Dosimetric Impact of Voluntary Deep Inspiration Breath Hold (DIBH) in Mediastinal Hodgkin Lymphomas: A Comparative Evaluation of Three Different Intensity Modulated Radiation Therapy (IMRT) Delivery Methods Using Voluntary DIBH and Free Breathing Techniques
Abstract
Simple Summary
Abstract
1. Introduction
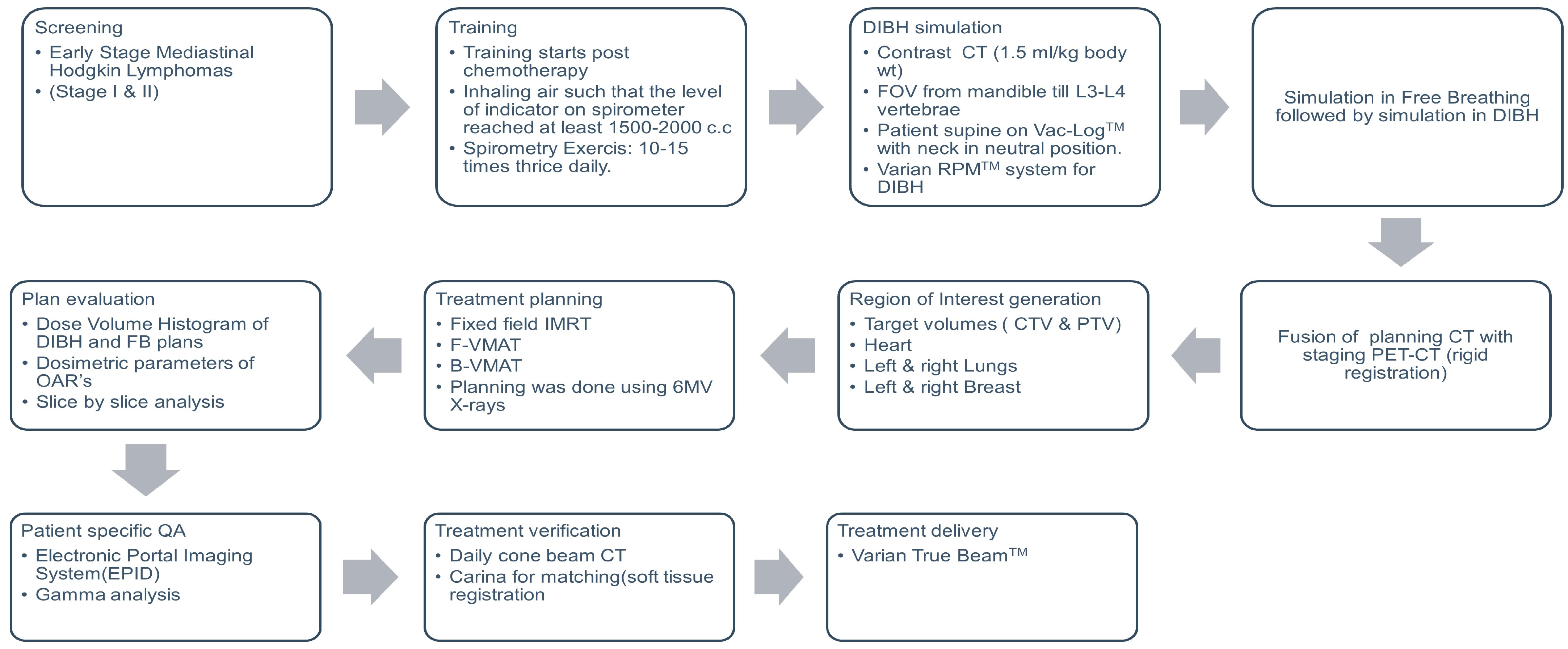
2. Materials and Methods
2.1. Patient Selection
2.2. DIBH Training and Simulation
2.3. Contouring
2.4. Treatment Planning
2.5. Treatment and Verification
2.6. Data Collection and Analysis
3. Results
3.1. Demographic and Treatment Profile of Patients
3.2. Volumes of PTV and OARs
3.3. Dosimetric Analysis
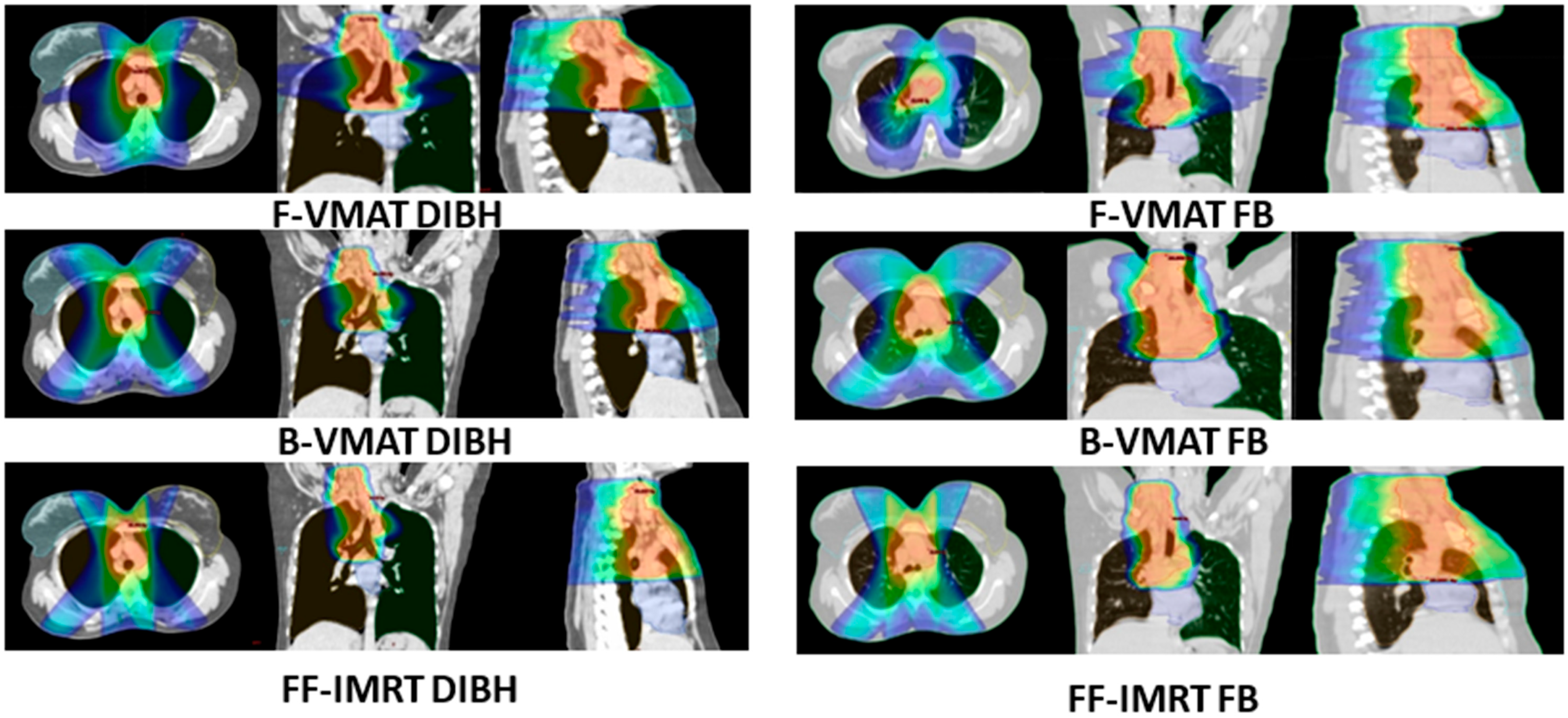
3.3.1. Plan Quality and Deliverability
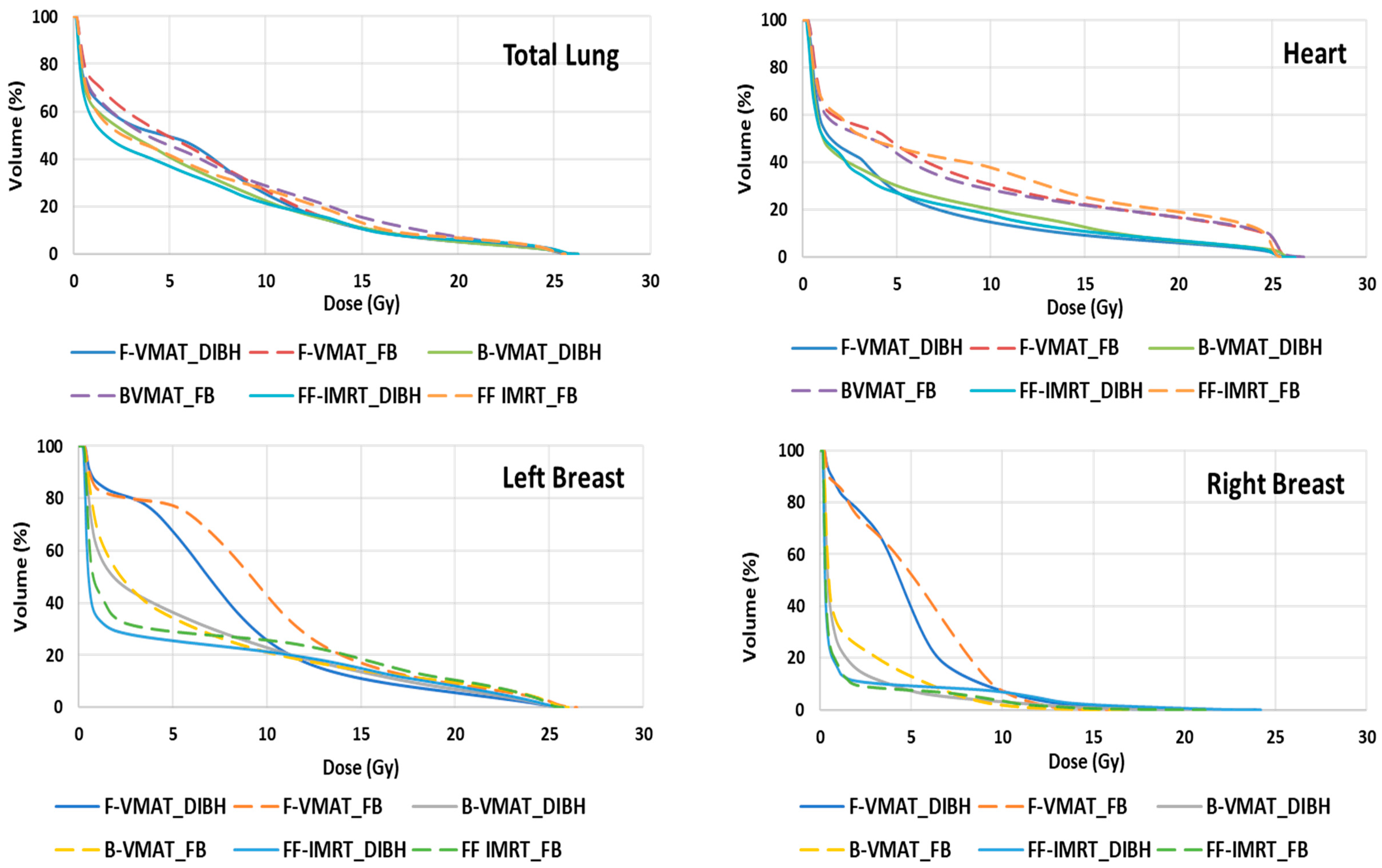
3.3.2. Doses to OARs
3.3.3. Heart
3.3.4. Lungs
3.3.5. Breast
3.3.6. Comparison of Three Different Radiotherapy Delivery Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wirth, A.; Mikhaeel, N.G.; Aleman, B.M.; Pinnix, C.C.; Constine, L.S.; Ricardi, U.; Illidge, T.M.; Eich, H.T.; Hoppe, B.S.; Dabaja, B.; et al. Involved Site Radiation Therapy in Adult Lymphomas: An Overview of International Lymphoma Radiation Oncology Group Guidelines. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 909–933. [Google Scholar] [CrossRef] [PubMed]
- Sasse, S.; Bröckelmann, P.J.; Goergen, H.; Plütschow, A.; Müller, H.; Kreissl, S.; Buerkle, C.; Borchmann, S.; Fuchs, M.; Borchmann, P.; et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: Updated analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 Trials. J. Clin. Oncol. 2017, 35, 1999–2007. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.C. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Specht, L.; Yahalom, J.; Illidge, T.; Berthelsen, A.K.; Constine, L.S.; Eich, H.T.; Girinsky, T.; Hoppe, R.T.; Mauch, P.; Mikhaeel, N.G.; et al. Modern radiation therapy for Hodgkin lymphoma: Field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 854–862. [Google Scholar] [CrossRef]
- Maraldo, M.V.; Aznar, M.C.; Vogelius, I.R.; Petersen, P.M.; Specht, L. Involved node radiation therapy: An effective alternative in early-stage Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1057–1065. [Google Scholar] [CrossRef]
- Laskar, S.; Kumar, D.P.; Khanna, N.; Menon, H.; Sengar, M.; Arora, B.; Gujral, S.; Shet, T.; Sridhar, E.; Rangarajan, V.; et al. Radiation therapy for early-stage unfavorable Hodgkin lymphoma: Is dose reduction feasible? Leuk. Lymphoma 2014, 55, 2356–2361. [Google Scholar] [CrossRef]
- Pern, V.; Zefkili, S.; Peurin, D.; Fourquet, A.; Kirova, Y. Can we reduce the toxicity of the mediastinal irradiation using new highly conformal techniques? J. Leuk. 2014, 2, 1000154. [Google Scholar]
- Paumier, A.; Khodari, W.; Beaudre, A.; Ghalibafian, M.; Blanchard, P.; Al Hamokles, H.; Bhari, M.; Lessard, N.; Girinsky, T. Intensity-modulated radiotherapy and involved-node concept in patients with Hodgkin lymphoma: Experience of the Gustave-Roussy Institute. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2011, 15, 709–715. [Google Scholar]
- Voong, K.R.; McSpadden, K.; Pinnix, C.C.; Shihadeh, F.; Reed, V.; Salehpour, M.R.; Arzu, I.; Wang, H.; Hodgson, D.; Garcia, J.; et al. Dosimetric advantages of a “butterfly” technique for intensity-modulated radiation therapy for young female patients with mediastinal Hodgkin’s lymphoma. Radiat. Oncol. 2014, 9, 94. [Google Scholar] [CrossRef]
- Buglione, M.; Guerini, A.E.; Filippi, A.R.; Spiazzi, L.; Pasinetti, N.; Magli, A.; Toraci, C.; Borghetti, P.; Triggiani, L.; Alghisi, A.; et al. A Systematic Review on Intensity Modulated Radiation Therapy for Mediastinal Hodgkin’s Lymphoma. Crit. Rev. Oncol./Hematol. 2021, 167, 103437. [Google Scholar] [CrossRef] [PubMed]
- Fiandra, C.; Filippi, A.R.; Catuzzo, P.; Botticella, A.; Ciammella, P.; Franco, P.; Borca, V.C.; Ragona, R.; Tofani, S.; Ricardi, U. Different IMRT solutions vs. 3D-Conformal Radiotherapy in early-stage Hodgkin’s lymphoma: Dosimetric comparison and clinical considerations. Radiat. Oncol. 2012, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Verbakel, W.F.; Cuijpers, J.P.; Slotman, B.J.; Senan, S. Dosimetric impact of interplay effect on RapidArc lung stereotactic treatment delivery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Berbeco, R.I.; Pope, C.J.; Jiang, S.B. Measurement of the interplay effect in lung IMRT treatment using EDR2 films. J. Appl. Clin. Med. Phys. 2006, 7, 33–42. [Google Scholar] [CrossRef]
- Ferini, G.; Molino, L.; Tripoli, A.; Valenti, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; Borzi, G.R. Anatomical Predictors of Dosimetric Advantages for Deep-inspiration-breath-hold 3D-conformal Radiotherapy Among Women with Left Breast Cancer. Anticancer Res. 2021, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Valenti, V.; Viola, A.; Umana, G.E.; Martorana, E. A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers 2022, 14, 3477. [Google Scholar] [CrossRef]
- Houlihan, O.A.; Rangaswamy, G.; Dunne, M.; Rohan, C.; O’Neill, L.; Chalke, S.; Daly, P.; Gillham, C.; McArdle, O. Deep inspiration breath hold versus free breathing technique in mediastinal radiotherapy for lymphoma. BJR Open 2021, 3, 20200067. [Google Scholar] [CrossRef]
- Petersen, P.M.; Aznar, M.C.; Berthelsen, A.K.; Loft, A.; Schut, D.A.; Maraldo, M.; Josipovic, M.; Klausen, T.L.; Andersen, F.L.; Specht, L. Prospective phase II trial of image-guided radiotherapy in Hodgkin lymphoma: Benefit of deep inspiration breath-hold. Acta Oncol. 2015, 54, 60–66. [Google Scholar] [CrossRef]
- Illidge, T.; Specht, L.; Yahalom, J.; Aleman, B.; Berthelsen, A.K.; Constine, L.; Dabaja, B.; Dharmarajan, K.; Ng, A.; Ricardi, U.; et al. Modern radiation therapy for nodal non-Hodgkin lymphoma—Target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 49–58. [Google Scholar] [CrossRef]
- Kataria, T.; Sharma, K.; Subramani, V.; Karrthick, K.P.; Bisht, S.S. Homogeneity Index: An objective tool for assessment of conformal radiation treatments. J. Med. Phys. 2012, 37, 207–213. [Google Scholar] [CrossRef]
- Wu, Q.; Mohan, R.; Morris, M.; Lauve, A.; Schmidt-Ullrich, R. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: Dosimetric results. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Younge, K.C.; Roberts, D.; Janes, L.A.; Anderson, C.; Moran, J.M.; Matuszak, M.M. Predicting deliverability of volumetric-modulated arc therapy (VMAT) plans using aperture complexity analysis. J. Appl. Clin. Med. Phys. 2016, 17, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Westerly, D.C.; Mackie, T.R.; Olivera, G.H.; Bentzen, S.M.; Patel, R.R.; Jaradat, H.; Tome, W.A.; Ritter, M.A.; Mehta, M.P. Integral radiation dose to normal structures with conformal external beam radiation. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Everett, A.S.; Hoppe, B.S.; Louis, D.; McDonald, A.M.; Morris, C.G.; Mendenhall, N.P.; Li, Z.; Flampouri, S. Comparison of Techniques for Involved-Site Radiation Therapy in Patients with Lower Mediastinal Lymphoma. Pract. Radiat. Oncol. 2019, 9, 426–434. [Google Scholar] [CrossRef]
- Starke, A.; Bowden, J.; Lynn, R.; Hall, K.; Hudson, K.; Rato, A.; Aldridge, E.; Robb, D.; Steele, P.; Brady, J.; et al. Comparison of butterfly volumetric modulated arc therapy to full arc with or without deep inspiration breath hold for the treatment of mediastinal lymphoma. Radiother. Oncol. 2018, 129, 449–455. [Google Scholar] [CrossRef]
- Dores, G.M.; Metayer, C.; Curtis, R.E. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: A population-based evaluation over 25 years. J. Clin. Oncol. 2002, 20, 3484–3494. [Google Scholar] [CrossRef]
- Aleman, B.M.; van den Belt-Dusebout, A.W.; Klokman, W.J.; van’t Veer, M.B.; Bartelink, H.; van Leeuwen, F.E. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J. Clin. Oncol. 2003, 21, 3431–3439. [Google Scholar] [CrossRef]
- Filippi, A.R.; Ragona, R.; Fusella, M.; Botticella, A.; Fiandra, C.; Ricardi, U. Changes in breast cancer risk associated with different volumes, doses, and techniques in female Hodgkin’s lymphoma patients treated with supra-diaphragmatic radiotherapy. Pract. Radiat. Oncol. 2013, 3, 216–222. [Google Scholar] [CrossRef]
- Begosh-Mayne, D.; Kumar, S.S.; Toffel, S.; Okunieff, P.; O’Dell, W. The dose-response characteristics of four NTCP models: Using a novel CT-based radiomic method to quantify radiation-induced lung density changes. Sci. Rep. 2020, 10, 10559. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S10–S19. [Google Scholar] [CrossRef]
- Palma, G.; Monti, S.; Conson, M.; Pacelli, R.; Cella, L. Normal tissue complication probability (NTCP) models for modern radiation therapy. Semin. Oncol. 2019, 46, 210–218. [Google Scholar] [CrossRef]
- Aznar, M.C.; Maraldo, M.V.; Schut, D.A.; Lundemann, M.; Brodin, N.P.; Vogelius, I.R.; Berthelsen, A.K.; Specht, L.; Petersen, P.M. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: Deep inspiration breath-hold, IMRT, or both? Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 169–174. [Google Scholar] [CrossRef]
| Characteristics | Number of Patients (n = 23) |
|---|---|
| Median (range) age in years | 27 (18–48) |
| Gender | |
| Males | 9 |
| Females | 14 |
| Planned dose | 25.2 Gy |
| Planned no. of fractions | 14 |
| Planned dose per fraction | 1.8 Gy |
| Technique used for treatment | |
| F-VMAT | 1 |
| B-VMAT | 21 |
| FF-IMRT | 1 |
| Target (PTV) and OAR Volumes | DIBH | FB | |||
|---|---|---|---|---|---|
| Mean (c.c.) | SEM | Mean (c.c.) | SEM | p-Value | |
| PTV Volume | 537.73 | 36.33 | 556.97 | 33.79 | 0.059 |
| Left Lung Volume | 1776.90 | 115.80 | 1047.78 | 73.85 | 0.000 |
| Right Lung Volume | 1993.07 | 129.96 | 1232.27 | 85.33 | 0.000 |
| Total Lungs Volume | 3771.21 | 244.63 | 2280.43 | 157.50 | 0.000 |
| Heart Volume | 444.03 | 28.58 | 504.42 | 32.51 | 0.000 |
| F-VMAT | Absolute Difference (Gy) | Relative Difference (%) | p-Value | B-VMAT | Absolute Difference (Gy) | Relative Difference (%) | p-Value | FF-IMRT | Absolute Difference (Gy) | Relative Difference (%) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV coverage | DIBH | 95.62 ± 0.06 | 0.090 | 0.094 | 0.183 | 95.44 ± 0.06 | 0.15 | 0.16 | 0.117 | 95.67 ± 0.06 | 0.01 | 0.01 | 0.90 |
| FB | 95.71 ± 0.07 | 95.59 ± 0.07 | 95.68 ± 0.06 | ||||||||||
| Homogeneity index (HI) | DIBH | 0.089 ± 0.0017 | 0.007 | 0 | 0.324 | 0.097 ± 0.002 | 0.01 | −4.74 | 0.143 | 0.090 ± 0.003 | −0.0038 | −4.42 | 0.20 |
| FB | 0.0869 ± 0.0023 | 0.093 ± 0.002 | 0.09 ± 0.002 | ||||||||||
| Conformity index (CI) | DIBH | 1.017 ± 0.004 | 0.01 | 0.99 | 0.505 | 1.034 ± 0.007 | 0.02 | 1.30 | 0.073 | 1.093 ± 0.007 | −0.0066 | −0.61 | 0.52 |
| FB | 1.014 ± 0.004 | 1.048 ± 0.005 | 1.087 ± 0.010 | ||||||||||
| Monitoring units (MUs) | DIBH | 635 ± 27 | 20 | 3.25 | 0.464 | 586 ± 19 | 61 | −11.62 | 0.013 | 1109 ± 46 | 17 | 1.51 | 0.90 |
| FB | 615 ± 16 | 525 ± 18 | 1126 ± 54 | ||||||||||
| Complexity | DIBH | 0.1632 ± 0.0073 | −0.0030 | −2.33 | 0.640 | 0.1517 ± 0.0062 | −0.0106 | −7.59 | 0.171 | 0.1625 ± 0.0056 | −0.0087 | −5.64 | 0.17 |
| FB | 0.1595 ± 0.0045 | 0.1410 ± 0.0044 | 0.1538 ± 0.0057 | ||||||||||
| Gamma passing rate (GPR) | DIBH | 99.37 ± 0.12 | 0.15 | −5.74 | 0.314 | 99.39 ± 0.14 | 0.48 | −2.86 | 0.304 | 99.43 ± 0.14 | −0.11 | −4.68 | 0.352 |
| FB | 99.52 ± 0.38 | 99.87 ± 0.44 | 99.32 ± 0.15 | ||||||||||
| F-VMAT | B-VMAT | FF-IMRT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Difference (Gy) | Relative Difference (%) | p-Value | Absolute Difference (Gy) | Relative Difference (%) | p-Value | Absolute Difference (Gy) | Relative Difference (%) | p-Value | ||||||
| LUNG Left | DIBH | Mean (Gy) | 5.64 ± 0.27 | 1.090 | 16.196 | 0.000 | 5.57 ± 0.27 | 1.44 | 20.53 | 0.000 | 5.33 ± 0.3 | 1.21 | 18.47 | 0.000 |
| FB | 6.73 ± 0.32 | 7.01 ± 0.36 | 6.53 ± 0.42 | |||||||||||
| DIBH | V5Gy [%] | 41.17 ± 8.72 | 5.770 | 12.292 | 0.017 | 37.98 ± 8.33 | 8.98 | 19.13 | 0.000 | 33.09 ± 2.04 | 7.72 | 18.92 | 0.000 | |
| FB | 46.94 ± 8.12 | 46.97 ± 1.9 | 40.81 ± 2.57 | |||||||||||
| DIBH | V10Gy [%] | 22.16 ± 1.84 | 5.983 | 21.259 | 0.000 | 22.77 ± 1.58 | 7.87 | 25.69 | 0.000 | 22.43 ± 1.46 | 5.11 | 18.57 | 0.001 | |
| FB | 28.14 ± 2.17 | 30.64 ± 2.08 | 27.54 ± 2.28 | |||||||||||
| DIBH | V20Gy [%] | 5.42 ± 0.51 | 1.86 | 25.55 | 0.002 | 5.85 ± 0.58 | 2.28 | 28.04 | 0.001 | 6.38 ± 0.67 | 2.27 | 26.24 | 0.003 | |
| FB | 7.28 ± 0.87 | 8.13 ± 1.0 | 8.65 ± 1.06 | |||||||||||
| DIBH | V25Gy [%] | 1.53 ± 0.15 | 0.666 | 30.277 | 0.005 | 1.57 ± 0.16 | 0.79 | 33.55 | 0.000 | 1.49 ± 0.18 | 0.42 | 21.98 | 0.045 | |
| FB | 2.2 ± 0.28 | 2.36 ± 0.28 | 1.91 ± 0.27 | |||||||||||
| LUNG Right | DIBH | Mean (Gy) | 5.73 ± 0.27 | 1.270 | 18.134 | 0.001 | 5.59 ± 0.29 | 1.52 | 21.38 | 0.000 | 5.40 ± 0.33 | 1.38 | 20.38 | 0.000 |
| FB | 7.01 ± 0.41 | 7.11 ± 0.45 | 6.78 ± 0.51 | |||||||||||
| DIBH | V5Gy [%] | 42.76 ± 2.23 | 9.442 | 18.088 | 0.003 | 38.08 ± 1.97 | 10.59 | 21.75 | 0.000 | 34.23 ± 2.49 | 8.79 | 20.43 | 0.000 | |
| FB | 52.20 ± 3.19 | 48.67 ± 3.04 | 43.01 ± 3.37 | |||||||||||
| DIBH | V10Gy [%] | 21.71 ± 1.52 | 5.491 | 20.185 | 0.003 | 22.49 ± 1.56 | 7.69 | 25.48 | 0.000 | 22.86 ± 1.57 | 6.24 | 21.43 | 0.001 | |
| FB | 27.21 ± 2.20 | 30.19 ± 2.34 | 29.10 ± 2.54 | |||||||||||
| DIBH | V20Gy [%] | 5.22 ± 0.46 | 1.81 | 25.75 | 0.005 | 5.74 ± 0.60 | 2.08 | 26.60 | 0.004 | 6.51 ± 0.63 | 2.22 | 25.43 | 0.010 | |
| FB | 7.03 ± 0.80 | 7.82 ± 0.93 | 8.73 ± 1.08 | |||||||||||
| DIBH | V25Gy [%] | 1.36 ± 0.13 | 0.725 | 34.859 | 0.003 | 1.43 ± 0.16 | 0.68 | 32.29 | 0.001 | 1.20 ± 0.14 | 0.46 | 27.59 | 0.000 | |
| FB | 2.08 ± 0.28 | 2.11 ± 0.26 | 1.66 ± 0.27 | |||||||||||
| LUNGS | DIBH | Mean (Gy) | 5.69 ± 0.24 | 1.185 | 17.242 | 0.000 | 5.58 ± 0.24 | 1.47 | 20.88 | 0.000 | 5.36 ± 0.26 | 1.30 | 19.50 | 0.000 |
| FB | 6.87 ± 0.33 | 7.05 ± 0.35 | 6.66 ± 0.40 | |||||||||||
| DIBH | V5Gy [%] | 41.31 ± 2.03 | 8.349 | 16.813 | 0.001 | 37.58 ± 1.71 | 9.99 | 21.00 | 0.000 | 33.83 ± 1.91 | 7.69 | 18.52 | 0.000 | |
| FB | 49.66 ± 2.24 | 47.57 ± 2.16 | 41.52 ± 2.65 | |||||||||||
| DIBH | V10Gy [%] | 21.21 ± 1.64 | 6.384 | 23.134 | 0.001 | 22.79 ± 1.3 | 7.49 | 24.74 | 0.000 | 22.62 ± 1.22 | 5.69 | 20.09 | 0.000 | |
| FB | 27.6 ± 1.92 | 30.28 ± 1.89 | 28.31 ± 2.0 | |||||||||||
| DIBH | V20Gy [%] | 5.33 ± 0.39 | 1.81 | 25.35 | 0.002 | 5.82 ± 0.50 | 2.09 | 26.42 | 0.000 | 6.46 ± 0.52 | 2.22 | 25.58 | 0.021 | |
| FB | 7.14 ± 0.69 | 7.91 ± 0.78 | 8.68 ± 0.88 | |||||||||||
| DIBH | V25Gy [%] | 1.47 ± 0.11 | 0.636 | 30.171 | 0.003 | 1.48 ± 0.10 | 0.76 | 33.95 | 0.000 | 1.32 ± 0.14 | 0.44 | 25.13 | 0.001 | |
| FB | 2.11 ± 0.23 | 2.24 ± 0.22 | 1.76 ± 0.24 | |||||||||||
| HEART | DIBH | Mean (Gy) | 4.96 ± 0.64 | 2.101 | 29.738 | 0.000 | 5.07 ± 0.63 | 2.54 | 33.37 | 0.000 | 5.74 ± 0.73 | 2.38 | 29.29 | 0.000 |
| FB | 7.07 ± 0.58 | 7.61 ± 0.63 | 8.11 ± 0.69 | |||||||||||
| DIBH | V5Gy [%] | 27.96 ± 4.08 | 9.130 | 24.613 | 0.002 | 26.39 ± 3.28 | 12.86 | 32.76 | 0.000 | 28.97 ± 4.02 | 12.91 | 30.82 | 0.000 | |
| FB | 37.09 ± 3.19 | 39.25 ± 3.58 | 41.87 ± 4.05 | |||||||||||
| DIBH | V10Gy [%] | 19.13 ± 3.10 | 7.382 | 27.849 | 0.003 | 18.52 ± 2.58 | 11.57 | 38.45 | 0.000 | 22.54 ± 3.41 | 10.25 | 31.25 | 0.000 | |
| FB | 26.51 ± 2.52 | 30.09 ± 2.71 | 32.78 ± 3.01 | |||||||||||
| DIBH | V15Gy [%] | 14.14 ± 2.49 | 6.253 | 30.658 | 0.002 | 14.21 ± 2.22 | 9.49 | 40.05 | 0.000 | 17.54 ± 2.67 | 8.39 | 32.36 | 0.000 | |
| FB | 20.4 ± 2.19 | 23.7 ± 2.31 | 25.93 ± 2.55 | |||||||||||
| BREAST Left | DIBH | Mean (Gy) | 5.15 ± 0.42 | 0.744 | 12.623 | 0.209 | 4.52 ± 0.37 | 0.86 | 15.97 | 0.092 | 4.44 ± 0.39 | 0.25 | 5.43 | 0.361 |
| FB | 5.89 ± 0.60 | 5.38 ± 0.56 | 4.70 ± 0.36 | |||||||||||
| DIBH | V4Gy [%] | 48.70 ± 5.91 | 4.201 | 7.942 | 0.588 | 38.23 ± 3.16 | 5.79 | 13.15 | 0.079 | 27.14 ± 2.54 | 1.88 | 6.47 | 0.269 | |
| FB | 52.90 ± 5.09 | 44.02 ± 4.05 | 29.02 ± 2.44 | |||||||||||
| DIBH | V10Gy [%] | 13.16 ± 1.39 | 6.481 | 32.993 | 0.331 | 14.97 ± 1.63 | 7.92 | 34.61 | 0.024 | 20.42 ± 2.01 | 1.82 | 8.17 | 0.272 | |
| FB | 19.65 ± 3.88 | 22.89 ± 3.54 | 22.24 ± 2.02 | |||||||||||
| BREAST Right | DIBH | Mean (Gy) | 4.23 ± 0.33 | 0.714 | 14.457 | 0.245 | 3.63 ± 0.41 | 0.84 | 18.68 | 0.004 | 3.26 ± 0.34 | 0.32 | 8.90 | 0.170 |
| FB | 4.94 ± 0.49 | 4.47 ± 0.53 | 3.57 ± 0.38 | |||||||||||
| DIBH | V4Gy [%] | 42.38 ± 5.38 | 5.223 | 10.972 | 0.523 | 33.71 ± 4.61 | 5.02 | 12.97 | 0.056 | 21.59 ± 2.52 | 1.82 | 7.75 | 0.295 | |
| FB | 47.60 ± 4.63 | 38.73 ± 4.23 | 23.41 ± 2.81 | |||||||||||
| DIBH | V10Gy [%] | 8.19 ± 0.97 | 5.437 | 39.912 | 0.030 | 12.13 ± 1.84 | 4.75 | 28.14 | 0.011 | 15.78 ± 1.94 | 1.26 | 7.37 | 0.551 | |
| FB | 13.62 ± 3.08 | 16.88 ± 3.02 | 17.04 ± 2.15 | |||||||||||
| Integral Dose | DIBH | 57,182.72 ± 3631.59 | −3088.07 | −5.71 | 0.128 | 54,898.85 ± 3355.85 | −1526.98 | −2.86 | 0.376 | 52,784.58 ± 3314.10 | −2361.46 | −4.68 | 0.160 | |
| FB | 54,094.65 ± 3404.70 | 53,371.87 ± 3131.36 | 50,423.12 ± 3153.61 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, S.; Patil, D.; Joshi, K.; Gamre, P.; Mishra, A.; Khairnar, S.; Kakoti, S.; Nayak, L.; Punatar, S.; Jain, J.; et al. Dosimetric Impact of Voluntary Deep Inspiration Breath Hold (DIBH) in Mediastinal Hodgkin Lymphomas: A Comparative Evaluation of Three Different Intensity Modulated Radiation Therapy (IMRT) Delivery Methods Using Voluntary DIBH and Free Breathing Techniques. Cancers 2024, 16, 690. https://doi.org/10.3390/cancers16040690
Mohanty S, Patil D, Joshi K, Gamre P, Mishra A, Khairnar S, Kakoti S, Nayak L, Punatar S, Jain J, et al. Dosimetric Impact of Voluntary Deep Inspiration Breath Hold (DIBH) in Mediastinal Hodgkin Lymphomas: A Comparative Evaluation of Three Different Intensity Modulated Radiation Therapy (IMRT) Delivery Methods Using Voluntary DIBH and Free Breathing Techniques. Cancers. 2024; 16(4):690. https://doi.org/10.3390/cancers16040690
Chicago/Turabian StyleMohanty, Samarpita, Divya Patil, Kishore Joshi, Poonam Gamre, Ajay Mishra, Sunil Khairnar, Sangeeta Kakoti, Lingaraj Nayak, Sachin Punatar, Jeevanshu Jain, and et al. 2024. "Dosimetric Impact of Voluntary Deep Inspiration Breath Hold (DIBH) in Mediastinal Hodgkin Lymphomas: A Comparative Evaluation of Three Different Intensity Modulated Radiation Therapy (IMRT) Delivery Methods Using Voluntary DIBH and Free Breathing Techniques" Cancers 16, no. 4: 690. https://doi.org/10.3390/cancers16040690
APA StyleMohanty, S., Patil, D., Joshi, K., Gamre, P., Mishra, A., Khairnar, S., Kakoti, S., Nayak, L., Punatar, S., Jain, J., Phurailatpam, R., & Goda, J. S. (2024). Dosimetric Impact of Voluntary Deep Inspiration Breath Hold (DIBH) in Mediastinal Hodgkin Lymphomas: A Comparative Evaluation of Three Different Intensity Modulated Radiation Therapy (IMRT) Delivery Methods Using Voluntary DIBH and Free Breathing Techniques. Cancers, 16(4), 690. https://doi.org/10.3390/cancers16040690





