Comprehensive Examination of Cholangiocarcinoma Patients Treated with Novel Targeted Therapies after Extended Molecular Profiling on Liquid Biopsies
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Statistical Considerations
3. Results
3.1. Baseline Characteristics
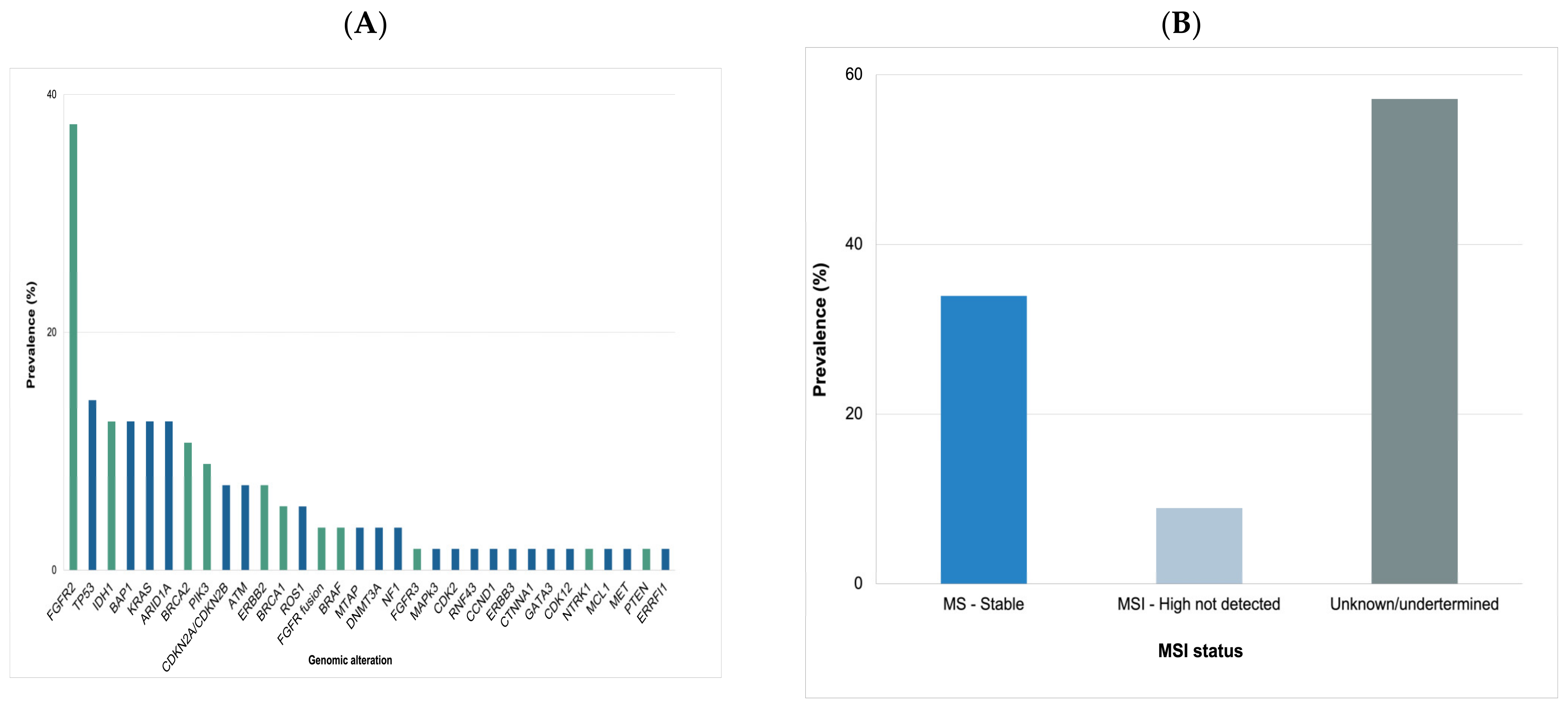
3.2. Molecular Analysis
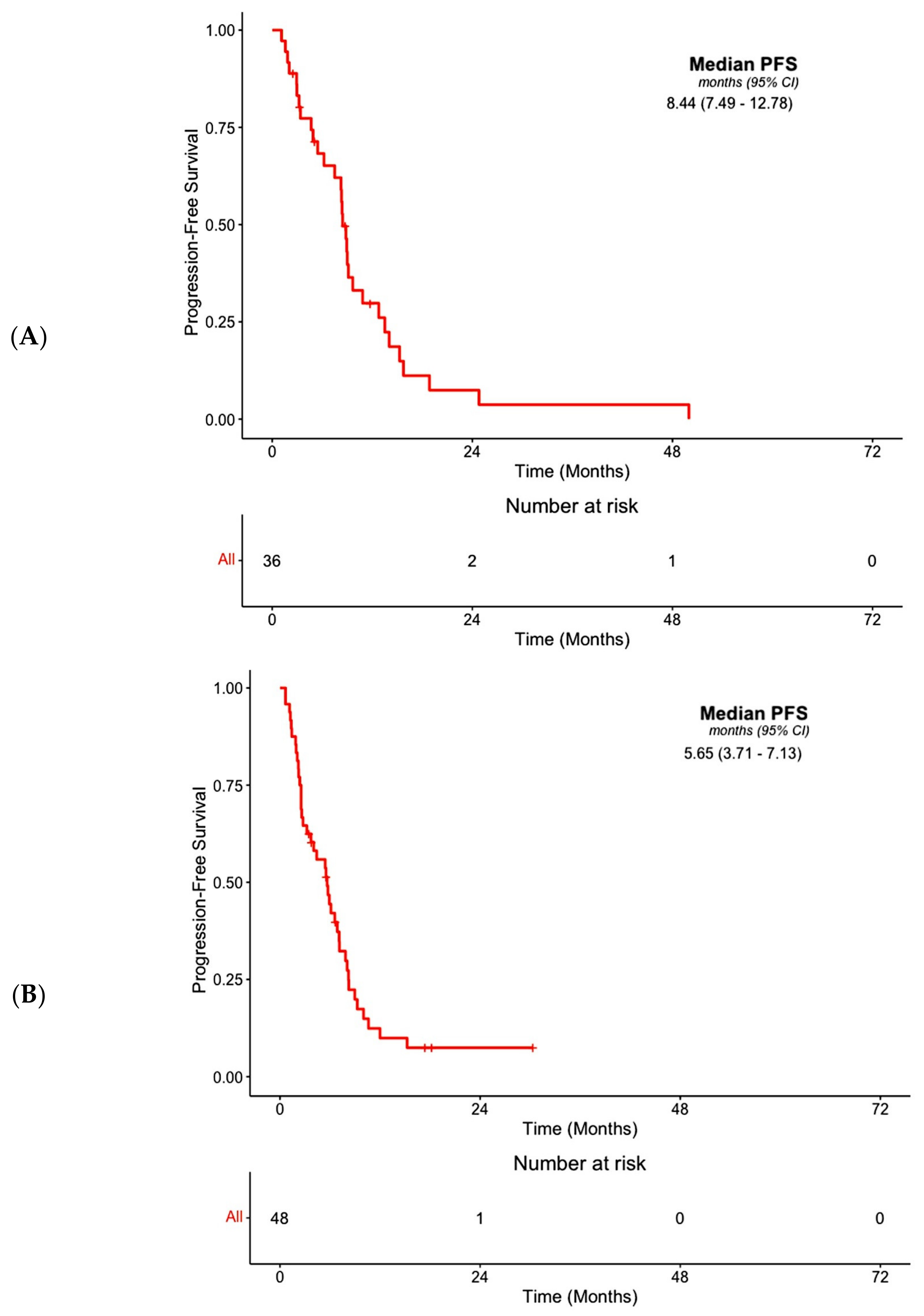
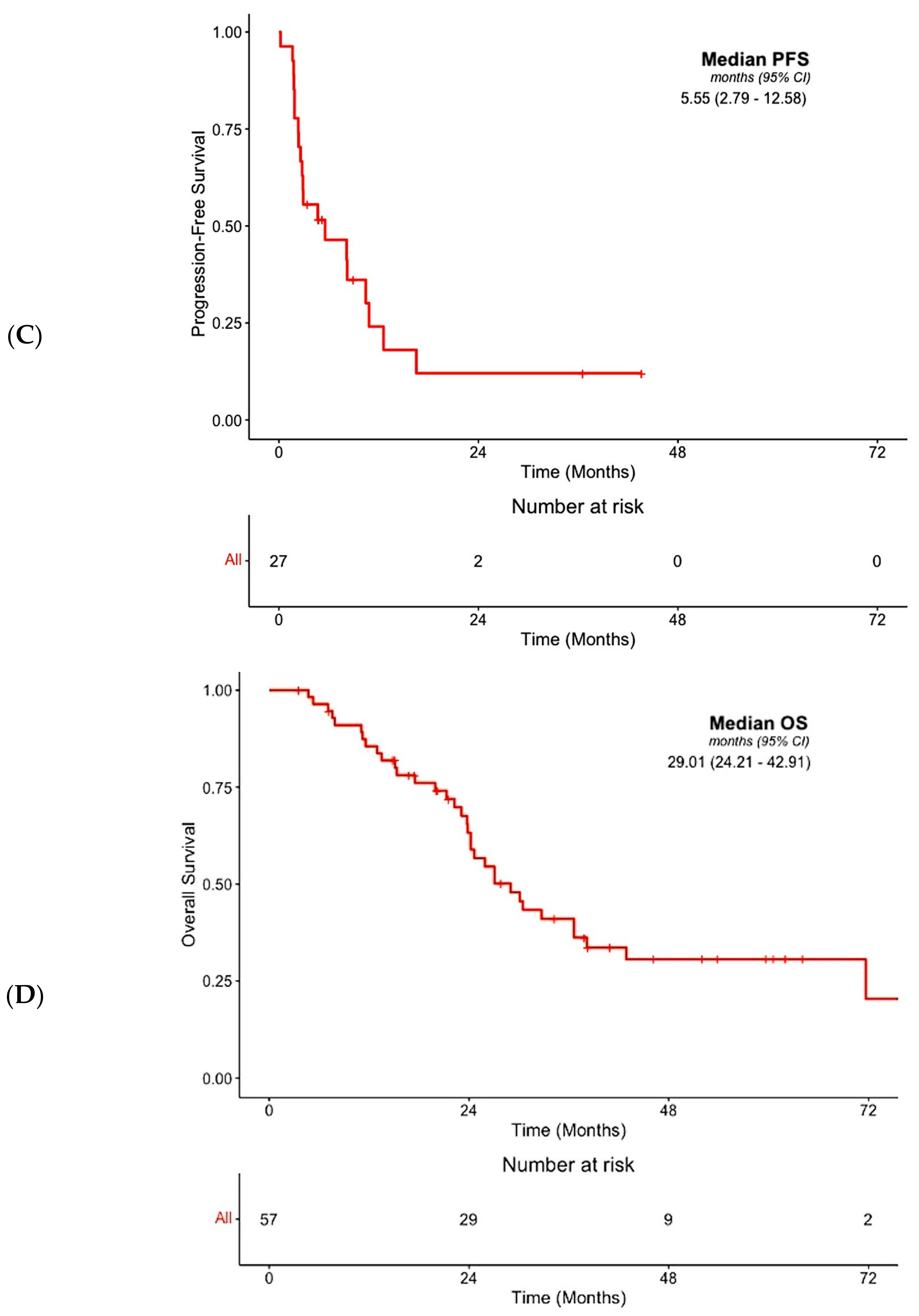
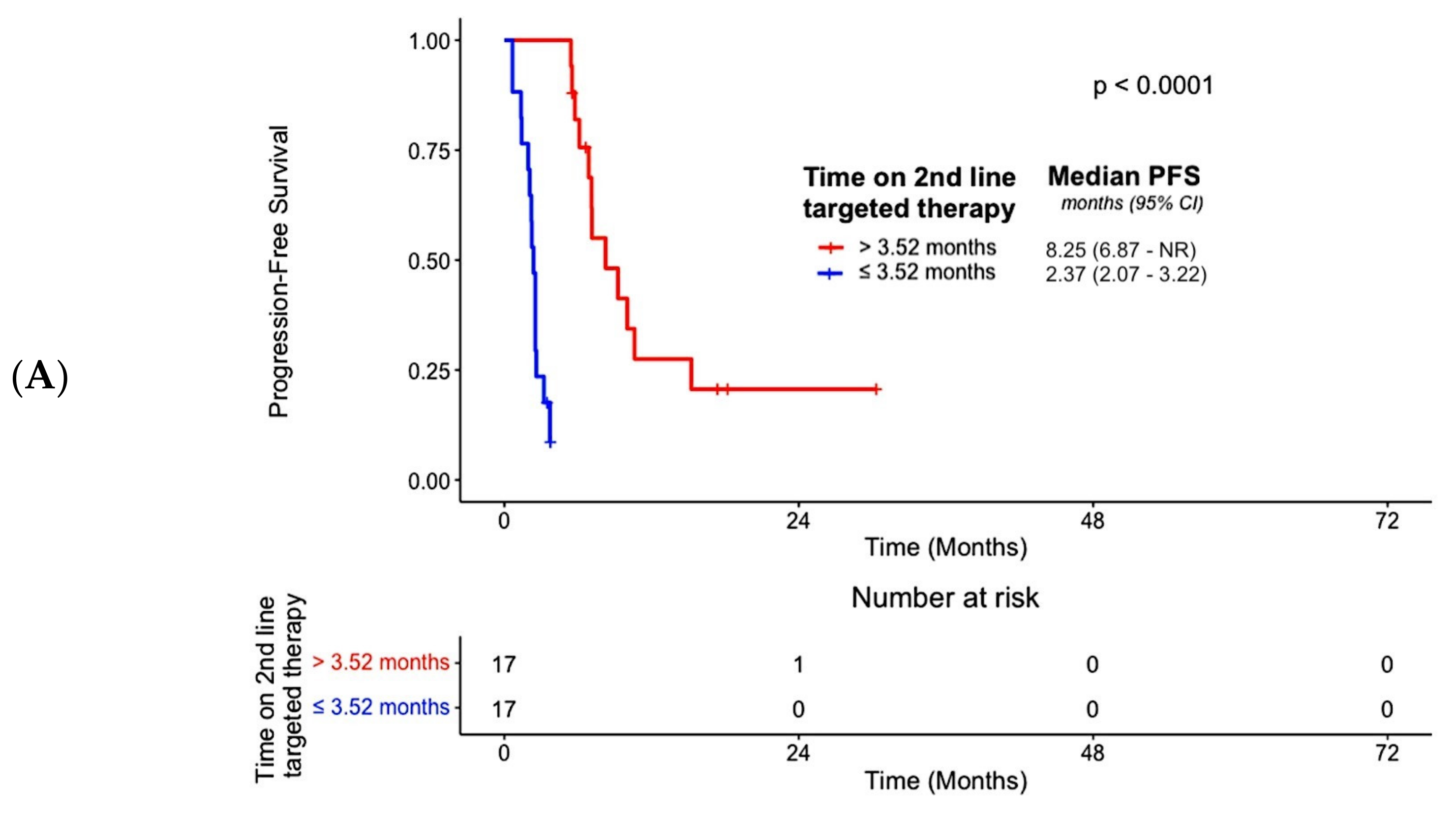
3.3. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.C.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in Combination with Gemcitabine and Cisplatin Compared with Gemcitabine and Cisplatin Alone for Patients with Advanced Biliary Tract Cancer (KEYNOTE-966): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma With IDH1 Mutation. JAMA Oncol 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/search/microsatellite%20instability (accessed on 16 January 2024).
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-Line FOLFOX Chemotherapy versus Active Symptom Control for Advanced Biliary Tract Cancer (ABC-06): A Phase 3, Open-Label, Randomised, Controlled Trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, S.; Borad, M.; Gallinson, D.; Murphy, A.; et al. O-2 Pemigatinib for Previously Treated Locally Advanced or Metastatic Cholangiocarcinoma: Final Results from FIGHT-202. Ann. Oncol. 2022, 33, S379. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017, 7, 252–263. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Pant, S.; Schuler, M.H.; Iyer, G.; Doi, T.; Qin, S.; Tabernero, J.; Arnold, D.; Gutierrez, M.; Prenen, H.; Folprecht, G.; et al. Efficacy and Safety of Erdafitinib in Adults with Cholangiocarcinoma (CCA) with Prespecified Fibroblast Growth Factor Receptor Alterations (FGFRalt) in the Phase 2 Open-Label, Single-Arm RAGNAR Trial: Expansion Cohort Results. J. Clin. Oncol. 2023, 41 (Suppl. S4), 610. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intra- and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Kalmár, A.; Galamb, O.; Szabó, G.; Pipek, O.; Medgyes-Horváth, A.; Barták, B.K.; Nagy, Z.B.; Szigeti, K.A.; Zsigrai, S.; Csabai, I.; et al. Patterns of Somatic Variants in Colorectal Adenoma and Carcinoma Tissue and Matched Plasma Samples from the Hungarian Oncogenome Program. Cancers 2023, 15, 907. [Google Scholar] [CrossRef]
- Wallander, K.; Haider, Z.; Jeggari, A.; Foroughi-Asl, H.; Gellerbring, A.; Lyander, A.; Chozhan, A.; Cuba Gyllensten, O.; Hägglund, M.; Wirta, V.; et al. Sensitive Detection of Cell-Free Tumour DNA Using Optimised Targeted Sequencing Can Predict Prognosis in Gastro-Oesophageal Cancer. Cancers 2023, 15, 1160. [Google Scholar] [CrossRef]
- Brocco, D.; Simeone, P.; Buca, D.; Marino, P.D.; De Tursi, M.; Grassadonia, A.; De Lellis, L.; Martino, M.T.; Veschi, S.; Iezzi, M.; et al. Blood Circulating CD133+ Extracellular Vesicles Predict Clinical Outcomes in Patients with Metastatic Colorectal Cancer. Cancers 2022, 14, 1357. [Google Scholar] [CrossRef]
- Kasi, P.M. Favorable Outcomes in FGFR Fusion-Positive Cholangiocarcinomas and Evolution on Treatment Noted on Circulating Tumor DNA Liquid Biopsies. Case Rep. Oncol. 2020, 13, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid Biopsy-Based Protein Biomarkers for Risk Prediction, Early Diagnosis, and Prognostication of Cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid Biopsies: Potential and Challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Storandt, M.H.; Kurniali, P.C.; Mahipal, A.; Jin, Z. Targeted Therapies in Advanced Cholangiocarcinoma. Life 2023, 13, 2066. [Google Scholar] [CrossRef]
- Jarada, T.N.; O’Sullivan, D.E.; Brenner, D.R.; Cheung, W.Y.; Boyne, D.J. Selection Bias in Real-World Data Studies Used to Support Health Technology Assessments: A Case Study in Metastatic Cancer. Curr. Oncol. 2023, 30, 1945–1953. [Google Scholar] [CrossRef]
- Koido, S.; Kan, S.; Yoshida, K.; Yoshizaki, S.; Takakura, K.; Namiki, Y.; Tsukinaga, S.; Odahara, S.; Kajihara, M.; Okamoto, M.; et al. Immunogenic Modulation of Cholangiocarcinoma Cells by Chemoimmunotherapy. Anticancer Res. 2014, 34, 6353–6361. [Google Scholar]
- Sawasdee, N.; Thepmalee, C.; Sujjitjoon, J.; Yongpitakwattana, P.; Junking, M.; Poungvarin, N.; Yenchitsomanus, P.; Panya, A. Gemcitabine Enhances Cytotoxic Activity of Effector T-Lymphocytes against Chemo-Resistant Cholangiocarcinoma Cells. Int. Immunopharmacol. 2020, 78, 106006. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Nakashima, A.; Kondo, N.; Sakabe, R.; Ohge, H.; Sueda, T. Prognostic Factors After Surgical Resection for Intrahepatic, Hilar, and Distal Cholangiocarcinoma. Ann. Surg. Oncol. 2011, 18, 651–658. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; Walter, T.; Horgan, A.M.; Amir, E.; Cleary, S.; McKeever, E.L.; Min, T.; Wallace, E.; Hedley, D.; Krzyzanowska, M.; et al. Outcome of Adjuvant Therapy in Biliary Tract Cancers. Am. J. Clin. Oncol. 2015, 38, 382. [Google Scholar] [CrossRef]
- Hester, C.; Nassour, I.; Adams-Huet, B.; Mathew, A.; Choti, M.A.; Minter, R.M.; Mansour, J.C.; Polanco, P.M.; Porembka, M.R.; Wang, S.C.; et al. Improved Survival in Surgically Resected Distal Cholangiocarcinoma Treated with Adjuvant Therapy: A Propensity Score Matched Analysis. J. Gastrointest. Surg. 2018, 22, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D’Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Fonkoua, L.A.K.; Mahipal, A.; Liao, C.-Y.; Fountzilas, C.; Li, D.; Pelster, M.S.; Goel, S.; Peng, P.; Sun, C.; et al. 95MO Tinengotinib in Patients with Advanced, Fibroblast Growth Factor Receptor (FGFR) Inhibitor Refractory/Relapsed Cholangiocarcinoma. Ann. Oncol. 2023, 34, S215–S216. [Google Scholar] [CrossRef]
- Shah, P.; Kendall, F.; Khozin, S.; Goosen, R.; Hu, J.; Laramie, J.; Ringel, M.; Schork, N. Artificial Intelligence and Machine Learning in Clinical Development: A Translational Perspective. Npj Digit. Med. 2019, 2, 69. [Google Scholar] [CrossRef]

| Total Number of Subjects | ||
|---|---|---|
| N | % | |
| Age at CCA diagnosis (years) | ||
| Median | 59 | |
| Range | 22–78 | |
| Interquartile range | 52–66 | |
| Gender | ||
| Male | 23 | 41 |
| Female | 33 | 59 |
| Smoking Status | ||
| Never Smoker | 13 | 23 |
| Current Smoker | 1 | 2 |
| Former Smoker | 11 | 20 |
| Unknown | 31 | 55 |
| ECOG Performance Status prior to commencing 1st targeted therapy | ||
| ECOG 0 | 5 | 9 |
| ECOG 1 | 18 | 32 |
| Unknown | 33 | 59 |
| CCA subtype | ||
| Hilar | 4 | 7 |
| Intrahepatic | 32 | 57 |
| Extrahepatic | 11 | 20 |
| Unknown | 9 | 16 |
| Stage at initial diagnosis | ||
| Localised | 26 | 46 |
| Metastatic | 26 | 46 |
| Unknown | 4 | 8 |
| Anatomic site of metastasis prior to commencing 1st targeted therapy | ||
| Lung | 17 | 30 |
| Liver | 36 | 64 |
| Bone | 5 | 9 |
| Brain | 1 | 2 |
| Lymph node | 18 | 32 |
| Prior treatment | ||
| Any CCA-related surgery | 23 | 41 |
| Radiation therapy | 3 | 5 |
| Co-morbidities | ||
| Hypertension | 11 | 20 |
| Hypercholesterolemia | 2 | 4 |
| Transient ischemic attack | 2 | 4 |
| Supraventricular tachycardia | 1 | 2 |
| Atrial fibrillation | 1 | 2 |
| Asthma | 3 | 5 |
| Chronic Obstructive Pulmonary Disease | 2 | 4 |
| Hypothyroidism | 3 | 5 |
| Right-sided gynecomastia | 1 | 2 |
| Anxiety/mixed anxiety and depressive disorder | 1 | 2 |
| Low mood | 1 | 2 |
| Migraine | 1 | 2 |
| Alzheimer’s disease | 1 | 2 |
| Celiac disease | 1 | 2 |
| Inflammatory Bowel Syndrome | 2 | 4 |
| Bowel adhesions | 1 | 2 |
| Ulcerative colitis | 1 | 2 |
| Acid reflux | 1 | 2 |
| Barrett’s oesophagus | 1 | 2 |
| Type 1 diabetes mellitus | 1 | 2 |
| Type 2 diabetes mellitus | 6 | 11 |
| Controlled HIV | 1 | 2 |
| Chronic Hepatitis C | 1 | 2 |
| Chronic Kidney Disease (Stage 1) | 1 | 2 |
| Chronic periodontitis | 1 | 2 |
| Osteoarthritis | 2 | 4 |
| Osteoporosis | 1 | 2 |
| Vitamin D deficiency | 1 | 2 |
| Alopecia | 1 | 2 |
| Eczema | 1 | 2 |
| Benign Prostatic Hyperplasia | 1 | 2 |
| Total Number of Subjects | ||
|---|---|---|
| N | % | |
| Number of lines of systemic therapy | ||
| 1st-line therapy | 1 | 2 |
| 2nd-line therapy | 25 | 45 |
| 3rd-line therapy | 21 | 38 |
| 4th-line therapy | 6 | 11 |
| 5th-line therapy | 0 | 0 |
| 6th-line therapy | 3 | 5 |
| 1st-Line systemic therapy | 56 | 100 |
| Chemotherapy | 52 | 93 |
| Chemotherapy + immunotherapy | 3 | 5 |
| Targeted agents | 1 | 2 |
| 2nd-Line systemic therapy | 55 | 100 |
| Chemotherapy | 20 | 36 |
| Targeted agents | 34 | 62 |
| Targeted agents + chemotherapy | 1 | 2 |
| 3rd-Line systemic therapy | 29 | 100 |
| Chemotherapy | 10 | 34 |
| Targeted agents | 17 | 59 |
| Targeted agents + chemotherapy | 1 | 3 |
| Targeted agents + immunotherapy | 1 | 3 |
| 4th-Line systemic therapy | 9 | 100 |
| Chemotherapy | 3 | 33 |
| Targeted agents | 6 | 67 |
| 5th-Line systemic therapy | 3 | 100 |
| Chemotherapy | 3 | 100 |
| 6th-Line systemic therapy | 3 | 100 |
| Chemotherapy | 1 | 33 |
| Targeted agents | 2 | 67 |
| Patients receiving 1st-line treatment (targeted agents) based on genomic profiling results | ||
| Yes | 1 | 100 |
| No | 0 | 0 |
| Patients receiving 2nd-line treatment (targeted agents) based on genomic profiling results | ||
| Yes | 32 | 91 |
| No | 3 | 9 |
| Patients receiving 3rd-line treatment (targeted agents) based on genomic profiling results | ||
| Yes | 16 | 84 |
| No | 3 | 16 |
| Patients receiving 4th-line treatment (targeted agents) based on genomic profiling results | ||
| Yes | 6 | 100 |
| No | 0 | 0 |
| Patients receiving 6th-line treatment (targeted agents) based on genomic profiling results | ||
| Yes | 2 | 100 |
| No | 0 | 0 |
| PFS (1st-Line Systemic Treatment) | PFS (2nd-Line Systemic Treatment) | PFS (3rd-Line Systemic Treatment) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate HR (95% CI) | p-Value | Adjusted HR (95% CI) a | p-Value | Univariate HR (95% CI) | p-Value | Adjusted HR (95% CI) a | p-Value | Univariate HR (95% CI) | p-Value | Adjusted HR (95% CI) a | p-Value | |
| Age (<70 years vs. ≥70 years) | 2.29 (0.78–6.76) | 0.13 | N/A | N/A | 0.56 (0.23–1.37) | 0.20 | N/A | N/A | 1.06 (0.31–3.67) | 0.93 | N/A | N/A |
| Gender (Male vs. Female) | 0.91 (0.43–1.94) | 0.82 | N/A | N/A | 0.85 (0.45–1.59) | 0.61 | N/A | N/A | 1.16 (0.48–2.81) | 0.75 | N/A | N/A |
| Smoking status (Current or former smoker vs. never smoker) | 1.73 (0.51–5.91) | 0.38 | N/A | N/A | 1.06 (0.43–2.62) | 0.91 | N/A | N/A | 1.19 (0.26–5.33) | 0.82 | N/A | N/A |
| CCA subtype | ||||||||||||
| Hilar | - | - | - | - | - | - | - | - | - | - | - | - |
| Intrahepatic | 0.75 (0.17–3.31) | 0.70 | N/A | N/A | 0.72 (0.25–2.10) | 0.55 | N/A | N/A | 0.22 (0.02–1.88) | 0.16 | N/A | N/A |
| Extrahepatic | 0.56 (0.11–3.07) | 0.53 | N/A | N/A | 0.56 (0.17–1.89) | 0.35 | N/A | N/A | 0.13 (0.01–1.37) | 0.09 | N/A | N/A |
| FGFR status (Pathogenic variant vs. wild type) | 2.27 (0.99–5.22) | 0.05 | N/A | N/A | 0.67 (0.35–1.29) | 0.23 | N/A | N/A | 0.74 (0.30–1.83) | 0.51 | N/A | N/A |
| BRCA status (Pathogenic variant vs. wild type) | 0.58 (0.23–1.46) | 0.25 | N/A | N/A | 1.64 (0.76–3.51) | 0.20 | N/A | N/A | 3.64 (1.25–10.57) | 0.02 | 3.99 (0.91–17.53) | 0.07 |
| Prior CCA-related surgical history | 0.98 (0.46–2.08) | 0.96 | N/A | N/A | 1.06 (0.56–2.00) | 0.86 | N/A | N/A | 1.12 (0.46–2.70) | 0.81 | N/A | N/A |
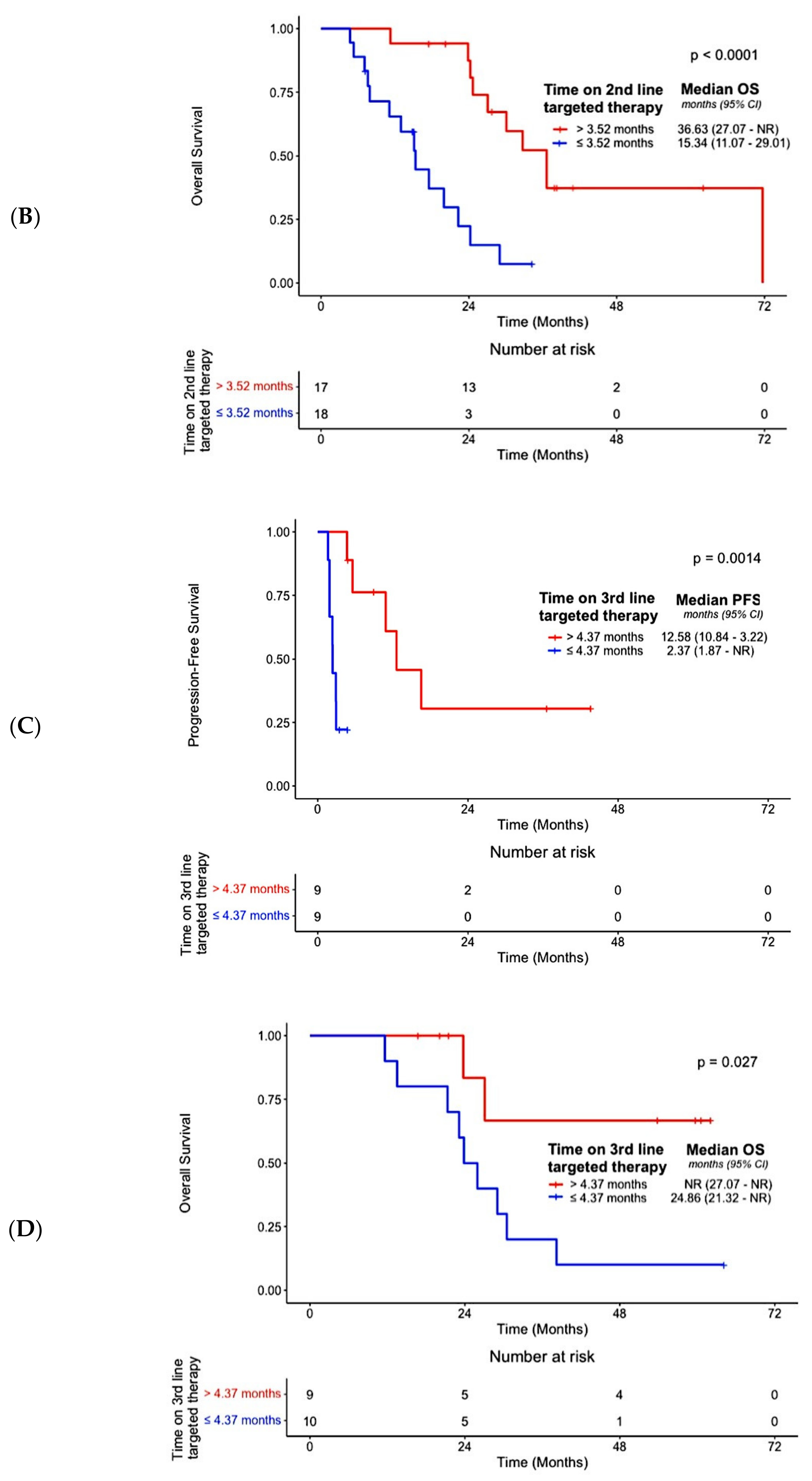
| Best overall response on 2nd-line targeted therapy (Responders vs. Non-Responders) | N/A | N/A | N/A | N/A | 0.07 (0.02–0.32) | <0.001 | 0.09 (0.02–0.43) | 0.002 | N/A | N/A | N/A | N/A |
| Nature of 2nd-line targeted therapy (Futibatinib vs. other targeted therapies) | N/A | N/A | N/A | N/A | 0.43 (0.20–0.96) | 0.04 | 0.11 (0.03–0.49) | 0.004 | N/A | N/A | N/A | N/A |
| Duration of 2nd-line targeted therapy (>3.52 months vs. ≤3.52 months) | N/A | N/A | N/A | N/A | N/A b | N/A b | N/A b | N/A b | N/A | N/A | N/A | N/A |
| Best overall response on 3rd-line targeted therapy (Responders vs. Non-Responders) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.18 (0.05–0.73) | 0.02 | 0.18 (0.02–0.67) | 0.02 |
| Nature of 3rd-line targeted therapy (Futibatinib vs. other targeted therapies) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1.79 (0.46–6.91) | 0.40 | N/A | N/A |
| Duration of 3rd-line targeted therapy (>4.37 months vs. ≤4.37 months) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.06 (0.007–0.53) | 0.01 | 0.06 (0.007–0.57) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, U.; Muhamad Faizul, E.; Howlett, S.; Amin, Z.; Hochhauser, D.; Shiu, K.-K.; Bridgewater, J.; Khan, K. Comprehensive Examination of Cholangiocarcinoma Patients Treated with Novel Targeted Therapies after Extended Molecular Profiling on Liquid Biopsies. Cancers 2024, 16, 697. https://doi.org/10.3390/cancers16040697
Mahmood U, Muhamad Faizul E, Howlett S, Amin Z, Hochhauser D, Shiu K-K, Bridgewater J, Khan K. Comprehensive Examination of Cholangiocarcinoma Patients Treated with Novel Targeted Therapies after Extended Molecular Profiling on Liquid Biopsies. Cancers. 2024; 16(4):697. https://doi.org/10.3390/cancers16040697
Chicago/Turabian StyleMahmood, Umair, Elisya Muhamad Faizul, Sarah Howlett, Zahir Amin, Daniel Hochhauser, Kai-Keen Shiu, John Bridgewater, and Khurum Khan. 2024. "Comprehensive Examination of Cholangiocarcinoma Patients Treated with Novel Targeted Therapies after Extended Molecular Profiling on Liquid Biopsies" Cancers 16, no. 4: 697. https://doi.org/10.3390/cancers16040697
APA StyleMahmood, U., Muhamad Faizul, E., Howlett, S., Amin, Z., Hochhauser, D., Shiu, K.-K., Bridgewater, J., & Khan, K. (2024). Comprehensive Examination of Cholangiocarcinoma Patients Treated with Novel Targeted Therapies after Extended Molecular Profiling on Liquid Biopsies. Cancers, 16(4), 697. https://doi.org/10.3390/cancers16040697







