positive Microbiological Cultures | % |
|---|---|---|
| Aspergillus | 6 | 0.9 |
| Candida albicans | 5 | 0.7 |
| Clostridium difficile | 6 | 0.9 |
| Escherichia coli | 55 | 8.6 |
| Enterococcus | 8 | 1.2 |
| Enterococcus faecium | 5 | 0.7 |
| Heamophilus influenzae | 4 | 0.6 |
| Klebsiella pneumoniae | 7 | 1.1 |
| Koagulase negative streptococcus | 21 | 3.3 |
| Mycobacterium tuberculosis | 5 | 0.7 |
| Pneumococcus | 7 | 1.1 |
| Proteus mirabilis | 7 | 1.1 |
| Pseudomonas aeruginosa | 17 | 2.6 |
| Staphylocuccus aureus | 22 | 3.4 |
| Staphylocuccus epidermidis | 13 | 2.0 |
| Staphylococcus | 9 | 1.4 |
| Stenotrophomonas maltophilia | 6 | 0.9 |
| Others | 57 | 8.9 |
| No pathogen detected | 378 | 59 |
| total | 638 | 100 |
| Parameter | χ2 | p | Hazard Ratio | 95%CI |
|---|---|---|---|---|
| Hb > 9 g/dL | 26.499 | <0.001 | 0.675 | 0.58–0.78 |
| ANC < 800/µL | 45.609 | <0.001 | 2.042 | 1.65–2.51 |
| WBC < 4000/µL | 0.024 | 0.875 | 1.013 | 0.85–1.19 |
| Pla < 50,000/µL | 0.395 | 0.529 | 1.060 | 0.88–1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, A.; Andresen, J.; Nachtkamp, K.; Kündgen, A.; Schulz, F.; Strupp, C.; Kobbe, G.; MacKenzie, C.; Timm, J.; Dietrich, S.; et al. Infectious Complications in Patients with Myelodysplastic Syndromes: A Report from the Düsseldorf MDS Registry. Cancers 2024, 16, 808. https://doi.org/10.3390/cancers16040808
Kasprzak A, Andresen J, Nachtkamp K, Kündgen A, Schulz F, Strupp C, Kobbe G, MacKenzie C, Timm J, Dietrich S, et al. Infectious Complications in Patients with Myelodysplastic Syndromes: A Report from the Düsseldorf MDS Registry. Cancers. 2024; 16(4):808. https://doi.org/10.3390/cancers16040808
Chicago/Turabian StyleKasprzak, Annika, Julia Andresen, Kathrin Nachtkamp, Andrea Kündgen, Felicitas Schulz, Corinna Strupp, Guido Kobbe, Colin MacKenzie, Jörg Timm, Sascha Dietrich, and et al. 2024. "Infectious Complications in Patients with Myelodysplastic Syndromes: A Report from the Düsseldorf MDS Registry" Cancers 16, no. 4: 808. https://doi.org/10.3390/cancers16040808
APA StyleKasprzak, A., Andresen, J., Nachtkamp, K., Kündgen, A., Schulz, F., Strupp, C., Kobbe, G., MacKenzie, C., Timm, J., Dietrich, S., Gattermann, N., & Germing, U. (2024). Infectious Complications in Patients with Myelodysplastic Syndromes: A Report from the Düsseldorf MDS Registry. Cancers, 16(4), 808. https://doi.org/10.3390/cancers16040808






