Procalcitonin for Early Detection of Pharyngocutaneous Fistula after Total Laryngectomy: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
Data Analysis
4. Discussion
- Diagnosis of infections.
- 2.
- Control of therapy and the resolution of bacterial infections.
- 3.
- Monitoring and control of patients at risk.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartoletti, M.; Antonelli, M.; Blasi, F.A.B.; Casagranda, I.; Chieregato, A.; Fumagalli, R.; Girardis, M.; Pieralli, F.; Plebani, M.; Rossolini, G.M.; et al. Procalcitonin-guided antibiotic therapy: An expert consensus. Clin. Chem. Lab. Med. 2018, 56, 1223–1229. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Iocca, O.; Copelli, C.; Ramieri, G.; Zocchi, J.; Savo, M.; Di Maio, P. Antibiotic prophylaxis in head and neck cancer surgery: Systematic review and Bayesian network meta-analysis. Head Neck 2022, 44, 254–261. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, J.H. Clinical utility of procalcitonin in severe odontogenic maxillofacial infection. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 3. [Google Scholar] [CrossRef]
- Koerdt, S.; Rommel, N.; Rohleder, N.H.; Sandig, S.; Frohwitter, G.; Steiner, T.; Wolff, K.D.; Kesting, M.R. Perioperative serum levels of procalcitonin, C-reactive protein, and leukocytes in head and neck free flaps. Int. J. Oral Maxillofac. Surg. 2017, 46, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Valenza, V.; Parrilla, C.; Galla, S.; Marchese, M.R.; Castaldi, P.; Almadori, G.; Paludetti, G. Pharyngocutaneous fistula onset after total laryngectomy: Scintigraphic analysis. Acta Otorhinolaryngol. Ital. 2009, 29, 242–244. [Google Scholar] [PubMed]
- Dilger, A.E.; Peters, A.T.; Wunderink, R.G.; Tan, B.K.; Kern, R.C.; Conley, D.B.; Welch, K.C.; Holl, J.L.; Smith, S.S. Procalcitonin as a Biomarker in Rhinosinusitis: A Systematic Review. Am. J. Rhinol. Allergy 2019, 33, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sager, R.; Kutz, A.; Mueller, B.; Schuetz, P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Jichao, S.; Cuida, M.; Liwei, S.; Jiani, L.; Dongdong, Z. Predictive value of procalcitonin level for pharyngocutaneous fistula after laryngectomy. Am. J. Otolaryngol.-Head Neck Med. Surg. 2023, 44, 103846. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.F.; Falcão, L.M.; Pinheiro, I.D. Procalcitonin as biomarker of infection: Implications for evaluation and treatment. Am. J. Ther. 2017, 24, e243–e249. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; De Corso, E.; Volante, M.; Almadori, G.; Paludetti, G. Postlaryngectomy pharyngocutaneous fistula: Incidence, predisposing factors, and therapy. Otolaryngol.-Head Neck Surg. 2005, 133, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Paudel, R.; Dogra, P.; Montgomery-Yates, A.A.; Yataco, A.C. Procalcitonin: A promising tool or just another overhyped test? Int. J. Med. Sci. 2020, 17, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and neck cancers, version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, G.; Di Giorgio, D.; Leone, F.; Pichi, B.; Campo, F.; De Virgilio, A.; Valentini, V.; Pellini, R. The T-shaped FST pharyngoplasty step-by-step closure technique. Head Neck 2022, 44, 2943–2946. [Google Scholar] [CrossRef] [PubMed]
- Pollaers, K.; Hinton-Bayre, A.; Friedland, P.L.; Farah, C.S. AJCC 8th Edition oral cavity squamous cell carcinoma staging–Is it an improvement on the AJCC 7th Edition? Oral Oncol. 2018, 82, 23–28. [Google Scholar] [CrossRef]
- Oberhofer, D.; Juras, J.; Pavičić, A.M.; Žurić, I.R.; Rumenjak, V. Comparison of C-reactive protein and procalcitonin as predictors of postoperative infectious complications after elective colorectal surgery. Croat. Med. J. 2012, 53, 612–619. [Google Scholar] [CrossRef]
- Almeida, A.B.; Faria, G.; Moreira, H.; Pinto-de-Sousa, J.; Correia-da-Silva, P.; Maia, J.C. Elevated serum C-reactive protein as a predictive factor for anastomotic leakage in colorectal surgery. Int. J. Surg. 2012, 10, 87–91. [Google Scholar] [CrossRef]
- Laifer, G.; Wasner, M.; Sendi, P.; Graber, P.; Gratzl, O.; Huber, P.; Fluckiger, U.; Zimmerli, W. Dynamics of serum procalcitonin in patients after major neurosurgery. Clin. Microbiol. Infect. 2005, 11, 679–681. [Google Scholar] [CrossRef][Green Version]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Kibe, S.; Adams, K.; Barlow, G. Diagnostic and prognostic biomarkers of sepsis in critical care. J. Antimicrob. Chemother. 2011, 66, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Ansaloni, L.; Bartoletti, M.; Catena, F.; Cardi, M.; Cortese, F.; Di Marzo, F.; Pea, F.; Plebani, M.; Rossolini, G.M.; et al. The role of procalcitonin in reducing antibiotics across the surgical pathway. World J. Emerg. Surg. 2021, 16, 15. [Google Scholar] [CrossRef]
- Scherl, C.; Kauffels, J.; Schützenberger, A.; Döllinger, M.; Bohr, C.; Dürr, S.; Fietkau, R.; Haderlein, M.; Koch, M.; Traxdorf, M.; et al. Secondary Tracheoesophageal Puncture After Laryngectomy Increases Complications With Shunt and Voice Prosthesis. Laryngoscope 2020, 130, E865–E873. [Google Scholar] [CrossRef]
- Covington, E.W.; Roberts, M.Z.; Dong, J. Procalcitonin Monitoring as a Guide for Antimicrobial Therapy: A Review of Current Literature. Pharmacotherapy 2018, 38, 569–581. [Google Scholar] [CrossRef]
- Tujula, B.; Hämäläinen, S.; Kokki, H.; Pulkki, K.; Kokki, M. Review of clinical practice guidelines on the use of procalcitonin in infections. Infect. Dis. 2020, 52, 227–234. [Google Scholar] [CrossRef]
- Creamer, A.W.; Kent, A.E.; Albur, M. Procalcitonin in respiratory disease: Use as a biomarker for diagnosis and guiding antibiotic therapy. Breathe 2019, 15, 296–304. [Google Scholar] [CrossRef]
- Lin, K.H.; Wang, F.L.; Wu, M.S.; Jiang, B.Y.; Kao, W.L.; Chao, H.Y.; Wu, J.Y.; Lee, C.C. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: A systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2014, 80, 72–78. [Google Scholar] [CrossRef]
- Domínguez-Comesaña, E.; Estevez-Fernández, S.M.; López-Gómez, V.; Ballinas-Miranda, J.; Domínguez-Fernández, R. Procalcitonin and C-reactive protein as early markers of postoperative intra-abdominal infection in patients operated on colorectal cancer. Int. J. Color. Dis. 2017, 32, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.; Hole, A.; Johnsen, H.; Åsberg, A.; Rydning, A.; Myrvold, H.E.; Bjerve, K.S. Reference intervals for procalcitonin and C-reactive protein after major abdominal surgery. Scand. J. Clin. Lab. Investig. 2002, 62, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mesolella, M.; Allosso, S.; Salerno, G.; Motta, G. Sport in the Laryngectomized Patient: A Literature Review and Single Case Presentation. J. Pers. Med. 2023, 13, 982. [Google Scholar] [CrossRef] [PubMed]
- Mesolella, M.; Allosso, S.; Di Lullo, A.M.; Ricciardiello, F.; Motta, G. Postoperative infectious complications in head and neck cancer surgery. Ann. Ital. Chir. 2022, 93, 637–647. [Google Scholar] [PubMed]
| Patients | Patho Logies | pTNM | Subsite | Sex | Age |
|---|---|---|---|---|---|
| 1 | SCC | pT3N0 | Glottis | F | 69 |
| 2 | SCC | pT4aN2b | Supraglottis | F | 74 |
| 3 | SCC | pT4aN2b | Glottis | F | 55 |
| 4 | SCC | pT4aN0 | Glottis | F | 63 |
| 5 | SCC | pT3N0 | Glottis | M | 71 |
| 6 | SCC | pT3N1 | Glottis | M | 77 |
| 7 | SCC | pT2N0 | Glottis | M | 80 |
| 8 | SCC | pT3N1 | Glottis | M | 64 |
| 9 | SCC | pT4aN0 | Glottis | M | 68 |
| 10 | SCC | pT4aN1 | Glottis | M | 67 |
| 11 | SCC | pT4aN0 | Glottis | M | 59 |
| 12 | SCC | pT3N1 | Supraglottis | M | 76 |
| 13 | SCC | pT4aN0 | Glottis | M | 63 |
| 14 | SCC | pT4aN1 | Glottis | M | 69 |
| 15 | SCC | pT2N0 | Glottis | M | 71 |
| 16 | SCC | pT4aN1 | Glottis | M | 77 |
| 17 | SCC | pT4aN0 | Glottis | M | 72 |
| 18 | SCC | pT3N0 | Glottis | M | 67 |
| 19 | SCC | pT4aN0 | Glottis | M | 65 |
| 20 | SCC | pT4aN0 | Glottis | M | 70 |
| 21 | SCC | pT3N2c | Supraglottis | M | 69 |
| 22 | SCC | pT3N0 | Glottis | M | 72 |
| 23 | SCC | pT4aN1 | Glottis | F | 69 |
| 24 | SCC | pT4aN0 | Glottis | M | 70 |
| 25 | SCC | pT3N0 | Glottis | M | 72 |
| 26 | SCC | pT2N0 | Glottis | M | 65 |
| 27 | SCC | pT3N1 | Supraglottis | F | 67 |
| Patients | Patho Logies | pTNM | Subsite | Sex | Age |
|---|---|---|---|---|---|
| 1 | SCC | pT4aN0 | Glottis | F | 69 |
| 2 | SCC | pT4aN0 | Subglottis | M | 66 |
| 3 | SCC | pT4aN1 | Glottis | M | 67 |
| 4 | SCC | pT4aN0 | Supraglottis | M | 65 |
| 5 | SCC | pT4aN2b | Glottis | M | 68 |
| 6 | SCC | pT3N0 | Glottis | M | 67 |
| 7 | SCC | pT4aN0 | Glottis | M | 67 |
| 8 | SCC | pT3N0 | Glottis | F | 66 |
| 9 | SCC | pT4aN1 | Glottis | M | 67 |
| Group 1: 27 Patients | |||
|---|---|---|---|
| mT0 | mT1 | mT2 | |
| Body temperature | 37.3 °C | 37.3 °C | 37.4° C |
| Leukocytosis | 12.14 × 103/mL | 13.12 × 103/mL | 13.43 × 103/mL |
| Positive ESR | 18/27 patients: m = 34 mm/h (66.67%) | 17/27 patients: m = 38 mm/h (74.07%) | 13/27 patients: m = 28 mm/h (48.55%) |
| Positive CPR | 15/27 patients: m = 30 mg/L (55.56%) | 17/27 patients: m = 32 mg/L (62.96%) | 15/27 patients: m = 22 mg/L (55.56%) |
| PCT in blood | negative | negative | Negative |
| PCT in saliva | negative | negative | negative |
| Group 2: 9 Patients | ||||
|---|---|---|---|---|
| mT0 | mT1 | mTX | mT2 | |
| Body temperature | 37.2 °C | 37.3 °C | 37.7° C | 37.3° C |
| Leukocytosis | 12.35 × 103/mL | 12.62 × 103/mL | 12.78 × 103/mL | 12.64 × 103/mL |
| Positive ESR | 6/9 patients: m = 33 mm/h (66.67%) | 7/9 patients: m = 34 mm/h (77.78%) | 8/9 patients: m = 34.80 mm/h (88.89%) | 6/9 patients: m = 29.50 mm/h (66.70%) |
| Positive CPR | 4/9 patients: m = 21.30 mg/L (44.44%) | 6/9 patients: m = 22.7 mg/L (66.67%) | 7/9 patients: m = 26.80 mg/L (77.78%) | 4/9 patients: m = 20.00 mg/L (44.44%) |
| PCT in blood and saliva | negative | 6/9 patients: m = 0.42 ng/mL (77.78%) | 8/9 patients: m = 0.63 ng/mL (66.67%) | 4/9 patients: m = 0.52 ng/mL (44.44%) |
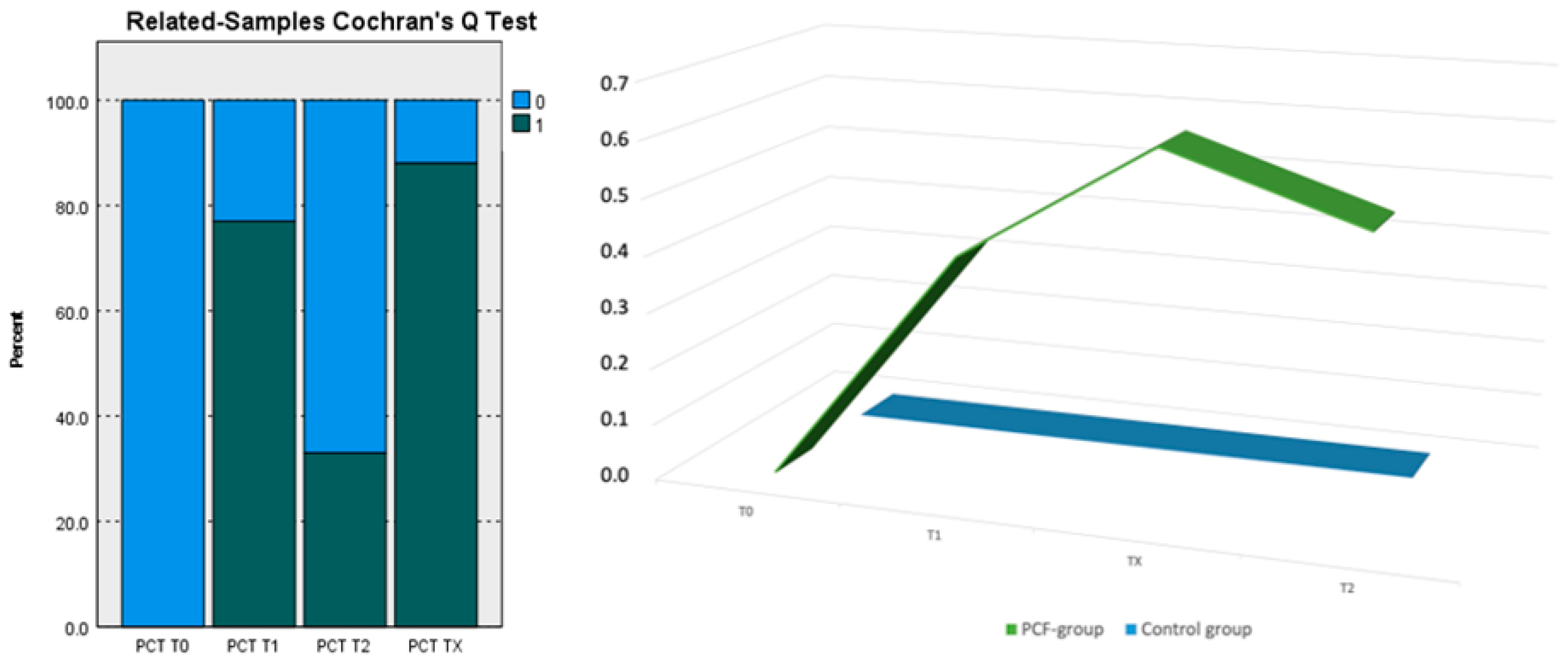
| Sample 1–Sample 2 | Test Statistic | Std. Error | Std. Test Statistic | Sig. |
|---|---|---|---|---|
| PCT T0-PCT T2 | −0.333 | 0.240 | −1.389 | 0.165 |
| PCT T0-PCT T1 | −0.778 | 0.240 | −3.240 | 0.001 |
| PCT T0-PCT TX | −0.889 | 0.240 | −3.703 | <0.001 |
| PCT T2-PCT T1 | 0.444 | 0.240 | 1.852 | 0.064 |
| PCT T2-PCT TX | −0.556 | 0.240 | −2.315 | 0.021 |
| PCT T1-PCT TX | −0.111 | 0.240 | −0.463 | 0.643 |
| Group | Successes | Trials | Proportion | Asymptotic Standard Error | Z | p-Value | |
|---|---|---|---|---|---|---|---|
| PCT T0 = 1 | =1 | 0 | 27 | 0.000 | 0.000 | - | - |
| =2 | 0 | 9 | 0.000 | 0.000 | - | - | |
| PCT T1 = 1 | =1 | 0 | 27 | 0.000 | 0.000 | −5.106 | <0.001 |
| =2 | 7 | 9 | 0.778 | 0.139 | |||
| PCT T2 = 1 | =1 | 0 | 27 | 0.000 | 0.000 | −3.133 | <0.002 |
| =2 | 3 | 9 | 0.333 | 0.157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesolella, M.; Allosso, S.; Petruzzi, G.; Evangelista, A.; Motta, G.; Motta, G. Procalcitonin for Early Detection of Pharyngocutaneous Fistula after Total Laryngectomy: A Pilot Study. Cancers 2024, 16, 768. https://doi.org/10.3390/cancers16040768
Mesolella M, Allosso S, Petruzzi G, Evangelista A, Motta G, Motta G. Procalcitonin for Early Detection of Pharyngocutaneous Fistula after Total Laryngectomy: A Pilot Study. Cancers. 2024; 16(4):768. https://doi.org/10.3390/cancers16040768
Chicago/Turabian StyleMesolella, Massimo, Salvatore Allosso, Gerardo Petruzzi, Antonietta Evangelista, Giovanni Motta, and Gaetano Motta. 2024. "Procalcitonin for Early Detection of Pharyngocutaneous Fistula after Total Laryngectomy: A Pilot Study" Cancers 16, no. 4: 768. https://doi.org/10.3390/cancers16040768
APA StyleMesolella, M., Allosso, S., Petruzzi, G., Evangelista, A., Motta, G., & Motta, G. (2024). Procalcitonin for Early Detection of Pharyngocutaneous Fistula after Total Laryngectomy: A Pilot Study. Cancers, 16(4), 768. https://doi.org/10.3390/cancers16040768










