Variant Allele Frequency Analysis of Circulating Tumor DNA as a Promising Tool in Assessing the Effectiveness of Treatment in Non-Small Cell Lung Carcinoma Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. cfDNA
2.1. ctDNA
2.2. cfDNA and ctDNA Differentiation
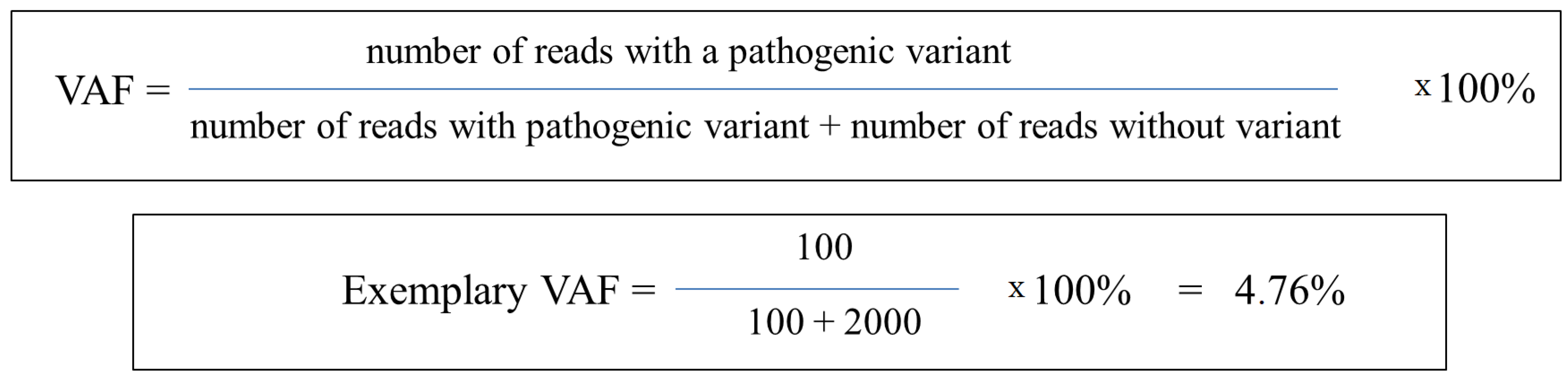
3. Variant Allele Frequency (VAF)
Usage of ctDNA VAF Analysis
4. Assessment of Therapy Effectiveness in NSCLC Patients Based on VAF
5. Technical Aspects of VAF Evaluation in NSCLC Patients
5.1. Biological Factors Affecting VAF Measurement
5.2. Appropriate Methodology Selection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACT | Adjuvant chemotherapy |
| ALK-TKI | ALK–tyrosine kinase inhibitor |
| BAL | Bronchoalveolar lavage |
| BSC | Best supportive care |
| BW | Bronchial washing |
| cfDNA | Circulating free DNA |
| ctDNA | Circulating tumor DNA |
| CNA | Copy number alteration |
| CNV | Copy number variation |
| CSF | Cerebrospinal fluid |
| EGFR-TKI | EGFR–tyrosine kinase inhibitor |
| FFPE | Formalin-fixed paraffin-embedded |
| ICI | Immune checkpoint inhibitor |
| LB | Liquid biopsy |
| MAF | Mutant allele frequency |
| MRD | Minimal residual disease |
| MSAF | Maximum somatic allele frequency |
| NET | Neutrophil extracellular trap |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| PD | Progressive disease |
| PFS | Progression-free survival |
| PR | Partial response |
| SD | Stable disease |
| SNV | Single nucleotide variant |
| TCGA | The Cancer Genome Atlas |
| TFE | Tumor Fraction Estimator |
| VAF | Variant allele frequency |
| WES | Whole exome sequencing |
| WGS | Whole genome sequencing |
References
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Deshpand, R.; Chandra, M.; Rauthan, A. Evolving Trends in Lung Cancer: Epidemiology, Diagnosis, and Management. Indian J. Cancer 2022, 59, 90. [Google Scholar] [CrossRef]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-Small Cell Lung Cancer (NSCLC): A Review of Risk Factors, Diagnosis, and Treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.E.-S.; Alhanafy, A.M.; Habib, M.S.E.-D.; Hagag, M.; Ibrahem, R.A.L. Serum Circulating Cell Free DNA as Potential Diagnostic and Prognostic Biomarker in Non Small Cell Lung Cancer. Biochem. Biophys. Rep. 2018, 15, 45–51. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic Recurrence Risk and Adjuvant Chemotherapy Benefit Prediction by ctDNA in Resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef]
- Filis, P.; Kyrochristos, I.; Korakaki, E.; Baltagiannis, E.G.; Thanos, D.; Roukos, D.H. Longitudinal ctDNA Profiling in Precision Oncology and Immunο-Oncology. Drug Discov. Today 2023, 28, 103540. [Google Scholar] [CrossRef]
- Kemper, M.; Krekeler, C.; Menck, K.; Lenz, G.; Evers, G.; Schulze, A.B.; Bleckmann, A. Liquid Biopsies in Lung Cancer. Cancers 2023, 15, 1430. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, H. Next-Generation Sequencing in Liquid Biopsy: Cancer Screening and Early Detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Garcia, J.; Kamps-Hughes, N.; Geiguer, F.; Couraud, S.; Sarver, B.; Payen, L.; Ionescu-Zanetti, C. Sensitivity, Specificity, and Accuracy of a Liquid Biopsy Approach Utilizing Molecular Amplification Pools. Sci. Rep. 2021, 11, 10761. [Google Scholar] [CrossRef] [PubMed]
- Pairawan, S.; Hess, K.; Janku, F.; Sanchez, N.; Shaw, K.; Eng, C.; Damodaran, S.; Javle, M.; Kaseb, A.; Hong, D.; et al. Cell-Free Circulating Tumor DNA Variant Allele Frequency Associates with Survival in Metastatic Cancer. Clin. Cancer Res. 2020, 26, 1924–1931. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Hughes, J.; van den Hout, A.; Burns, C.; Woodhouse, R.; Dennis, L.; Hegde, P.; Oxnard, G.R.; Vietz, C. Driver Mutation Variant Allele Frequency in Circulating Tumor DNA and Association with Clinical Outcome in Patients with Non–Small Cell Lung Cancer and EGFR- and KRAS-Mutated Tumors. J. Mol. Diagn. 2022, 24, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Kuziora, M.; Brohawn, P.Z.; Higgs, B.W.; Gupta, A.; Dennis, P.A.; Ranade, K. Early Reduction in ctDNA Predicts Survival in Patients with Lung and Bladder Cancer Treated with Durvalumab. Clin. Cancer Res. 2018, 24, 6212–6222. [Google Scholar] [CrossRef]
- Snyder, M.W.; Kircher, M.; Hill, A.J.; Daza, R.M.; Shendure, J. Cell-Free DNA Comprises an In Vivo Nucleosome Footprint That Informs Its Tissues-Of-Origin. Cell 2016, 164, 57–68. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.R.; Yan, Y.H.; Zhang, J.X.; Chu, T.; Kwong, L.N.; Patel, A.A.; Zhang, D.Y. Limitations and Opportunities of Technologies for the Analysis of Cell-Free DNA in Cancer Diagnostics. Nat. Biomed. Eng. 2022, 6, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar] [CrossRef]
- Papadopoulos, N. Pathophysiology of ctDNA Release into the Circulation and Its Characteristics: What Is Important for Clinical Applications. In Tumor Liquid Biopsies; Schaffner, F., Merlin, J.-L., Von Bubnoff, N., Eds.; Recent Results in Cancer Research; Springer International Publishing: Cham, Switzerland, 2020; Volume 215, pp. 163–180. ISBN 978-3-030-26438-3. [Google Scholar]
- Shen, H.; Jin, Y.; Zhao, H.; Wu, M.; Zhang, K.; Wei, Z.; Wang, X.; Wang, Z.; Li, Y.; Yang, F.; et al. Potential Clinical Utility of Liquid Biopsy in Early-Stage Non-Small Cell Lung Cancer. BMC Med. 2022, 20, 480. [Google Scholar] [CrossRef]
- Aucamp, J.; Bronkhorst, A.J.; Badenhorst, C.P.S.; Pretorius, P.J. The Diverse Origins of Circulating Cell-free DNA in the Human Body: A Critical Re-evaluation of the Literature. Biol. Rev. 2018, 93, 1649–1683. [Google Scholar] [CrossRef]
- Bardelli, A.; Pantel, K. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell 2017, 31, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Filipska, M.; Rosell, R. Mutated Circulating Tumor DNA as a Liquid Biopsy in Lung Cancer Detection and Treatment. Mol. Oncol. 2021, 15, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-C.; Nguyen, T.H.; Phan, T.H.; Tran, T.-H.T.; Pham, T.T.T.; Ho, T.D.; Nguyen, H.H.T.; Duong, M.-L.; Nguyen, C.M.; Nguyen, Q.-T.B.; et al. Fragment Length Profiles of Cancer Mutations Enhance Detection of Circulating Tumor DNA in Patients with Early-Stage Hepatocellular Carcinoma. BMC Cancer 2023, 23, 233. [Google Scholar] [CrossRef] [PubMed]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Uemura, M.; Fujita, M.; Maejima, K.; Koh, Y.; Matsushita, M.; Nakano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; et al. Clinical Significance of the Mutational Landscape and Fragmentation of Circulating Tumor DNA in Renal Cell Carcinoma. Cancer Sci. 2019, 110, 617–628. [Google Scholar] [CrossRef]
- Jiang, P.; Lo, Y.M.D. The Long and Short of Circulating Cell-Free DNA and the Ins and Outs of Molecular Diagnostics. Trends Genet. 2016, 32, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Pisapia, P.; Pepe, F.; Russo, G.; Buono, M.; Russo, A.; Gomez, J.; Khorshid, O.; Mack, P.C.; Rolfo, C.; et al. The Evolving Role of Liquid Biopsy in Lung Cancer. Lung Cancer 2022, 172, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra2. [Google Scholar] [CrossRef]
- Sánchez-Herrero, E.; Serna-Blasco, R.; Robado de Lope, L.; González-Rumayor, V.; Romero, A.; Provencio, M. Circulating Tumor DNA as a Cancer Biomarker: An Overview of Biological Features and Factors That May Impact on ctDNA Analysis. Front. Oncol. 2022, 12, 943253. [Google Scholar] [CrossRef]
- Gale, D.; Heider, K.; Ruiz-Valdepenas, A.; Hackinger, S.; Perry, M.; Marsico, G.; Rundell, V.; Wulff, J.; Sharma, G.; Knock, H.; et al. Residual ctDNA after Treatment Predicts Early Relapse in Patients with Early-Stage Non-Small Cell Lung Cancer. Ann. Oncol. 2022, 33, 500–510. [Google Scholar] [CrossRef]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid Biopsies: Potential and Challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef]
- FDA. FDA Approves Liquid Biopsy Next-Generation Sequencing Companion Diagnostic Test; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Hu, Y.; Ulrich, B.C.; Supplee, J.; Kuang, Y.; Lizotte, P.H.; Feeney, N.B.; Guibert, N.M.; Awad, M.M.; Wong, K.-K.; Jänne, P.A.; et al. False-Positive Plasma Genotyping Due to Clonal Hematopoiesis. Clin. Cancer Res. 2018, 24, 4437–4443. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-Intensity Sequencing Reveals the Sources of Plasma Circulating Cell-Free DNA Variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Arisi, M.F.; Dotan, E.; Fernandez, S.V. Circulating Tumor DNA in Precision Oncology and Its Applications in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4441. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive Human Cell-Type Methylation Atlas Reveals Origins of Circulating Cell-Free DNA in Health and Disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wei, W.; Ye, Z.; Zheng, J.; Xu, R. Liquid Biopsy of Methylation Biomarkers in Cell-Free DNA. Trends Mol. Med. 2021, 27, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Diep, D.; Plongthongkum, N.; Fung, H.-L.; Zhang, K.; Zhang, K. Identification of Methylation Haplotype Blocks Aids in Deconvolution of Heterogeneous Tissue Samples and Tumor Tissue-of-Origin Mapping from Plasma DNA. Nat. Genet. 2017, 49, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhao, Q.; Wei, W.; Zheng, L.; Yi, S.; Li, G.; Wang, W.; Sheng, H.; Pu, H.; Mo, H.; et al. Circulating Tumor DNA Methylation Profiles Enable Early Diagnosis, Prognosis Prediction, and Screening for Colorectal Cancer. Sci. Transl. Med. 2020, 12, eaax7533. [Google Scholar] [CrossRef]
- Lianidou, E. Detection and Relevance of Epigenetic Markers on ctDNA: Recent Advances and Future Outlook. Mol. Oncol. 2021, 15, 1683–1700. [Google Scholar] [CrossRef]
- Jiang, P.; Chan, C.W.M.; Chan, K.C.A.; Cheng, S.H.; Wong, J.; Wong, V.W.-S.; Wong, G.L.H.; Chan, S.L.; Mok, T.S.K.; Chan, H.L.Y.; et al. Lengthening and Shortening of Plasma DNA in Hepatocellular Carcinoma Patients. Proc. Natl. Acad. Sci. USA 2015, 112, E1317–E1325. [Google Scholar] [CrossRef]
- Sivapalan, L.; Iams, W.T.; Belcaid, Z.; Scott, S.C.; Niknafs, N.; Balan, A.; White, J.R.; Kopparapu, P.; Cann, C.; Landon, B.V.; et al. Dynamics of Sequence and Structural Cell-Free DNA Landscapes in Small-Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.P.; Xiao, J.; Pavlick, D.C.; Guo, C.; Yang, L.; Jin, D.X.; Fendler, B.; Severson, E.; Killian, J.K.; Hiemenz, M.; et al. Circulating Cell-Free DNA Yield and Circulating-Tumor DNA Quantity from Liquid Biopsies of 12,139 Cancer Patients. Clin. Chem. 2021, 67, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, Y.; Zhuo, M.; Chen, X.; Xie, Y.; Duan, J.; Bai, H.; Hao, S.; Yu, Z.; Yi, Y.; et al. Maximum Somatic Allele Frequency-Adjusted Blood-Based Tumor Mutational Burden Predicts the Efficacy of Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer. Cancers 2022, 14, 5649. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Welsh, A.; Chung, J.H.; Pavlick, D.; Bernicker, E.H.; Creelan, B.C.; Forcier, B.; Ross, J.S.; Stephens, P.J.; Ali, S.M.; et al. Hybrid Capture-Based Genomic Profiling of Circulating Tumor DNA from Patients with Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Paul, S.M.; Yun, C.; Hu, S.; Shen, V.; Velcheti, V.; Mok, T.S.; Gandara, D.R.; Chae, Y.K.; Schleifman, E.; et al. Abstract CT194: Exploratory Subgroup Analysis of Atezolizumab (Atezo) Clinical Characteristics in Patients (Pts) with Low Circulating Tumor DNA (ctDNA) in B-F1RST—A Phase II Trial Evaluating Blood-Based Tumor Mutational Burden (bTMB) in NSCLC. Cancer Res. 2019, 79, CT194. [Google Scholar] [CrossRef]
- Chen, Y.; Seeruttun, S.R.; Wu, X.; Wang, Z. Maximum Somatic Allele Frequency in Combination With Blood-Based Tumor Mutational Burden to Predict the Efficacy of Atezolizumab in Advanced Non-Small Cell Lung Cancer: A Pooled Analysis of the Randomized POPLAR and OAK Studies. Front. Oncol. 2019, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-Based Tumor Mutational Burden as a Predictor of Clinical Benefit in Non-Small-Cell Lung Cancer Patients Treated with Atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Brash, D.E. Next-Generation Sequencing Methodologies to Detect Low-Frequency Mutations: “Catch Me If You Can”. Mutat. Res./Rev. Mutat. Res. 2023, 792, 108471. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Boscolo Bielo, L.; Trapani, D.; Repetto, M.; Crimini, E.; Valenza, C.; Belli, C.; Criscitiello, C.; Marra, A.; Subbiah, V.; Curigliano, G. Variant Allele Frequency: A Decision-Making Tool in Precision Oncology? Trends Cancer 2023, 9, 1058–1068. [Google Scholar] [CrossRef]
- Manca, P.; Corallo, S.; Lonardi, S.; Fucà, G.; Busico, A.; Leone, A.G.; Corti, F.; Antoniotti, C.; Procaccio, L.; Smiroldo, V.; et al. Variant Allele Frequency in Baseline Circulating Tumour DNA to Measure Tumour Burden and to Stratify Outcomes in Patients with RAS Wild-Type Metastatic Colorectal Cancer: A Translational Objective of the Valentino Study. Br. J. Cancer 2022, 126, 449–455. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Facchinetti, F.; DiToro, D.F.; Baiev, I.; Majeed, U.; Reyes, S.; Chen, C.; Zhang, K.; Sharman, R.; Uson Junior, P.L.S.; et al. The Clinical Landscape of Cell-Free DNA Alterations in 1671 Patients with Advanced Biliary Tract Cancer. Ann. Oncol. 2022, 33, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, C.A.; Gale, D.; Piskorz, A.M.; Biggs, H.; Hodgkin, C.; Addley, H.; Freeman, S.; Moyle, P.; Sala, E.; Sayal, K.; et al. Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study. PLoS Med. 2016, 13, e1002198. [Google Scholar] [CrossRef]
- Kujala, J.; Hartikainen, J.M.; Tengström, M.; Sironen, R.; Kosma, V.-M.; Mannermaa, A. High Mutation Burden of Circulating Cell-Free DNA in Early-Stage Breast Cancer Patients Is Associated with a Poor Relapse-Free Survival. Cancer Med. 2020, 9, 5922–5931. [Google Scholar] [CrossRef]
- Dentro, S.C.; Leshchiner, I.; Haase, K.; Tarabichi, M.; Wintersinger, J.; Deshwar, A.G.; Yu, K.; Rubanova, Y.; Macintyre, G.; Demeulemeester, J.; et al. Characterizing Genetic Intra-Tumor Heterogeneity across 2658 Human Cancer Genomes. Cell 2021, 184, 2239–2254.e39. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Favero, F.; de Bruin, E.C.; Birkbak, N.J.; Szallasi, Z.; Swanton, C. Clonal Status of Actionable Driver Events and the Timing of Mutational Processes in Cancer Evolution. Sci. Transl. Med. 2015, 7, 283ra54. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, L.; Whalen, J.; D’Aco, K.; Wu, J.; Gustafson, C.B.; Solovieff, N.; Su, F.; Leary, R.J.; Campbell, C.D.; Balbin, O.A. Clonal Hematopoiesis Detection in Patients with Cancer Using Cell-Free DNA Sequencing. Sci. Transl. Med. 2023, 15, eabm8729. [Google Scholar] [CrossRef]
- Shin, H.-T.; Choi, Y.-L.; Yun, J.W.; Kim, N.K.D.; Kim, S.-Y.; Jeon, H.J.; Nam, J.-Y.; Lee, C.; Ryu, D.; Kim, S.C.; et al. Prevalence and Detection of Low-Allele-Fraction Variants in Clinical Cancer Samples. Nat. Commun. 2017, 8, 1377. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Raphael, B.J.; Dobson, J.R.; Oesper, L.; Vandin, F. Identifying Driver Mutations in Sequenced Cancer Genomes: Computational Approaches to Enable Precision Medicine. Genome Med. 2014, 6, 5. [Google Scholar] [CrossRef]
- Wodarz, D.; Newell, A.C.; Komarova, N.L. Passenger Mutations Can Accelerate Tumour Suppressor Gene Inactivation in Cancer Evolution. J. R. Soc. Interface 2018, 15, 20170967. [Google Scholar] [CrossRef]
- Kumar, S.; Warrell, J.; Li, S.; McGillivray, P.D.; Meyerson, W.; Salichos, L.; Harmanci, A.; Martinez-Fundichely, A.; Chan, C.W.Y.; Nielsen, M.M.; et al. Passenger Mutations in 2500 Cancer Genomes: Overall Molecular Functional Impact and Consequences. Cell 2020, 180, 915–927.e16. [Google Scholar] [CrossRef]
- Salvadores, M.; Mas-Ponte, D.; Supek, F. Passenger Mutations Accurately Classify Human Tumors. PLoS Comput. Biol. 2019, 15, e1006953. [Google Scholar] [CrossRef]
- Pavel, A.B.; Korolev, K.S. Genetic Load Makes Cancer Cells More Sensitive to Common Drugs: Evidence from Cancer Cell Line Encyclopedia. Sci. Rep. 2017, 7, 1938. [Google Scholar] [CrossRef] [PubMed]
- McFarland, C.D.; Yaglom, J.A.; Wojtkowiak, J.W.; Scott, J.G.; Morse, D.L.; Sherman, M.Y.; Mirny, L.A. The Damaging Effect of Passenger Mutations on Cancer Progression. Cancer Res. 2017, 77, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Li, M.; He, Y.; Kinzler, K.W.; Vogelstein, B.; Dressman, D. BEAMing: Single-Molecule PCR on Microparticles in Water-in-Oil Emulsions. Nat. Methods 2006, 3, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Denis, J.A.; Guillerm, E.; Coulet, F.; Larsen, A.K.; Lacorte, J.-M. The Role of BEAMing and Digital PCR for Multiplexed Analysis in Molecular Oncology in the Era of Next-Generation Sequencing. Mol. Diagn. Ther. 2017, 21, 587–600. [Google Scholar] [CrossRef]
- Warburton, L.; Calapre, L.; Pereira, M.R.; Reid, A.; Robinson, C.; Amanuel, B.; Ziman, M.; Millward, M.; Gray, E. Circulating Tumour DNA in Advanced Melanoma Patients Ceasing PD1 Inhibition in the Absence of Disease Progression. Cancers 2020, 12, 3486. [Google Scholar] [CrossRef]
- Casanova-Salas, I.; Athie, A.; Boutros, P.C.; Del Re, M.; Miyamoto, D.T.; Pienta, K.J.; Posadas, E.M.; Sowalsky, A.G.; Stenzl, A.; Wyatt, A.W.; et al. Quantitative and Qualitative Analysis of Blood-Based Liquid Biopsies to Inform Clinical Decision-Making in Prostate Cancer. Eur. Urol. 2021, 79, 762–771. [Google Scholar] [CrossRef]
- Shields, M.D.; Chen, K.; Dutcher, G.; Patel, I.; Pellini, B. Making the Rounds: Exploring the Role of Circulating Tumor DNA (ctDNA) in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 9006. [Google Scholar] [CrossRef]
- Plasma and Serum Preparation. Available online: https://www.thermofisher.com/uk/en/home/references/protocols/cell-and-tissue-analysis/elisa-protocol/elisa-sample-preparation-protocols/plasma-and-serum-preparation.html (accessed on 5 February 2024).
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef]
- Parpart-Li, S.; Bartlett, B.; Popoli, M.; Adleff, V.; Tucker, L.; Steinberg, R.; Georgiadis, A.; Phallen, J.; Brahmer, J.; Azad, N.; et al. The Effect of Preservative and Temperature on the Analysis of Circulating Tumor DNA. Clin. Cancer Res. 2017, 23, 2471–2477. [Google Scholar] [CrossRef]
- Pittella-Silva, F.; Chin, Y.M.; Chan, H.T.; Nagayama, S.; Miyauchi, E.; Low, S.-K.; Nakamura, Y. Plasma or Serum: Which Is Preferable for Mutation Detection in Liquid Biopsy? Clin. Chem. 2020, 66, 946–957. [Google Scholar] [CrossRef]
- Pös, Z.; Pös, O.; Styk, J.; Mocova, A.; Strieskova, L.; Budis, J.; Kadasi, L.; Radvanszky, J.; Szemes, T. Technical and Methodological Aspects of Cell-Free Nucleic Acids Analyzes. Int. J. Mol. Sci. 2020, 21, 8634. [Google Scholar] [CrossRef]
- Thress, K.S.; Brant, R.; Carr, T.H.; Dearden, S.; Jenkins, S.; Brown, H.; Hammett, T.; Cantarini, M.; Barrett, J.C. EGFR Mutation Detection in ctDNA from NSCLC Patient Plasma: A Cross-Platform Comparison of Leading Technologies to Support the Clinical Development of AZD9291. Lung Cancer 2015, 90, 509–515. [Google Scholar] [CrossRef]
- Lebofsky, R.; Decraene, C.; Bernard, V.; Kamal, M.; Blin, A.; Leroy, Q.; Rio Frio, T.; Pierron, G.; Callens, C.; Bieche, I.; et al. Circulating Tumor DNA as a Non-invasive Substitute to Metastasis Biopsy for Tumor Genotyping and Personalized Medicine in a Prospective Trial across All Tumor Types. Mol. Oncol. 2015, 9, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Scilla, K.A.; Rolfo, C. The Role of Circulating Tumor DNA in Lung Cancer: Mutational Analysis, Diagnosis, and Surveillance Now and into the Future. Curr. Treat. Options Oncol. 2019, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.; Lebow, E.S.; Yeh, R.; Das, J.P.; Namakydoust, A.; Paik, P.K.; Chaft, J.E.; Jayakumaran, G.; Rose Brannon, A.; Benayed, R.; et al. Overall Survival with Circulating Tumor DNA-Guided Therapy in Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Guan, Y.; Xu, Y.; Han, Y.; Wu, C.; Bao, C.; Zhou, B.; Wang, H.; Zhang, M.; Liu, W.; et al. The Diagnostic and Prognostic Usage of Circulating Tumor DNA in Operable Hepatocellular Carcinoma. Am. J. Transl. Res. 2019, 11, 6462–6474. [Google Scholar] [PubMed]
- Van Velzen, M.J.M.; Creemers, A.; Van Den Ende, T.; Schokker, S.; Krausz, S.; Reinten, R.J.; Dijk, F.; Van Noesel, C.J.M.; Halfwerk, H.; Meijer, S.L.; et al. Circulating Tumor DNA Predicts Outcome in Metastatic Gastroesophageal Cancer. Gastric Cancer 2022, 25, 906–915. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; Van Der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Wang, J.; Zhao, X.; Zhang, T.; Yang, Y.; Pang, J.; Ou, Q.; Wu, L.; Xu, X.; et al. Unrevealing the Therapeutic Benefits of Radiotherapy and Consolidation Immunotherapy Using ctDNA-Defined Tumor Clonality in Unresectable Locally Advanced Non-Small Cell Lung Cancer. Cancer Lett. 2023, 582, 216569. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Narayan, A.; Kole, A.J.; Decker, R.H.; Teysir, J.; Carriero, N.J.; Lee, A.; Nemati, R.; Nath, S.K.; Mane, S.M.; et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin. Cancer Res. 2018, 24, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Yang, H.; Liu, X.-Y.; Chen, Z.-G.; Wang, Y.; Fong, W.P.; Hu, M.-T.; Zheng, Y.-C.; Zheng, Y.; Li, B.-K.; et al. Dynamic Monitoring of Circulating Tumor DNA to Predict Prognosis and Efficacy of Adjuvant Chemotherapy after Resection of Colorectal Liver Metastases. Theranostics 2021, 11, 7018–7028. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C. Best Practices for Variant Calling in Clinical Sequencing. Genome Med. 2020, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Jiang, L.; Dong, X.; Gu, K.; Pan, Y.; Shi, Q.; Zhang, G.; Wang, H.; Zhang, X.; Yang, N.; et al. Utilization of Circulating Cell-Free DNA Profiling to Guide First-Line Chemotherapy in Advanced Lung Squamous Cell Carcinoma. Theranostics 2021, 11, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.M.; Nishimura, K.K.; Zariffa, N.; Thompson, J.C.; Hoering, A.; Cilento, V.; Rosenthal, A.; Anagnostou, V.; Baden, J.; Beaver, J.A.; et al. Changes in Circulating Tumor DNA Reflect Clinical Benefit Across Multiple Studies of Patients With Non–Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. JCO Precis. Oncol. 2022, 6, e2100372. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Carpenter, E.L.; Silva, B.A.; Rosenstein, J.; Chien, A.L.; Quinn, K.; Espenschied, C.R.; Mak, A.; Kiedrowski, L.A.; Lefterova, M.; et al. Serial Monitoring of Circulating Tumor DNA by Next-Generation Gene Sequencing as a Biomarker of Response and Survival in Patients With Advanced NSCLC Receiving Pembrolizumab-Based Therapy. JCO Precis. Oncol. 2021, 5, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Zulato, E.; Del Bianco, P.; Nardo, G.; Attili, I.; Pavan, A.; Boscolo Bragadin, A.; Marra, L.; Pasello, G.; Fassan, M.; Calabrese, F.; et al. Longitudinal Liquid Biopsy Anticipates Hyperprogression and Early Death in Advanced Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. Br. J. Cancer 2022, 127, 2034–2042. [Google Scholar] [CrossRef]
- Bewicke-Copley, F.; Arjun Kumar, E.; Palladino, G.; Korfi, K.; Wang, J. Applications and Analysis of Targeted Genomic Sequencing in Cancer Studies. Comput. Struct. Biotechnol. J. 2019, 17, 1348–1359. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Liu, G.; Chen, S.; Xu, M.; Song, L.; Wang, Y. ctDNA Concentration, MIKI67 Mutations and Hyper-Progressive Disease Related Gene Mutations Are Prognostic Markers for Camrelizumab and Apatinib Combined Multiline Treatment in Advanced NSCLC. Front. Oncol. 2020, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Xu, X.; Jiang, T.; Cheng, Y.; Chen, G.; Pan, Y.; Fang, Y.; Wang, Q.; Huang, Y.; et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Srivastava, M.; Reck, M.; Wakelee, H.; Altorki, N.K.; Vallieres, E.; Liersch, R.; Harada, M.; Tanaka, H.; Hamm, J.T.; et al. 1O IMpower010: ctDNA Status in Patients (Pts) with Resected NSCLC Who Received Adjuvant Chemotherapy (Chemo) Followed by Atezolizumab (Atezo) or Best Supportive Care (BSC). Immuno-Oncol. Technol. 2022, 16, 100106. [Google Scholar] [CrossRef]
- Soo, R.A.; Martini, J.-F.; Van Der Wekken, A.J.; Teraoka, S.; Ferrara, R.; Shaw, A.T.; Shepard, D.; Calella, A.M.; Polli, A.; Toffalorio, F.; et al. Early Circulating Tumor DNA Dynamics and Efficacy of Lorlatinib in Patients With Treatment-Naive, Advanced, ALK-Positive NSCLC. J. Thorac. Oncol. 2023, 18, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.; Cui, W.; Popat, S. Crizotinib-Resistant ROS1 G2101A Mutation Associated With Sensitivity to Lorlatinib in ROS1-Rearranged NSCLC: Case Report. JTO Clin. Res. Rep. 2022, 3, 100376. [Google Scholar] [CrossRef]
- Beagan, J.J.; Bach, S.; Van Boerdonk, R.A.; Van Dijk, E.; Thunnissen, E.; Van Den Broek, D.; Weiss, J.; Kazemier, G.; Pegtel, D.M.; Bahce, I.; et al. Circulating Tumor DNA Analysis of EGFR-Mutant Non-Small Cell Lung Cancer Patients Receiving Osimertinib Following Previous Tyrosine Kinase Inhibitor Treatment. Lung Cancer 2020, 145, 173–180. [Google Scholar] [CrossRef]
- Vaclova, T.; Grazini, U.; Ward, L.; O’Neill, D.; Markovets, A.; Huang, X.; Chmielecki, J.; Hartmaier, R.; Thress, K.S.; Smith, P.D.; et al. Clinical Impact of Subclonal EGFR T790M Mutations in Advanced-Stage EGFR-Mutant Non-Small-Cell Lung Cancers. Nat. Commun. 2021, 12, 1780. [Google Scholar] [CrossRef]
- Ai, X.; Cui, J.; Zhang, J.; Chen, R.; Lin, W.; Xie, C.; Liu, A.; Zhang, J.; Yang, W.; Hu, X.; et al. Clonal Architecture of EGFR Mutation Predicts the Efficacy of EGFR-Tyrosine Kinase Inhibitors in Advanced NSCLC: A Prospective Multicenter Study (NCT03059641). Clin. Cancer Res. 2021, 27, 704–712. [Google Scholar] [CrossRef]
- Lam, V.K.; Zhang, J.; Wu, C.C.; Tran, H.T.; Li, L.; Diao, L.; Wang, J.; Rinsurongkawong, W.; Raymond, V.M.; Lanman, R.B.; et al. Genotype-Specific Differences in Circulating Tumor DNA Levels in Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 601–609. [Google Scholar] [CrossRef]
- Friedlaender, A.; Tsantoulis, P.; Chevallier, M.; De Vito, C.; Addeo, A. The Impact of Variant Allele Frequency in EGFR Mutated NSCLC Patients on Targeted Therapy. Front. Oncol. 2021, 11, 644472. [Google Scholar] [CrossRef]
- Angeles, A.K.; Christopoulos, P.; Yuan, Z.; Bauer, S.; Janke, F.; Ogrodnik, S.J.; Reck, M.; Schlesner, M.; Meister, M.; Schneider, M.A.; et al. Early Identification of Disease Progression in ALK-Rearranged Lung Cancer Using Circulating Tumor DNA Analysis. NPJ Precis. Oncol. 2021, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Dietz, S.; Christopoulos, P.; Yuan, Z.; Angeles, A.K.; Gu, L.; Volckmar, A.-L.; Ogrodnik, S.J.; Janke, F.; Fratte, C.D.; Zemojtel, T.; et al. Longitudinal Therapy Monitoring of ALK-Positive Lung Cancer by Combined Copy Number and Targeted Mutation Profiling of Cell-Free DNA. EBioMedicine 2020, 62, 103103. [Google Scholar] [CrossRef]
- The TRACERx consortium; The PEACE consortium; Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; et al. Phylogenetic ctDNA Analysis Depicts Early-Stage Lung Cancer Evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Belloum, Y.; Janning, M.; Mohme, M.; Simon, R.; Kropidlowski, J.; Sartori, A.; Irwin, D.; Westphal, M.; Lamszus, K.; Loges, S.; et al. Discovery of Targetable Genetic Alterations in NSCLC Patients with Different Metastatic Patterns Using a MassARRAY-Based Circulating Tumor DNA Assay. Cells 2020, 9, 2337. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Hendifar, A.; Gangi, A.; Zaghiyan, K.; Atkins, K.; Nasseri, Y.; Murrell, Z.; Figueiredo, J.C.; Salvy, S.; Haile, R.; et al. Clinical Applications of Minimal Residual Disease Assessments by Tumor-Informed and Tumor-Uninformed Circulating Tumor DNA in Colorectal Cancer. Cancers 2021, 13, 4547. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, F.; Shen, H.; Wang, C.; Li, X.; Chervova, O.; Wu, S.; Qiu, F.; Peng, D.; Zhu, X.; et al. Individualized Tumor-Informed Circulating Tumor DNA Analysis for Postoperative Monitoring of Non-Small Cell Lung Cancer. Cancer Cell 2023, 41, 1749–1762.e6. [Google Scholar] [CrossRef]
- Cheng, L.; Gao, G.; Zhao, C.; Wang, H.; Yao, C.; Yu, H.; Yao, J.; Li, F.; Guo, L.; Jian, Q.; et al. Personalized Circulating Tumor DNA Detection to Monitor Immunotherapy Efficacy and Predict Outcome in Locally Advanced or Metastatic Non-small Cell Lung Cancer. Cancer Med. 2023, 12, 14317–14326. [Google Scholar] [CrossRef]
- Plagnol, V.; Woodhouse, S.; Howarth, K.; Lensing, S.; Smith, M.; Epstein, M.; Madi, M.; Smalley, S.; Leroy, C.; Hinton, J.; et al. Analytical Validation of a next Generation Sequencing Liquid Biopsy Assay for High Sensitivity Broad Molecular Profiling. PLoS ONE 2018, 13, e0193802. [Google Scholar] [CrossRef]
- González de Aledo-Castillo, J.M.; Serhir-Sgheiri, S.; Calbet-Llopart, N.; Arcocha, A.; Jares, P.; Reguart, N.; Puig-Butillé, J.A. Technical Evaluation of the COBAS EGFR Semiquantitative Index (SQI) for Plasma cfDNA Testing in NSCLC Patients with EGFR Exon 19 Deletions. Diagnostics 2021, 11, 1319. [Google Scholar] [CrossRef]
- Ryu, J.-S.; Lim, J.H.; Lee, M.K.; Lee, S.J.; Kim, H.-J.; Kim, M.J.; Park, M.H.; Kim, J.S.; Nam, H.-S.; Park, N.; et al. Feasibility of Bronchial Washing Fluid-Based Approach to Early-Stage Lung Cancer Diagnosis. Oncologist 2019, 24, e603–e606. [Google Scholar] [CrossRef] [PubMed]
- Otake, S.; Goto, T.; Higuchi, R.; Nakagomi, T.; Hirotsu, Y.; Amemiya, K.; Oyama, T.; Mochizuki, H.; Omata, M. The Diagnostic Utility of Cell-Free DNA from Ex Vivo Bronchoalveolar Lavage Fluid in Lung Cancer. Cancers 2022, 14, 1764. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.S.; Hui, A.B.-Y.; Chabon, J.J.; Esfahani, M.S.; Stehr, H.; Nabet, B.Y.; Zhou, L.; Chaudhuri, A.A.; Benson, J.; Ayers, K.; et al. Genomic Profiling of Bronchoalveolar Lavage Fluid in Lung Cancer. Cancer Res. 2022, 82, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, X.; Li, D.; Chen, K.; Zhan, Q.; Ge, M.; Zhou, X.; Liang, X.; Guan, M. Digital PCR-Based Detection of EGFR Mutations in Paired Plasma and CSF Samples of Lung Adenocarcinoma Patients with Central Nervous System Metastases. Target. Oncol. 2019, 14, 343–350. [Google Scholar] [CrossRef]
| Clinical Trials ID (Duration) | Title | Main Location (Sponsor) | Number of Participants (Trial Type) | Primary Outcomes |
|---|---|---|---|---|
| NCT05708599 (02.2023–02.2028) | A Study to Compare Tissue and Liquid Biopsies in People With Different Types of Cancer | Germany (Boehringer Ingelheim) | 180 (Interventional) | The mean VAF of mutations in ctDNA samples over the timescale of the patient’s treatment course |
| NCT05429320 (06.2022–06.2025 | A Study of Local Ablative Therapy (LAT) in People With Non-Small Cell Lung Cancer (NSCLC) | USA (Memorial Sloan Kettering Cancer Center) | 117 (Interventional) | Measure the reduction in mean VAF by 6 months after local ablative therapy |
| NCT05921474 (04.2023–12.2023) | Detection of Circulating Tumor DNA After Stereotactic Ablative Radiotherapy in Patients With Unbiopsied Lung Tumors (SABR-DETECT) | Canada (Lawson Health Research Institute) | 100 (Observational) | Increases in VAF or quantifiable ctDNA from baseline to post-treatment samples (in patients with detectable ctDNA at baseline) |
| NCT05221372 (02.2017–01.2031) | ProSpecTive sAmpling in dRiver muTation Pulmonary Oncology Patients on Tyrosine Kinase Inhibitors (START-TKI) | Netherlands (Erasmus Medical Center) | 1300 (Observational) | The relative presence of primary mutations and resistance mutations in plasma levels under the treatment of a small molecule kinase inhibitor until the progression of disease measured in VAF |
| NCT04122833 (09.2019–12.2024) | Impact of Concomitant Genetic Alterations in EGFR Mutated Adenocarcinoma by NGS Analysis: A Multicenter Study | South Korea (Konkuk University Medical Center) | 80 (Observational) | The correlation between the change in VAF and drug response in matched tumor tissues before and after TKI treatment |
| NCT05102110 (12.2021–12/2023) | Feasibility Study to Investigate Rectal Mucus in Aero-Digestive Tract Cancer (ORI-EGI-03) | United Kingdom (Origin Sciences) | 300 (Observational) | The correlation of SNP allele frequency in genes associated with known aero-digestive cancers in paired samples of tumour type and rectal mucus |
| NCT05254795 (04.2022–12.2036) | Precision Medicine Randomized Clinical Trial Comparing Molecular Tumor Board Assisted Care to Usual Care (PRiMAL) | USA (Jill M Kolesar) | 500 (Interventional) | The association of ctDNA VAF with 1-year overall survival |
| NCT05782361 (05.2023–02.2028) | POTENT-Tepotinib in Combination With Pembrolizumab in NSCLC | United Kingdom (Institute of Cancer Research) | 38 (Interventional) | The determination of allele frequency of genomic aberrations including, but not limited to, the MET, EGFR, BRAF, and KRAS genes in plasma |
| NCT03778229 (01.2019–05.2025) | Osimertinib Plus Savolitinib in EGFRm+/ΔMET+ NSCLC Following Prior Osimertinib (SAVANNAH) | USA (AstraZeneca) | 360 (Interventional) | Total clearance of EGFR mutations at 6 weeks after osimertinib and savolitinib therapy initiation (the percentage and absolute change from baseline in EGFR mutation allele frequencies) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galant, N.; Nicoś, M.; Kuźnar-Kamińska, B.; Krawczyk, P. Variant Allele Frequency Analysis of Circulating Tumor DNA as a Promising Tool in Assessing the Effectiveness of Treatment in Non-Small Cell Lung Carcinoma Patients. Cancers 2024, 16, 782. https://doi.org/10.3390/cancers16040782
Galant N, Nicoś M, Kuźnar-Kamińska B, Krawczyk P. Variant Allele Frequency Analysis of Circulating Tumor DNA as a Promising Tool in Assessing the Effectiveness of Treatment in Non-Small Cell Lung Carcinoma Patients. Cancers. 2024; 16(4):782. https://doi.org/10.3390/cancers16040782
Chicago/Turabian StyleGalant, Natalia, Marcin Nicoś, Barbara Kuźnar-Kamińska, and Paweł Krawczyk. 2024. "Variant Allele Frequency Analysis of Circulating Tumor DNA as a Promising Tool in Assessing the Effectiveness of Treatment in Non-Small Cell Lung Carcinoma Patients" Cancers 16, no. 4: 782. https://doi.org/10.3390/cancers16040782
APA StyleGalant, N., Nicoś, M., Kuźnar-Kamińska, B., & Krawczyk, P. (2024). Variant Allele Frequency Analysis of Circulating Tumor DNA as a Promising Tool in Assessing the Effectiveness of Treatment in Non-Small Cell Lung Carcinoma Patients. Cancers, 16(4), 782. https://doi.org/10.3390/cancers16040782






