Interval Cancer in Population-Based Colorectal Screening Programmes: Incidence and Characteristics of Tumours
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. CRIBEA-CIN Project
2.2. Study Variables
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC). “Global Cancer Observatory”. Available online: https://gco.iarc.fr/ (accessed on 23 January 2024).
- Heresbach, D.; Manfredi, S.; D’halluin, P.N.; Bretagne, J.F.; Branger, B. Review in depth and meta-analysis of controlled trials on colorectal cancer screening by faecal occult blood test. Eur. J. Gastroenterol. Hepatol. 2006, 18, 427–433. [Google Scholar] [CrossRef]
- Zauber, A.G. The impact of screening on colorectal cancer mortality and incidence: Has it really made a difference? Dig. Dis. Sci. 2015, 60, 681–691. [Google Scholar] [CrossRef]
- Wieten, E.; Schreuders, E.H.; Grobbee, E.J.; Nieboer, D.; Bramer, W.M.; Lansdorp-Vogelaar, I.; Bruno, M.J.; Kuipers, E.J.; Spaander, M.C.W. Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: A systematic review and meta-analysis. Gut 2019, 68, 873–881. [Google Scholar] [CrossRef]
- Segnan, N.; Patnick, J.; Karsa, L. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis, 1st ed.; Publications Office of the European Union, European Commission: Luxembourg, 2010. [Google Scholar]
- Parra-Blanco, A.; Gimeno-García, A.Z.; Quintero, E.; Nicolás, D.; Moreno, S.G.; Jiménez, A.; Hernández-Guerra, M.; Carrillo-Palau, M.; Eishi, Y.; López-Bastida, J. Diagnostic accuracy of immunochemical versus guaiac faecal occult blood tests for colorectal cancer screening. J. Gastroenterol. 2010, 45, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Guittet, L.; Bouvier, V.; Mariotte, N.; Vallee, J.P.; Arsène, D.; Boutreux, S.; Tichet, J.; Launoy, G. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut 2007, 56, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Portillo Villares, I.; Arana-Arri, E.; Idigoras Rubio, I.; Espinás Piñol, J.A.; Pérez Riquelme, F.; de la Vega Prieto, M.; González Aledo, A.; Oceja Setien, E.; Vanaclocha Espi, M.; Ibáñez Cabanell, J.; et al. Lesions Detected in Six Spanish Colorectal Cancer Screening Population Based Programmes. CRIBEA Project. Rev. Esp. Salud Publica 2017, 91, e201701021. [Google Scholar] [PubMed]
- Smith, A.; Young, G.P.; Cole, S.R.; Bampton, P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006, 107, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Bretagne, J.F.; Carlo, A.; Piette, C.; Rousseau, C.; Cosson, M.; Lièvre, A. Significant decrease in interval colorectal cancer incidence after implementing immunochemical testing in a multiple-round guaiac-based screening programme. Br. J. Cancer 2021, 125, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Vanaclocha-Espi, M.; Ibáñez, J.; Molina-Barceló, A.; Valverde-Roig, M.J.; Nolasco, A.; Pérez-Riquelme, F.; de la Vega, M.; Portillo, I.; Salas, D. Optimal cut-off value for detecting colorectal cancer with fecal immunochemical tests according to age and sex. PLoS ONE 2021, 16, e0254021. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.D.; Bramble, M.G.; Rees, C.J.; Lee, T.J.; Bradburn, D.M.; Mills, S.J. Comparison of screen-detected and interval colorectal cancers in the Bowel Cancer Screening Programme. Br. J. Cancer 2012, 107, 417–421. [Google Scholar] [CrossRef]
- Garcia, M.; Domènech, X.; Vidal, C.; Torné, E.; Milà, N.; Binefa, G.; Benito, L.; Moreno, V. Interval cancers in a population-based screening program for colorectal cancer in catalonia, Spain. Gastroenterol. Res. Pract. 2015, 2015, 672410. [Google Scholar] [CrossRef] [PubMed]
- Burón, A.; Macià, F.; Andreu, M.; Pellisé, M.; Castells, X.; Grau, J. Population-based colorectal cancer screening: Interval cancers and relationship with the quantitative faecal immunological for hemoglobin. Med. Clin. 2019, 152, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.J.C.; McClements, P.; Watling, C.; Libby, G.; Weller, D.; Brewster, D.H.; Black, R.; Carey, F.A.; Fraser, C.G. Interval cancers in a FOBT-based colorectal cancer population screening programme: Implications for stage, gender and tumour site. Gut 2012, 61, 576–581. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, W.; Hoeck, S.; Peeters, M.; Van Hal, G.; Francart, J.; De Brabander, I. Occurrence and characteristics of faecal immunochemical screen-detected cancers vs non-screen-detected cancers: Results from a Flemish colorectal cancer screening programme. United Eur. Gastroenterol. J. 2020, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Vanaclocha-Espi, M.; Ibáñez, J.; Molina-Barceló, A.; Valverde-Roig, M.J.; Pérez, E.; Nolasco, A.; de la Vega, M.; de la Lastra-Bosch, I.D.; Oceja, M.E.; Espinàs, J.A.; et al. Risk factors for severe complications of colonoscopy in screening programs. Prev. Med. 2019, 118, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Chilton, A. Quality Assurance Guidelines for Colonoscopy; NHS BCSP Publication: London, UK, 2011; Volume 6, pp. 1–24. [Google Scholar]
- Chubak, J.; Bogart, A.; Fuller, S.; Laing, S.S.; Green, B.B. Uptake and positive predictive value of fecal occult blood tests: A randomized controlled trial. Prev. Med. 2013, 57, 671–678. [Google Scholar] [CrossRef]
- van Rossum, L.G.; van Rijn, A.F.; Laheij, R.J.; van Oijen, M.G.; Fockens, P.; van Krieken, H.H.; Verbeek, A.L.; Jansen, J.B.; Dekker, E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008, 135, 82–90. [Google Scholar] [CrossRef]
- Guo, F.; De Brabander, I.; Francart, J.; Candeur, M.; Polus, M.; van Eycken, L.; Brenner, H. Benefits of switching from guaiac-based faecal occult blood to faecal immunochemical testing: Experience from the Wallonia-Brussels colorectal cancer screening programme. Br. J. Cancer 2020, 122, 1109–1117. [Google Scholar] [CrossRef]
- Grobbee, E.J.; Wisse, P.H.A.; Schreuders, E.H.; van Roon, A.; van Dam, L.; Zauber, A.G.; Lansdorp-Vogelaar, I.; Bramer, W.; Berhane, S.; Deeks, J.J.; et al. Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals. Cochrane Database Syst. Rev. 2022, 6, CD009276. [Google Scholar] [CrossRef]
- Bujanda, L.; Sarasqueta, C.; Castells, A.; Pellisé, M.; Cubiella, J.; Gil, I.; Cosme, A.; Arana-Arri, E.; Mar, I.; Idigoras, I.; et al. Colorectal cancer in a second round after a negative faecal immunochemical test. Eur. J. Gastroenterol. Hepatol. 2015, 27, 813–818. [Google Scholar] [CrossRef]
- Ribe, S.G.; Botteri, E.; Løberg, M.; Randel, K.R.; Kalager, M.; Nilsen, J.A.; Gulichsen, E.H.; Holme, Ø. Impact of time between faecal immunochemical tests in colorectal cancer screening on screening results: A natural experiment. Int. J. Cancer 2023, 152, 1414–1424. [Google Scholar] [CrossRef]
- Breekveldt, E.C.H.; Toes-Zoutendijk, E.; van de Schootbrugge-Vandermeer, H.J.; de Jonge, L.; Kooyker, A.I.; Spaander, M.C.W.; van Vuuren, A.J.; van Kemenade, F.J.; Ramakers, C.; Dekker, E.; et al. Factors associated with interval colorectal cancer after negative FIT: Results of two screening rounds in the Dutch FIT-based CRC screening program. Int. J. Cancer 2023, 152, 1536–1546. [Google Scholar] [CrossRef]
- Portillo, I.; Arana-Arri, E.; Idigoras, I.; Bilbao, I.; Martínez-Indart, L.; Bujanda, L.; Gutierrez-Ibarluzea, I. Colorectal and interval cancers of the Colorectal Cancer Screening Program in the Basque Country (Spain). World J. Gastroenterol. 2017, 23, 2731–2742. [Google Scholar] [CrossRef]
- Van Hal, G.; Janssens, S.; de Schutter, H. Optimizing the colorectal cancer screening programme using faecal immunochemical test (FIT) in Flanders, Belgium from the “interval cancer” perspective. Br. J. Cancer. 2022, 126, 1091–1099. [Google Scholar] [CrossRef]
- Digby, J.; Fraser, C.G.; Carey, F.A.; Lang, J.; Stanners, G.; Steele, R.J. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J. Med. Screen. 2016, 23, 130–134. [Google Scholar] [CrossRef] [PubMed]
- van der Vlugt, M.; Grobbee, E.J.; Bossuyt, P.M.M.; Bos, A.; Bongers, E.; Spijker, W.; Kuipers, E.J.; Lansdorp-Vogelaar, I.; Spaander, M.C.W.; Dekker, E. Interval Colorectal Cancer Incidence Among Subjects Undergoing Multiple Rounds of Fecal Immunochemical Testing. Gastroenterology 2017, 153, 439–447. [Google Scholar] [CrossRef]
- Blanks, R.; Burón Pust, A.; Alison, R.; He, E.; Barnes, I.; Patnick, J.; Reeves, G.K.; Floud, S.; Beral, V.; Green, J. Screen-detected and interval colorectal cancers in England: Associations with lifestyle and other factors in women in a large UK prospective cohort. Int. J. Cancer 2019, 145, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.S.; Sexton, K.; Frankish, P.; Hulme-Moir, M.; Bissett, I.; Parry, S. Interval colorectal cancers after negative faecal immunochemical test in the New Zealand Bowel Screening Pilot. BMJ Open Gastroenterol. 2023, 10, 24. [Google Scholar] [CrossRef]
| Whole Sample | gFOBT | FIT | ||||
|---|---|---|---|---|---|---|
| N | Rate * | N | Rate * | N | Rate * | |
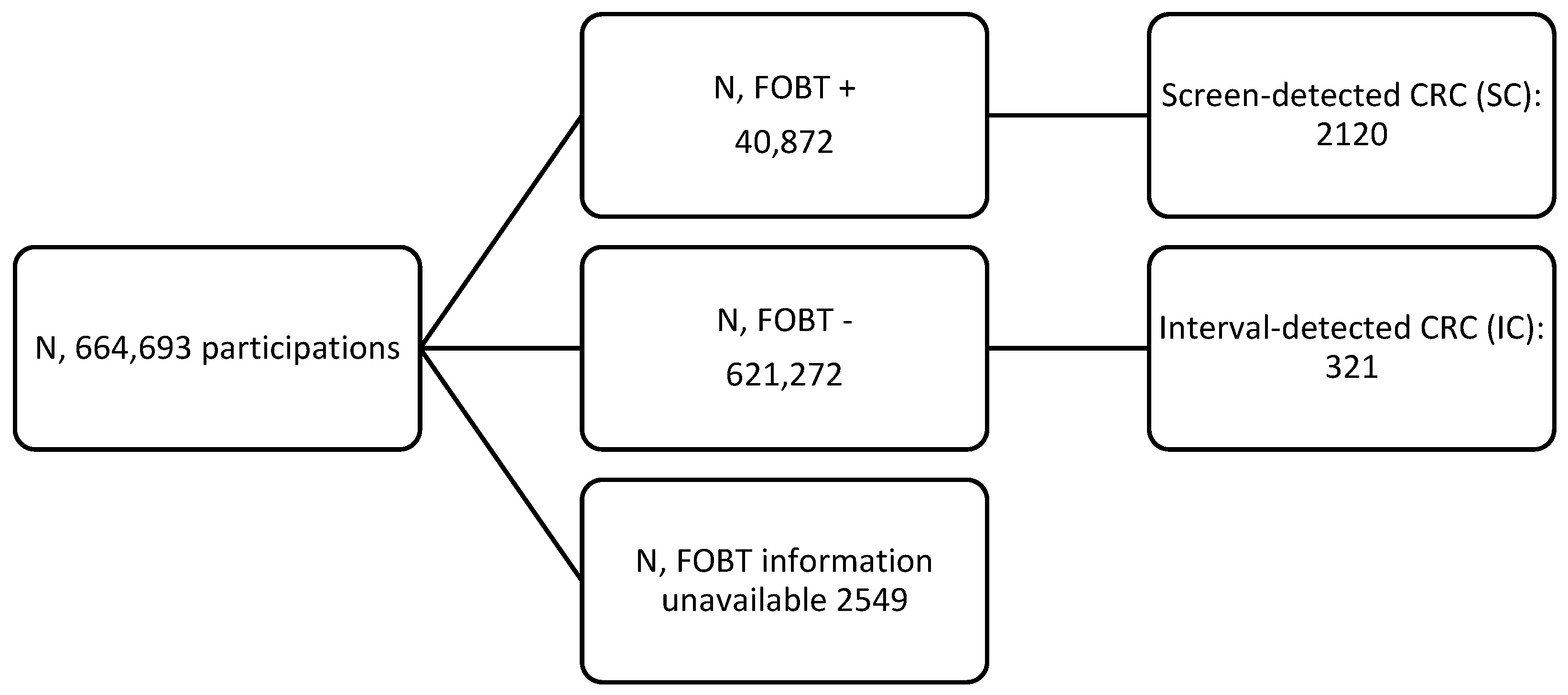
| People screened | 488,136 | |||||
| FOBTs performed | 664,693 | 106,064 | 558,629 | |||
| In initial screening | 488,136 | 79,137 | 408,999 | |||
| In subsequent screening | 176,557 | 26,927 | 149,630 | |||
| SC | 2120 | 3.19 | 144 | 1.36 | 1976 | 3.54 |
| In initial screening | 1704 | 3.49 | 106 | 1.34 | 1598 | 3.91 |
| In subsequent screening | 416 | 2.36 | 38 | 1.41 | 378 | 2.53 |
| IC | 321 | 0.48 | 123 | 1.16 | 198 | 0.35 |
| In initial screening | 258 | 0.52 | 98 | 1.24 | 160 | 0.39 |
| In subsequent screening | 63 | 0.36 | 25 | 0.93 | 38 | 0.25 |
| Time to diagnosis (med ± SD) | 14.04 ± 6.38 | 14.43 ± 6.37 | 13.80 ± 6.38 | |||
| <12 months | 116 (36.1) | 41 (33.3) | 75 (37.9) | |||
| 12–24 months | 205 (63.9) | 82 (66.7) | 123 (62.1) | |||
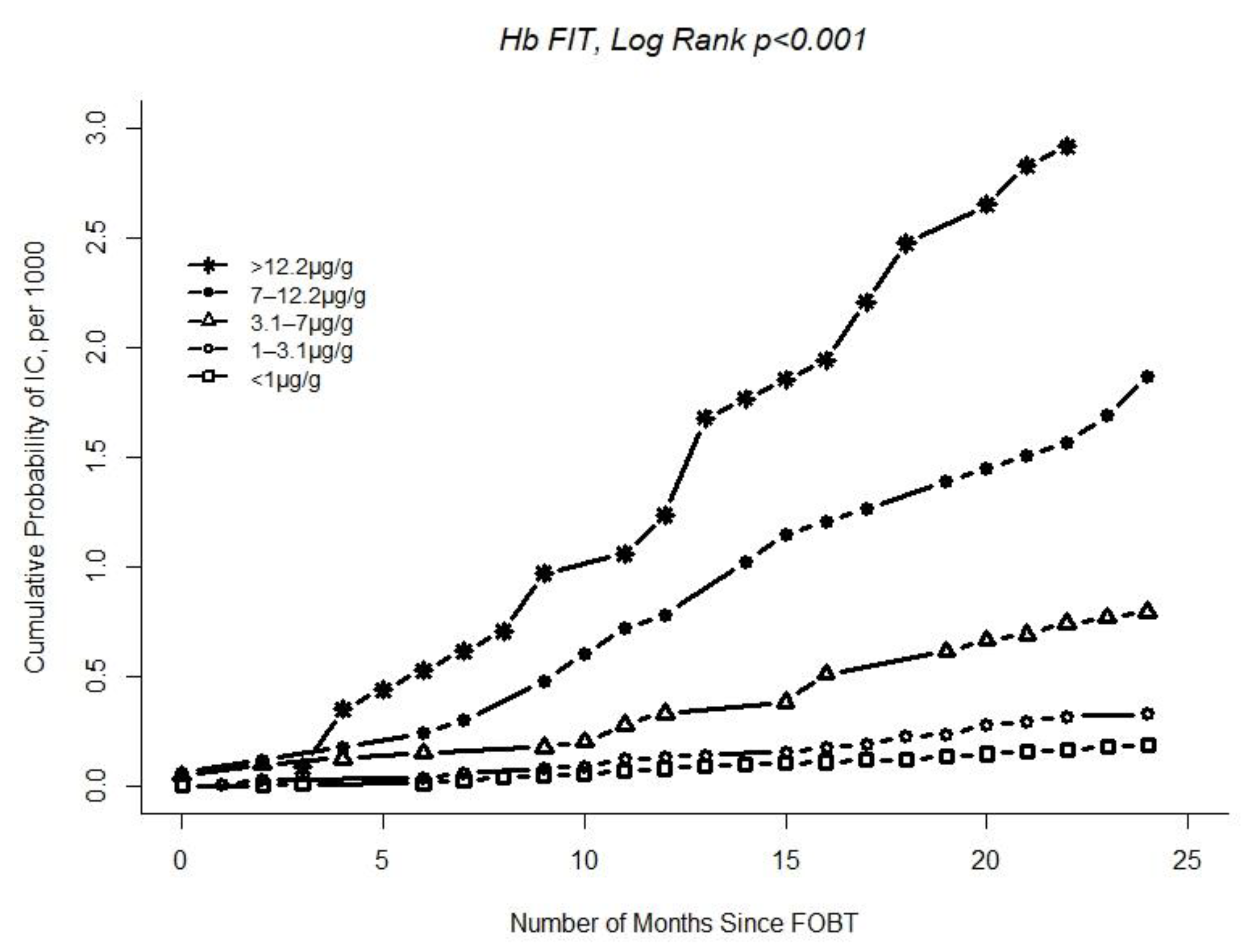
| IC according to Hb in negative FIT (6 unknown) | ||||||
| <1 μg/g | 65 (33.9%) | |||||
| 1–3.1 μg/g | 32 (16.7%) | |||||
| 3.1–7 μg/g | 31 (16.1%) | |||||
| 7–12.2 μg/g | 31 (16.1%) | |||||
| >12.2 μg/g | 33 (17.2%) | |||||
| Negative FOBTs | Only Negative FITs | |||||
|---|---|---|---|---|---|---|
| No. Negative FOBT | IC | HR (95% CI) | No. Negative FIT | IC | HR (95% CI) | |
| Age | ||||||
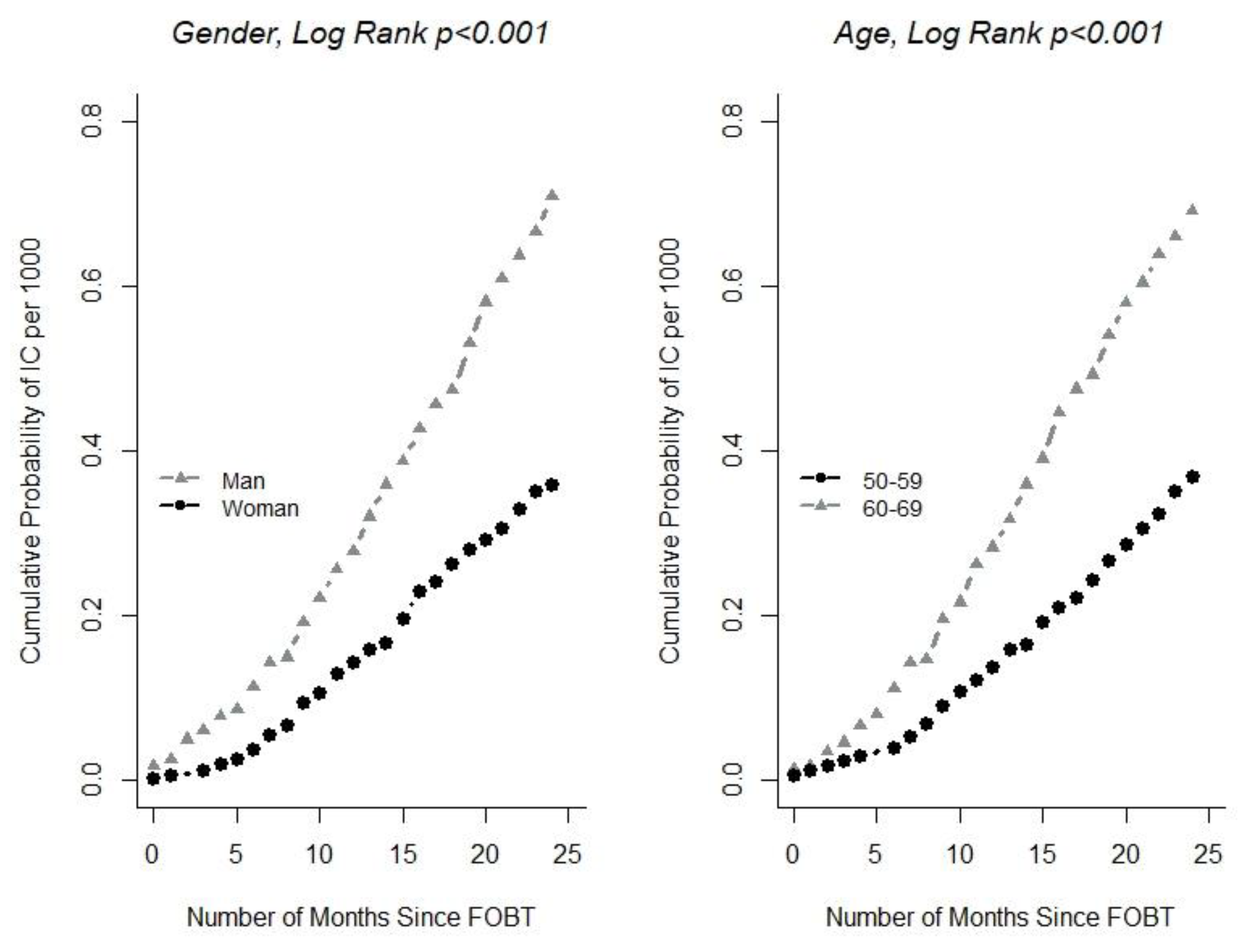
| 50–59 | 334,293 | 123 | 1 | 288,262 | 83 | 1 |
| 60–69 | 286,619 | 198 | 1.81 (1.44–2.27) | 229,843 | 115 | 1.68 (1.26–2.24) |
| Sex | ||||||
| Female | 339,859 | 122 | 1 | 283,948 | 74 | 1 |
| Male | 281,053 | 199 | 1.95 (1.56–2.45) | 234,157 | 124 | 1.84 (1.37–2.46) |
| FOBT type | ||||||
| Guaiac | 102,807 | 123 | 1 | |||
| FIT | 518,105 | 198 | 0.34 (0.27–0.43) | |||
| Type of participation | ||||||
| Initial | 454,021 | 258 | 1 | 377,547 | 160 | 1 |
| Subsequent | 166,864 | 63 | 0.62 (0.47–0.82) | 140,531 | 38 | 0.65 (0.45–0.92) |
| Hb in faeces | ||||||
| <1 μg/g | 341,837 | 65 | 1 | |||
| 1–3.1 μg/g | 97,043 | 32 | 1.75 (1.15–2.68) | |||
| 3.1–7 μg/g | 38,950 | 31 | 3.91 (2.55–6.00) | |||
| 7–12.2 μg/g | 16,567 | 31 | 9.03 (5.88–13.86) | |||
| >12.2 μg/g | 11,306 | 33 | 13.73 (9.01–20.91) | |||
| IC vs. SC | IC < 12 m vs. SC | IC 12–24 m vs. SC | |||||
|---|---|---|---|---|---|---|---|
| IC n (%) | SC n (%) | OR (95% CI) | IC n (%) | OR (95% CI) | IC n (%) | OR (95% CI) | |
| Sex | |||||||
| Female | 120 (37.4) | 715 (33.7) | ref | 49 (38.6) | ref | 71 (36.6) | ref |
| Male | 201 (62.6) | 1405 (66.3) | 0.98 (0.65–1.49) | 78 (61.4) | 1.14 (0.62– 2.17) | 123 (63.4) | 0.92 (0.56–1.55) |
| Age | |||||||
| 50–59 | 123 (38.3) | 790 (38.3) | Ref | 46 (36.2) | Ref | 77 (39.7) | ref |
| 60–69 | 198 (61.7) | 1330 (61.7) | 0.85 (0.57–1.27) | 81 (63.8) | 1.00 (0.54–1.86) | 117 (60.3) | 0.80 (0.5–1.31) |
| Type of screening | |||||||
| Initial | 258 (80.4) | 1704 (80.4) | ref | 100 (78.7) | ref | 158 (81.4) | Ref |
| Subsequent | 63 (19.6) | 416 (19.6) | 1.36 (0.79–2.26) | 27 (21.3) | 2.02 (0.96–4.04) | 36 (18.6) | 0.96 (0.45–1.89) |
| Location | |||||||
| Sigmoid or rectum | 177 (60.0) | 1095 (69.0) | ref | 66 (56.9) | ref | 111 (62.0) | ref |
| Caecum | 49 (16.6) | 92 (5,8) | 2.92(1.62–5.12) | 16 (13.8) | 1.82 (0.62–4.60) | 33 (18.4) | 3.49 (1.34–4.76) |
| Ascending | 27 (9.2) | 126 (7.9) | 1.05 (0.49–2.06) | 15 (12.9) | 1.96 (0.79–4.47) | 12 (6.7) | 0.42 (0.10–1.25) |
| Transverse | 30 (10.2) | 179 (11.3) | 1.07 (0.55–1.94) | 13 (11.2) | 1.33 (0.51–3.09) | 17 (9.5) | 0.96 (0.40–2.03) |
| Descending | 12 (4.1) | 97 (6.0) | 0.33 (0.08–1.09) | 6 (5.2) | 0.48 (0.07–2.04) | 6 (3.4) | 0.31 (0.05–1.27) |
| Tumour size, mm | 1.20 (1.16–1.25) | 1.18 (1.14–1.24) | 1.19 (1.15–1.25) | ||||
| Morphology | |||||||
| Adenocarcinoma NOS | 269 (93.4) | 2003 (97.8) | ref | 107 (92.2) | ref | 162 (94.2) | ref |
| Other * | 19 (6.6) | 45 (2.2) | 2.37 (1.11–4.79) | 9 (7.8) | 3.65 (1.35–8.81) | 10 (5.8) | 1.62 (0.55–4.06) |
| Stage | |||||||
| I | 47 (18.1) | 998 (50.9) | ref | 16 (14.5) | ref | 31 (20.7) | ref |
| II | 58 (22.3) | 382 (19.5) | 2.08 (1.17–3.73) | 30 (27.3) | 3.03 (1.29–7.60) | 28 (18.7) | 1.40 (0.66–2.94) |
| III | 81 (31.2) | 450 (22.9) | 2.51 (1.48–4.35) | 30 (27.3) | 2.14 (0.89–5.43) | 51 (34.0) | 2.49 (1.34–4.76) |
| IV | 74 (28.5) | 131 (6.7) | 4.55 (2.39–8.62) | 34 (30.9) | 5.56 (2.06–15.27) | 40 (26.7) | 3.90 (1.81–8.30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanaclocha-Espí, M.; Pinto-Carbó, M.; Ibáñez, J.; Valverde-Roig, M.J.; Portillo, I.; Pérez-Riquelme, F.; de la Vega, M.; Castán-Cameo, S.; Salas, D.; Molina-Barceló, A. Interval Cancer in Population-Based Colorectal Screening Programmes: Incidence and Characteristics of Tumours. Cancers 2024, 16, 769. https://doi.org/10.3390/cancers16040769
Vanaclocha-Espí M, Pinto-Carbó M, Ibáñez J, Valverde-Roig MJ, Portillo I, Pérez-Riquelme F, de la Vega M, Castán-Cameo S, Salas D, Molina-Barceló A. Interval Cancer in Population-Based Colorectal Screening Programmes: Incidence and Characteristics of Tumours. Cancers. 2024; 16(4):769. https://doi.org/10.3390/cancers16040769
Chicago/Turabian StyleVanaclocha-Espí, Mercedes, Marina Pinto-Carbó, Josefa Ibáñez, María José Valverde-Roig, Isabel Portillo, Francisco Pérez-Riquelme, Mariola de la Vega, Susana Castán-Cameo, Dolores Salas, and Ana Molina-Barceló. 2024. "Interval Cancer in Population-Based Colorectal Screening Programmes: Incidence and Characteristics of Tumours" Cancers 16, no. 4: 769. https://doi.org/10.3390/cancers16040769
APA StyleVanaclocha-Espí, M., Pinto-Carbó, M., Ibáñez, J., Valverde-Roig, M. J., Portillo, I., Pérez-Riquelme, F., de la Vega, M., Castán-Cameo, S., Salas, D., & Molina-Barceló, A. (2024). Interval Cancer in Population-Based Colorectal Screening Programmes: Incidence and Characteristics of Tumours. Cancers, 16(4), 769. https://doi.org/10.3390/cancers16040769






