Subtype-Specific Survival of Young Women with Breast Cancer and Its Interaction with the Germline BRCA Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Patient Cohort
2.2. Pathology Findings
- TN (Triple-negative: hormone receptor-negative and Her2/neu-negative);
- HR+/Her2− (hormone receptor-positive, Her2/neu-negative);
- HR+/Her2+ (hormone receptor-positive, Her2/neu-positive);
- Her2+/HR− (Her2/neu-positive, hormone receptor-negative).
2.3. Germline Testing
- gBRCAm (pathogenic variant in BRCA1 or BRCA2);
- gBRCAwt (wild type, meaning no pathogenic variant in BRCA1 or BRCA2);
- No data (not tested or missing due to testing in an external institution).
2.4. Tumor and Nodal Stage and Clinical Variables
2.5. Survival Analysis and Survival Endpoints
2.6. Statistical Analysis
3. Results
3.1. Histopathological and Clinical Features
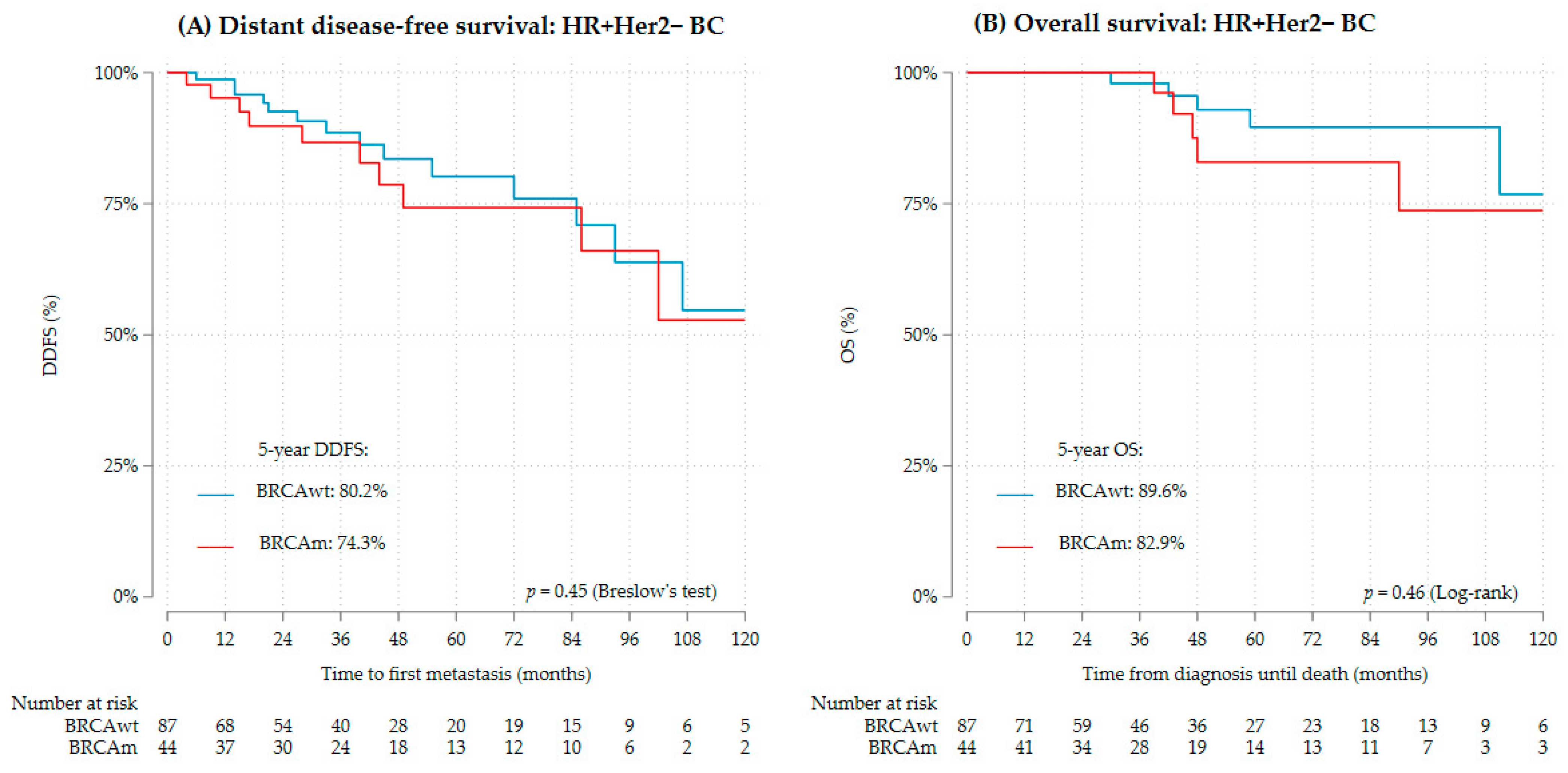
3.2. Distant Disease-Free Survival and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubsky, P.C.; Gnant, M.F.; Taucher, S.; Roka, S.; Kandioler, D.; Pichler-Gebhard, B.; Agstner, I.; Seifert, M.; Sevelda, P.; Jakesz, R. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin. Breast Cancer 2002, 3, 65–72. [Google Scholar] [CrossRef]
- Maggard, M.A.; O’Connell, J.B.; Lane, K.E.; Liu, J.H.; Etzioni, D.A.; Ko, C.Y. Do young breast cancer patients have worse outcomes? J. Surg. Res. 2003, 113, 109–113. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Hughes, M.E.; Warner, E.T.; Ottesen, R.A.; Wong, Y.-N.; Edge, S.B.; Theriault, R.L.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J. Clin. Oncol. 2016, 34, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Jiang, Y.-Z.; Yu, K.-D.; Shao, Z.-M. Different Patterns in the Prognostic Value of Age for Breast Cancer-Specific Mortality Depending on Hormone Receptor Status: A SEER Population-Based Analysis. Ann. Surg. Oncol. 2015, 22, 1102–1110. [Google Scholar] [CrossRef]
- Cathcart-Rake, E.J.; Ruddy, K.J.; Bleyer, A.; Johnson, R.H. Breast Cancer in Adolescent and Young Adult Women Under the Age of 40 Years. JCO Oncol. Pract. 2021, 17, 305–313. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, A.J.; Schmidt, M.K.; van ‘t Veer, L.J.; Tollenaar, R.A.; van Leeuwen, F.E. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: What’s the evidence? A systematic review with meta-analysis. PLoS ONE 2015, 10, e0120189. [Google Scholar] [CrossRef]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef]
- Guzmán-Arocho, Y.D.; Rosenberg, S.M.; Garber, J.E.; Vardeh, H.; Poorvu, P.D.; Ruddy, K.J.; Kirkner, G.; Snow, C.; Tamimi, R.M.; Peppercorn, J.; et al. Clinicopathological features and BRCA1 and BRCA2 mutation status in a prospective cohort of young women with breast cancer. Br. J. Cancer 2022, 126, 302–309. [Google Scholar] [CrossRef]
- Vocka, M.; Zimovjanova, M.; Bielcikova, Z.; Tesarova, P.; Petruzelka, L.; Mateju, M.; Krizova, L.; Kotlas, J.; Soukupova, J.; Janatova, M.; et al. Estrogen Receptor Status Oppositely Modifies Breast Cancer Prognosis in BRCA1/BRCA2 Mutation Carriers Versus Non-Carriers. Cancers 2019, 11, 738. [Google Scholar] [CrossRef]
- Lambertini, M.; Ceppi, M.; Hamy, A.-S.; Caron, O.; Poorvu, P.D.; Carrasco, E.; Grinshpun, A.; Punie, K.; Rousset-Jablonski, C.; Ferrari, A.; et al. Clinical behavior and outcomes of breast cancer in young women with germline BRCA pathogenic variants. npj Breast Cancer 2021, 7, 16. [Google Scholar] [CrossRef]
- Loibl, S.; Jackisch, C.; Lederer, B.; Untch, M.; Paepke, S.; Kümmel, S.; Schneeweiss, A.; Huober, J.; Hilfrich, J.; Hanusch, C.; et al. Outcome after neoadjuvant chemotherapy in young breast cancer patients: A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res. Treat. 2015, 152, 377–387. [Google Scholar] [CrossRef]
- Liu, Z.; Sahli, Z.; Wang, Y.; Wolff, A.C.; Cope, L.M.; Umbricht, C.B. Young age at diagnosis is associated with worse prognosis in the Luminal A breast cancer subtype: A retrospective institutional cohort study. Breast Cancer Res. Treat. 2018, 172, 689–702. [Google Scholar] [CrossRef]
- Gupta, S.; Nair, N.S.; Hawaldar, R. Abstract GS5-01: Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: A phase III randomized controlled trial. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Robert Koch Institute. Datenbankabfrage mit Schätzung der Inzidenz von Krebs in Deutschland auf Basis der epidemiologischen Landeskrebsregisterdaten für 2015–2018; Robert Koch Institute: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Desantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Ruddy, K.J.; Gelber, S.; Shin, J.; Garber, J.E.; Rosenberg, R.; Przypysny, M.; Partridge, A.H. Genetic testing in young women with breast cancer: Results from a Web-based survey. Ann. Oncol. 2010, 21, 741–747. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Ruddy, K.J.; Tamimi, R.M.; Gelber, S.; Schapira, L.; Come, S.; Borges, V.F.; Larsen, B.; Garber, J.E.; Partridge, A.H. BRCA1 and BRCA2 Mutation Testing in Young Women with Breast Cancer. JAMA Oncol. 2016, 2, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Tesch, M.E.; Partridge, A.H. Treatment of Breast Cancer in Young Adults. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Gentilini, O.; et al. ESO–ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann. Oncol. 2022, 33, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Mailliez, A.; D’Hondt, V.; Lusque, A.; Caron, O.; Cabel, L.; Goncalves, A.; Debled, M.; Gladieff, L.; Ferrero, J.M.; Petit, T.; et al. Survival outcomes of metastatic breast cancer patients by germline BRCA1/2 status in a large multicenter real-world database. Int. J. Cancer 2023, 152, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Fielding, S.; Loibl, S.; Janni, W.; Clark, E.; Franzoi, M.A.; Fumagalli, D.; Caballero, C.; Arecco, L.; Salomoni, S.; et al. Impact of Age on Clinical Outcomes and Efficacy of Adjuvant Dual Anti-HER2 Targeted Therapy. J. Natl. Cancer Inst. 2022, 114, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Booser, D.; Valero, V.; Murray, J.L.; Koenig, K.; Esteva, F.J.; Ueno, N.T.; Zhang, J.; Shi, W.; Qi, Y.; et al. Estrogen Receptor (ER) mRNA and ER-Related Gene Expression in Breast Cancers That Are 1% to 10% ER-Positive by Immunohistochemistry. J. Clin. Oncol. 2012, 30, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhong, X.; Fan, Y.; Wu, Y.; Zheng, H.; Luo, T. Clinical characteristics and survival outcome of patients with estrogen receptor low positive breast cancer. Breast 2022, 63, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.L.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, W.; Wali, V.B.; Pongor, L.S.; Li, C.; Lau, R.; Győrffy, B.; Lifton, R.P.; Symmans, W.F.; Pusztai, L.; et al. Predictors of Chemosensitivity in Triple Negative Breast Cancer: An Integrated Genomic Analysis. PLoS Med. 2016, 13, e1002193. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Hahnen, E.; Lederer, B.; Hauke, J.; Loibl, S.; Kröber, S.; Schneeweiss, A.; Denkert, C.; Fasching, P.A.; Blohmer, J.U.; Jackisch, C.; et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer. JAMA Oncol. 2017, 3, 1378. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Almeida-Santos, T. The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers—Systematic review and meta-analysis. Hered. Cancer Clin. Pract. 2019, 17, 11. [Google Scholar] [CrossRef]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; Lederer, B.; Denkert, C.; Schneeweiss, A.; Braun, S.; et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response—Final results from GeparSixto. Ann. Oncol. 2018, 29, 2341–2347. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann. Oncol. 2022, 33, 384–394. [Google Scholar] [CrossRef]
- Tung, N.; Arun, B.; Hacker, M.R.; Hofstatter, E.; Toppmeyer, D.L.; Isakoff, S.J.; Borges, V.; Legare, R.D.; Isaacs, C.; Wolff, A.C.; et al. TBCRC 031: Randomized Phase II Study of Neoadjuvant Cisplatin Versus Doxorubicin-Cyclophosphamide in Germline BRCA Carriers with HER2-Negative Breast Cancer (the INFORM trial). J. Clin. Oncol. 2020, 38, 1539–1548. [Google Scholar] [CrossRef]
- Metzger-Filho, O.; Collier, K.; Asad, S.; Ansell, P.J.; Watson, M.; Bae, J.; Cherian, M.; O’Shaughnessy, J.; Untch, M.; Rugo, H.S.; et al. Matched cohort study of germline BRCA mutation carriers with triple negative breast cancer in brightness. npj Breast Cancer 2021, 7, 142. [Google Scholar] [CrossRef]
- Saha, P.; Regan, M.M.; Pagani, O.; Francis, P.A.; Walley, B.A.; Ribi, K.; Bernhard, J.; Luo, W.; Gómez, H.L.; Burstein, H.J.; et al. Treatment Efficacy, Adherence, and Quality of Life Among Women Younger Than 35 Years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J. Clin. Oncol. 2017, 35, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; De Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.1, 2018 AWMF Registernummer: 032-045OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (accessed on 8 May 2022).


| Patient Characteristics * | Total—n (%) (n = 473) | Clinical Subtype—n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Triple Negative (n = 148, 31.3%) | HR+/Her2− (n = 232, 49.0%) | HR+/Her2+ (n = 65, 13.7%) | Her2+/HR− (n = 28, 5.9%) | ||||||||
| age at initial diagnosis | ≤25 | 14 | (3.0) | 4 | (2.7) | 6 | (2.6) | 4 | (6.2) | 0 | (0.0) |
| 26–30 | 70 | (14.8) | 29 | (19.6) | 27 | (11.6) | 9 | (13.8) | 5 | (17.9) | |
| 31–35 | 184 | (38.8) | 71 | (48.0) | 78 | (33.5) | 24 | (36.9) | 11 | (39.3) | |
| 36–39 | 206 | (43.5) | 44 | (29.7) | 122 | (52.4) | 28 | (43.1) | 12 | (42.9) | |
| median age (range) | 35 | (22–39) | 34 | (22–39) | 36 | (22–39) | 35 | (24–39) | 35 | (26–39) | |
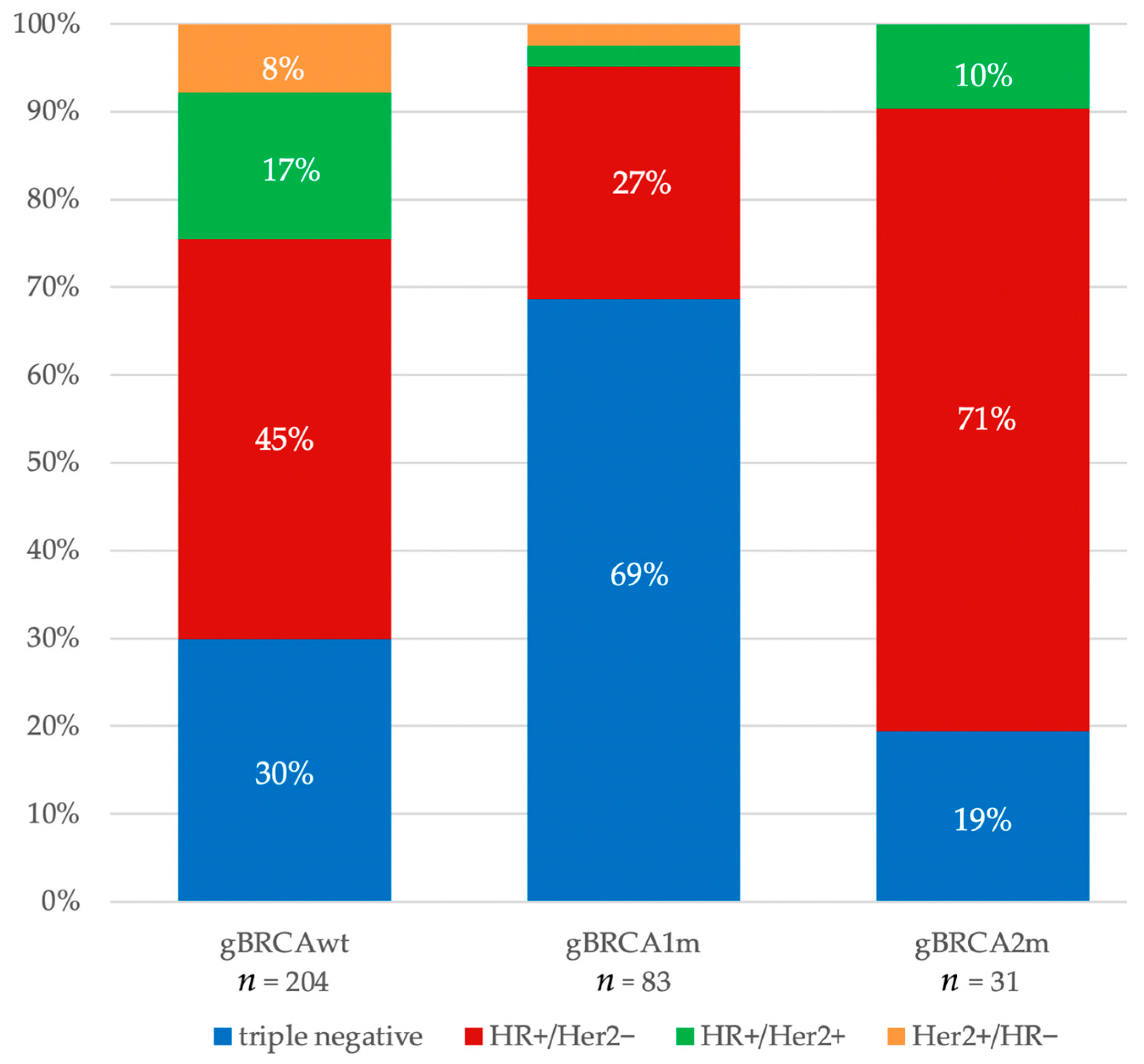
| germline BRCA status | gBRCAwt | 204 | (43.1) | 61 | (41.2) | 93 | (40.1) | 34 | (52.3) | 16 | (57.1) |
| gBRCA1m | 83 | (17.5) | 57 | (38.5) | 22 | (9.5) | 2 | (3.1) | 2 | (7.1) | |
| gBRCA2m | 31 | (6.6) | 6 | (4.1) | 22 | (9.5) | 3 | (4.6) | 0 | (0.0) | |
| gBRCA1m and gBRCA2m | 1 | (0.2) | 1 | (0.7) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |
| not tested/missing | 154 | (32.6) | 23 | (15.5) | 95 | (40.9) | 26 | (40.0) | 10 | (35.7) | |
| histopathology | NST/invasive ductal | 392 | (87.9) | 125 | (88.7) | 185 | (84.9) | 57 | (93.4) | 25 | (96.2) |
| lobular | 23 | (5.1) | 1 | (0.7) | 19 | (8.7) | 3 | (4.9) | 0 | (0.0) | |
| medullary | 11 | (2.5) | 9 | (6.4) | 2 | (0.9) | 0 | (0.0) | 0 | (0.0) | |
| mucinous | 7 | (1.6) | 0 | (0.0) | 7 | (3.2) | 0 | (0.0) | 0 | (0.0) | |
| metaplastic | 7 | (1.6) | 6 | (4.3) | 1 | (0.5) | 0 | (0.0) | 0 | (0.0) | |
| other | 6 | (1.3) | 0 | (0.0) | 4 | (1.8) | 1 | (1.6) | 1 | (3.8) | |
| missing | 27 | 7 | 14 | 4 | 2 | ||||||
| T stage | T1 | 169 | (37.7) | 45 | (32.1) | 91 | (41.0) | 26 | (44.1) | 7 | (25.9) |
| T2 | 207 | (46.2) | 75 | (53.6) | 95 | (42.8) | 25 | (42.4) | 12 | (44.4) | |
| T3 | 54 | (12.1) | 15 | (10.7) | 28 | (12.6) | 6 | (10.2) | 5 | (18.5) | |
| T4 | 18 | (4.0) | 5 | (3.6) | 8 | (3.6) | 2 | (3.4) | 3 | (11.1) | |
| missing | 25 | 8 | 10 | 6 | 1 | ||||||
| nodal involvement | N0 | 222 | (49.6) | 76 | (54.7) | 102 | (46.2) | 36 | (59.0) | 8 | (29.6) |
| N+ | 226 | (50.4) | 63 | (45.3) | 119 | (53.8) | 25 | (41.0) | 19 | (70.4) | |
| missing | 25 | 9 | 11 | 4 | 1 | ||||||
| primary metastasis | M0 | 433 | (92.5) | 143 | (97.3) | 212 | (91.8) | 53 | (85.5) | 25 | (89.3) |
| M1 | 35 | (7.5) | 4 | (2.7) | 19 | (8.2) | 9 | (14.5) | 3 | (10.7) | |
| missing | 5 | 1 | 1 | 3 | 0 | ||||||
| tumor grading | G1 | 23 | (5.2) | 0 | (0.0) | 20 | (90) | 3 | (5.3) | 0 | (0.0) |
| G2 | 179 | (40.3) | 21 | (15.1) | 123 | (55.2) | 27 | (47.4) | 8 | (32.0) | |
| G3 | 242 | (54.5) | 118 | (84.9) | 80 | (35.9) | 27 | (47.4) | 17 | (68.0) | |
| missing | 29 | 9 | 9 | 8 | 3 | ||||||
| Ki67 proliferative index | low (≤15%) | 105 | (24.4) | 3 | (2.3) | 84 | (38.9) | 14 | (23.0) | 4 | (18.2) |
| intermediate | 138 | (32.0) | 28 | (21.2) | 76 | (35.2) | 27 | (44.3) | 7 | (31.8) | |
| high (>35%) | 188 | (43.6) | 101 | (76.5) | 56 | (25.9) | 20 | (32.8) | 11 | (50.0) | |
| missing | 42 | 16 | 16 | 4 | 6 | ||||||
| chemotherapy treatment of first occurrence | no | 70 | (15.0) | 7 | (4.8) | 60 | (26.2) | 3 | (4.8) | 0 | (0.0) |
| yes | 397 | (85.0) | 140 | (95.2) | 169 | (73.8) | 60 | (95.2) | 28 | (100.0) | |
| missing | 6 | 1 | 3 | 2 | 0 | ||||||
| use of platinum com-pounds in chemotherapy of first occurrence | no (other chemo) | 309 | (81.3) | 84 | (61.8) | 157 | (97.5) | 49 | (87.5) | 19 | (70.4) |
| yes | 71 | (18.7) | 52 | (38.2) | 4 | (2.5) | 7 | (12.5) | 8 | (29.6) | |
| missing | 17 | 4 | 8 | 4 | 1 | ||||||
| Factor | Levels | DDFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| age | 1.04 (0.96, 1.14) | 0.332 | 1.08 (0.97, 1.20) | 0.173 | |
| germline BRCA status | gBRCAm | 1.00 | 1.00 | ||
| gBRCAwt | 2.39 (1.17, 4.90) | 0.017 | 7.19 (2.10, 24.61) | 0.002 | |
| T stage | T1 | 1.00 | 0.004 | 1.00 | 0.071 |
| T2 | 5.49 (1.66, 18.19) | 0.005 | 3.76 (1.10, 12.87) | 0.035 | |
| T3/T4 | 9.26 (2.45, 35.00) | 0.001 | 5.11 (1.14, 23.00) | 0.033 | |
| nodal involvement | N0 | 1.00 | 1.00 | ||
| N+ | 3.71 (1.79, 7.69) | <0.001 | 3.90 (1.54, 9.86) | 0.004 | |
| tumor grading | G1/G2 | 1.00 | 1.00 | ||
| G3 | 0.76 (0.33, 1.74) | 0.514 | 1.42 (0.43, 4.75) | 0.566 | |
| Ki67 proliferative | low/intermediate (≤35%) | 1.00 | 1.00 | ||
| index | high (>35%) | 2.29 (0.95, 5.53) | 0.066 | 2.06 (0.74, 5.69) | 0.166 |
| platinum-based | yes | 1.00 | 1.00 | ||
| chemotherapy | other chemo | 1.71 (0.89, 3.31) | 0.109 | 1.12 (0.48, 2.60) | 0.793 |
| Factor | Levels | DDFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| germline BRCA status | gBRCAm | 1.00 | 1.00 | ||
| gBRCAwt | 2.06 (0.98, 4.34) | 0.057 | 6.38 (1.81, 22.49) | 0.004 | |
| nodal status | N0 | 1.00 | 1.00 | ||
| N+ | 5.03 (2.03, 12.44) | <0.001 | 6.42 (1.82, 22.60) | 0.004 | |
| Factor | Levels | DDFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| age | 0.98 (0.92, 1.05) | 0.630 | 0.96 (0.88, 1.04) | 0.291 | |
| germline BRCA status | gBRCAwt | 1.00 | 1.00 | ||
| gBRCAm | 1.26 (0.56, 2.85) | 0.572 | 1.55 (0.48, 5.13) | 0.464 | |
| T stage | T1 | 1.00 | 0.130 | 1.00 | 0.408 |
| T2 | 1.29 (0.67, 2.49) | 0.438 | 1.28 (0.51, 3.20) | 0.598 | |
| T3/T4 | 2.32 (1.02, 5.26) | 0.044 | 2.14 (0.70, 6.56) | 0.182 | |
| nodal involvement | N0 | 1.00 | 1.00 | ||
| N+ | 1.27 (0.70, 2.29) | 0.431 | 1.59 (0.66, 3.83) | 0.306 | |
| tumor grading | G1/G2 | 1.00 | 1.00 | ||
| G3 | 1.96 (1.11, 3.46) | 0.020 | 3.14 (1.34, 7.34) | 0.008 | |
| Ki67 proliferative | low/intermediate (≤35%) | 1.00 | 1.00 | ||
| index | high (>35%) | 1.18 (0.60, 2.29) | 0.635 | 0.95 (0.35, 2.59) | 0.917 |
| chemotherapy | no chemo | 1.00 | 1.00 | ||
| yes | 1.25 (0.64, 2.43) | 0.513 | 1.78 (0.61, 5.17) | 0.291 | |
| Factor | Levels | DDFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| germline BRCA status | gBRCAwt | 1.00 | 1.00 | ||
| gBRCAm | 1.71 (0.73, 4.00) | 0.217 | 1.66 (0.50, 5.53) | 0.411 | |
| T stage | T1 | 1.00 | |||
| T2 | 1.02 (0.40, 2.61) | 0.969 | - | - | |
| T3/T4 | 3.58 (1.04, 12.27) | 0.042 | |||
| tumor grading | G1/G2 | 1.00 | 1.00 | ||
| G3 | 2.37 (0.99, 5.71) | 0.054 | 1.74 (0.53, 5.75) | 0.368 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hage, A.M.; Gebert, P.; Blohmer, J.-U.; Hedayati, E.; Speiser, D.; Karsten, M.M. Subtype-Specific Survival of Young Women with Breast Cancer and Its Interaction with the Germline BRCA Status. Cancers 2024, 16, 738. https://doi.org/10.3390/cancers16040738
Hage AM, Gebert P, Blohmer J-U, Hedayati E, Speiser D, Karsten MM. Subtype-Specific Survival of Young Women with Breast Cancer and Its Interaction with the Germline BRCA Status. Cancers. 2024; 16(4):738. https://doi.org/10.3390/cancers16040738
Chicago/Turabian StyleHage, Anna Maria, Pimrapat Gebert, Jens-Uwe Blohmer, Elham Hedayati, Dorothee Speiser, and Maria Margarete Karsten. 2024. "Subtype-Specific Survival of Young Women with Breast Cancer and Its Interaction with the Germline BRCA Status" Cancers 16, no. 4: 738. https://doi.org/10.3390/cancers16040738
APA StyleHage, A. M., Gebert, P., Blohmer, J.-U., Hedayati, E., Speiser, D., & Karsten, M. M. (2024). Subtype-Specific Survival of Young Women with Breast Cancer and Its Interaction with the Germline BRCA Status. Cancers, 16(4), 738. https://doi.org/10.3390/cancers16040738






