Reasons for Treatment Discontinuation and Their Effect on Outcomes of Immunotherapy in Southwest Finland: A Retrospective, Real-World Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Treatment Characteristics
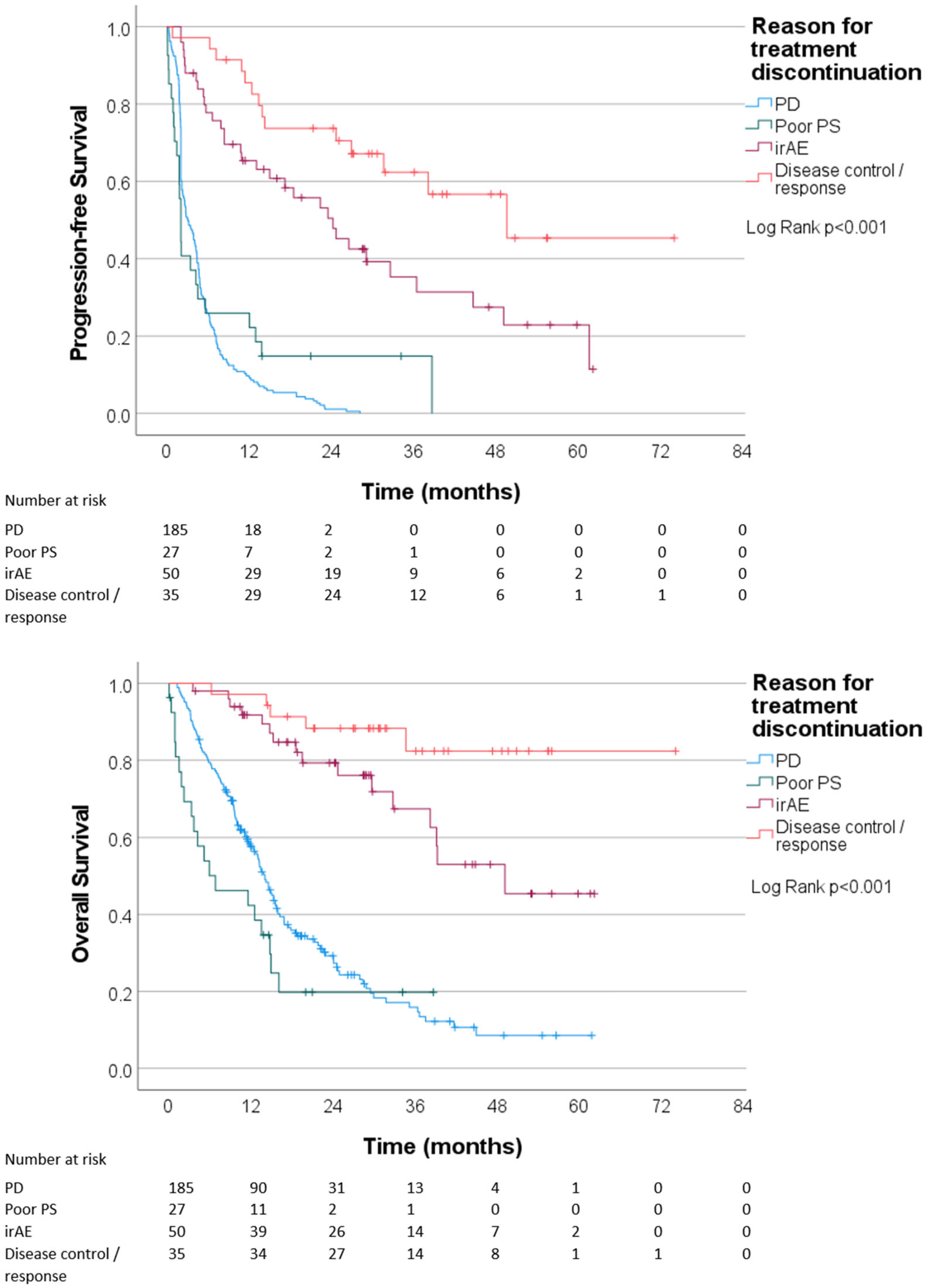
3.2. Reasons for Treatment Discontinuation
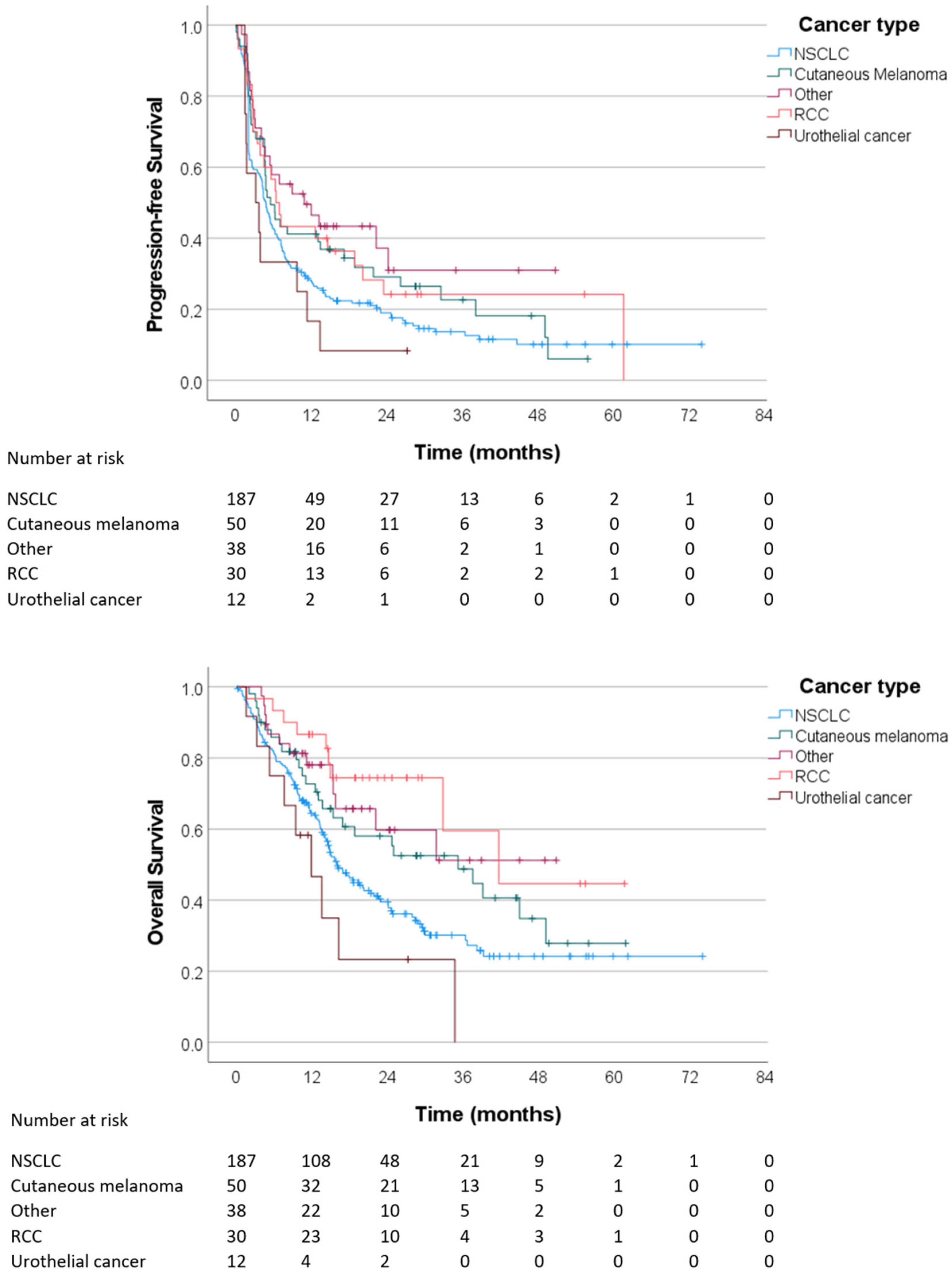
3.3. Outcomes of Immunotherapy
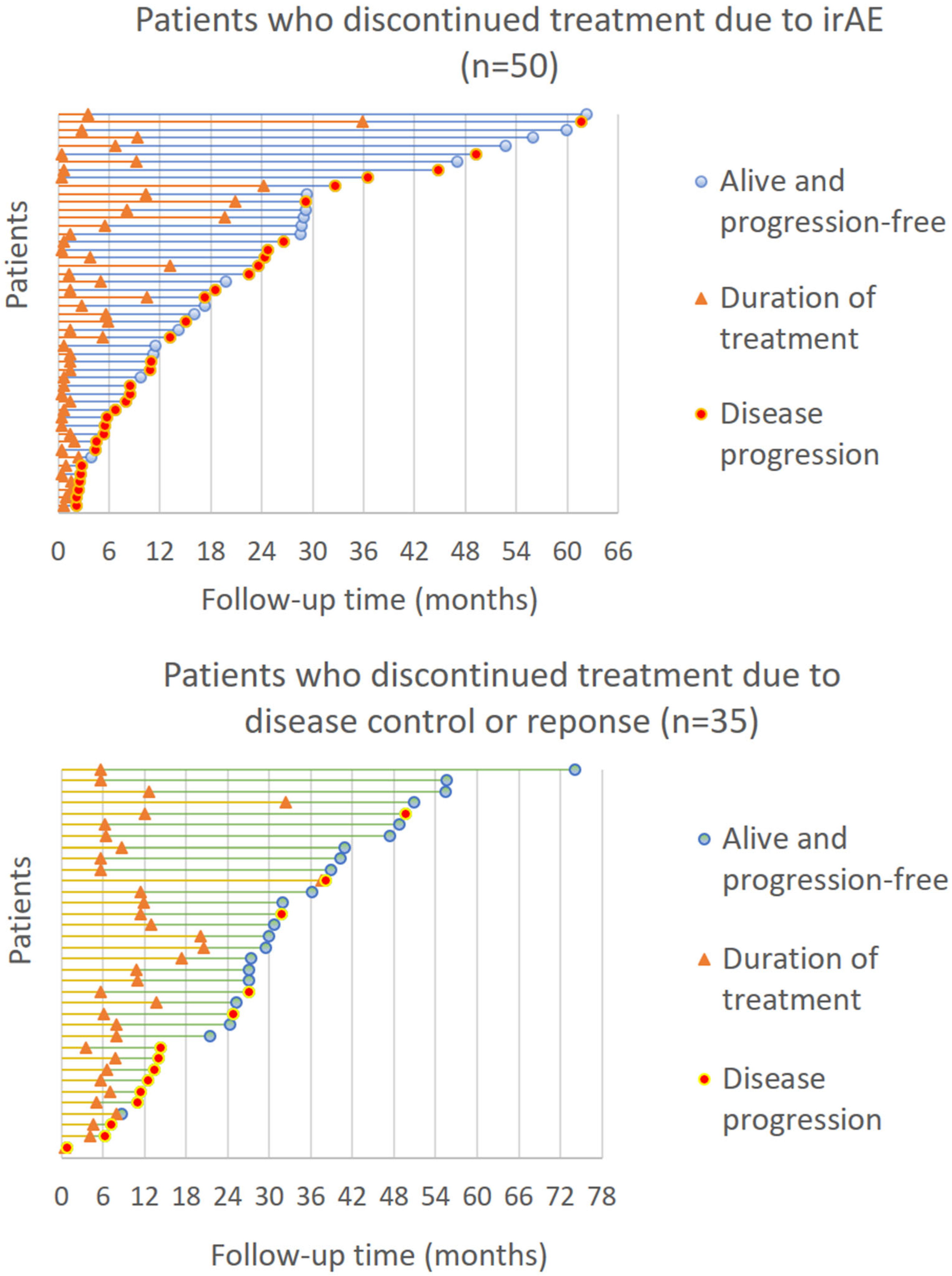
3.4. Disease Progression after Treatment Discontinuation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/homepage (accessed on 1 November 2023).
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab Versus Everolimus in Patients with Advanced Renal Cell Carcinoma: Updated Results with Long-Term Follow-Up of the Randomized, Open-Label, Phase 3 CheckMate 025 Trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes with Nivolumab in Patients with Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020, 38, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Basak, E.A.; Vermeer, N.S.; de Joode, K.; Hurkmans, D.P.; Velthuis, D.E.M.; Oomen-de Hoop, E.; Schreurs, M.W.; Bins, S.; Koolen, S.L.; Debets, R.; et al. Associations between patient and disease characteristics and severe adverse events during immune checkpoint inhibitor treatment: An observational study. Eur. J. Cancer 2022, 174, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Karhapää, H.; Mäkelä, S.; Laurén, H.; Jaakkola, M.; Schalin-Jäntti, C.; Hernberg, M. Immune checkpoint inhibitors, endocrine adverse events, and outcomes of melanoma. Endocr. Connect. 2022, 11, e210562. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Robert, C.; Hwu, W.J.; Hamid, O.; Ribas, A.; Weber, J.S.; Daud, A.I.; Hodi, F.S.; Wolchok, J.D.; Mitchell, T.C.; Hersey, P.; et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur. J. Cancer 2021, 144, 182–191. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Plimack, E.R.; Soulières, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Shah, A.Y.; Suárez, C.; Hamzaj, A.; Porta, C.; Hocking, C.M.; et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.K.; Suijkerbuijk, K.P.M.; de Boer, A.; van Dartel, M.; Hilarius, D.L.; Pasmooij, A.M.G.; van Zeijl, M.C.; Aarts, M.J.; Berkmortel, F.W.v.D.; Blank, C.U.; et al. Long-term survival of patients with advanced melanoma treated with BRAF-MEK inhibitors. Melanoma Res. 2022, 32, 460–468. [Google Scholar] [CrossRef]
- Donia, M.; Kimper-Karl, M.L.; Høyer, K.L.; Bastholt, L.; Schmidt, H.; Svane, I.M. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur. J. Cancer 2017, 74, 89–95. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. RECIST working group. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Frelaut, M.; Le Tourneau, C.; Borcoman, E. Hyperprogression under Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2674. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Filleron, T.; Virazels, M.; Dufau, C.; Milhès, J.; Pagès, C.; Olivier, P.; Ayyoub, M.; Mounier, M.; Lusque, A.; et al. Combining Nivolumab and Ipilimumab with Infliximab or Certolizumab in Patients with Advanced Melanoma: First Results of a Phase Ib Clinical Trial. Clin. Cancer Res. 2021, 27, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.; Montazari, E.; Spillson, C.; Bentebibel, S.E.; Awiwi, M.; Elsayes, K.M.; Gao, J.; Altan, M.; Wong, M.K.K.; Glitza, I.C.; et al. Tocilizumab in combination with ipilimumab and nivolumab in solid tumors. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS9600. [Google Scholar] [CrossRef]
- Shalata, W.; Zolnoorian, J.; Migliozzi, G.; Jama, A.A.; Dudnik, Y.; Cohen, A.Y.; Meirovitz, A.; Yakobson, A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. Int. J. Mol. Sci. 2023, 24, 5938. [Google Scholar] [CrossRef]
- Mäkelä, S.; Kohtamäki, L.; Laukka, M.; Juteau, S.; Hernberg, M. Limited-duration anti-PD-1 therapy for patients with metastatic melanoma. Acta Oncol. 2020, 59, 438–443. [Google Scholar] [CrossRef]
- Jansen, Y.J.L.; Rozeman, E.A.; Mason, R.; Goldinger, S.M.; Geukes Foppen, M.H.; Hoejberg, L.; Schmidt, H.; van Thienen, J.V.; Haanen, J.B.A.G.; Tiainen, L.; et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann. Oncol. 2019, 30, 1154–1161. [Google Scholar] [CrossRef]
- Sun, L.; Bleiberg, B.; Hwang, W.T.; Marmarelis, M.E.; Langer, C.J.; Singh, A.; Cohen, R.B.; Mamtani, R.; Aggarwal, C. Association Between Duration of Immunotherapy and Overall Survival in Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2023, 9, 1075–1082. [Google Scholar] [CrossRef]
- Seremet, T.; Jansen, Y.; Planken, S.; Njimi, H.; Delaunoy, M.; El Housni, H.; Awada, G.; Schwarze, J.K.; Keyaerts, M.; Everaert, H.; et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J. Transl. Med. 2019, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Mattila, K.E.; Mäkelä, S.; Kytölä, S.; Andersson, E.; Vihinen, P.; Ramadan, S.; Skyttä, T.; Tiainen, L.; Vuoristo, M.-S.; Tyynelä-Korhonen, K.; et al. Circulating tumor DNA is a prognostic biomarker in metastatic melanoma patients treated with chemoimmunotherapy and BRAF inhibitor. Acta Oncol. 2022, 61, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef] [PubMed]
| Variable | Result |
|---|---|
| Median age | 67 (27–90) years |
| Sex: Male | 197 (62%) |
| Sex: Female | 120 (38%) |
| ECOG performance status: 0 | 88 (28%) |
| ECOG performance status: 1 | 203 (64%) |
| ECOG performance status: ≥2 | 26 (8%) |
| Cancer type: Non-small cell lung cancer | 187 (59%) |
| Cancer type: Cutaneous melanoma | 50 (16%) |
| Cancer type: Renal cell carcinoma | 30 (9%) |
| Cancer type: Urothelial cancer | 12 (4%) |
| Cancer type: Other | 38 (12%) |
| Brain metastases prior to ICI: not present | 296 (93%) |
| Brain metastases prior to ICI: present | 21 (7%) |
| Treatment type: PD-1/L1 inhibitor | 276 (87%) |
| Treatment type: CTLA-4 + PD-1 inhibitor | 13 (4%) |
| Treatment type: PD-1/L1 + chemotherapy | 27 (9%) |
| Treatment type: CTLA-4 inhibitor | 1 (0.3%) |
| Treatment line: First-line | 141 (44%) |
| Treatment line: Later line | 176 (56%) |
| Median duration of treatment | 2.8 (0.5–37.5) months |
| Duration of treatment: <6 months | 240 (76%) |
| Duration of treatment: 6–12 months | 43 (14%) |
| Duration of treatment: >12 months | 34 (11%) |
| Received systemic cancer treatment after ICI | 165 (52%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtanen, S.; Pihlman, H.; Silvoniemi, M.; Vihinen, P.; Jaakkola, P.; Mattila, K.E. Reasons for Treatment Discontinuation and Their Effect on Outcomes of Immunotherapy in Southwest Finland: A Retrospective, Real-World Cohort Study. Cancers 2024, 16, 709. https://doi.org/10.3390/cancers16040709
Virtanen S, Pihlman H, Silvoniemi M, Vihinen P, Jaakkola P, Mattila KE. Reasons for Treatment Discontinuation and Their Effect on Outcomes of Immunotherapy in Southwest Finland: A Retrospective, Real-World Cohort Study. Cancers. 2024; 16(4):709. https://doi.org/10.3390/cancers16040709
Chicago/Turabian StyleVirtanen, Saana, Heidi Pihlman, Maria Silvoniemi, Pia Vihinen, Panu Jaakkola, and Kalle E. Mattila. 2024. "Reasons for Treatment Discontinuation and Their Effect on Outcomes of Immunotherapy in Southwest Finland: A Retrospective, Real-World Cohort Study" Cancers 16, no. 4: 709. https://doi.org/10.3390/cancers16040709
APA StyleVirtanen, S., Pihlman, H., Silvoniemi, M., Vihinen, P., Jaakkola, P., & Mattila, K. E. (2024). Reasons for Treatment Discontinuation and Their Effect on Outcomes of Immunotherapy in Southwest Finland: A Retrospective, Real-World Cohort Study. Cancers, 16(4), 709. https://doi.org/10.3390/cancers16040709






