Kaposi’s Sarcoma: Evaluation of Clinical Features, Treatment Outcomes, and Prognosis in a Single-Center Retrospective Case Series
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Diagnosis and Treatment
2.4. Data Collection
2.5. Statistical Analysis
2.6. Ethics Considerations
3. Results
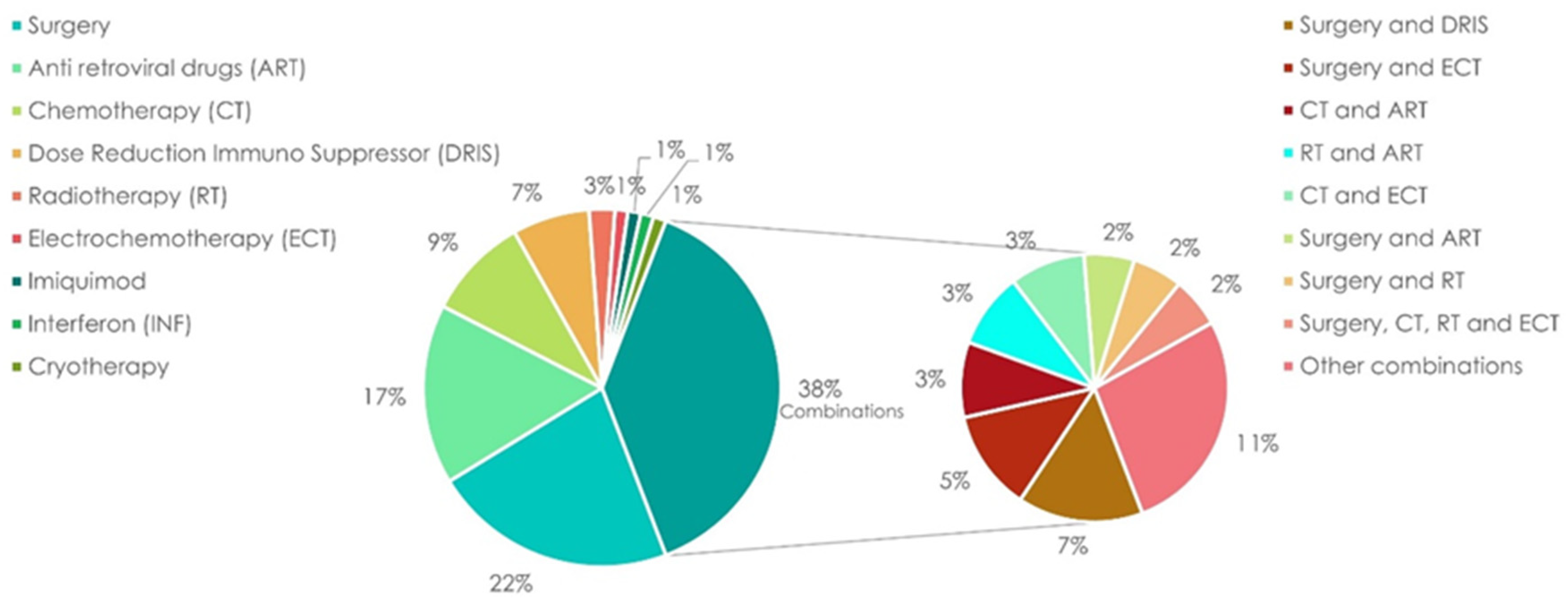
3.1. Treatment
3.2. Response and Relapse
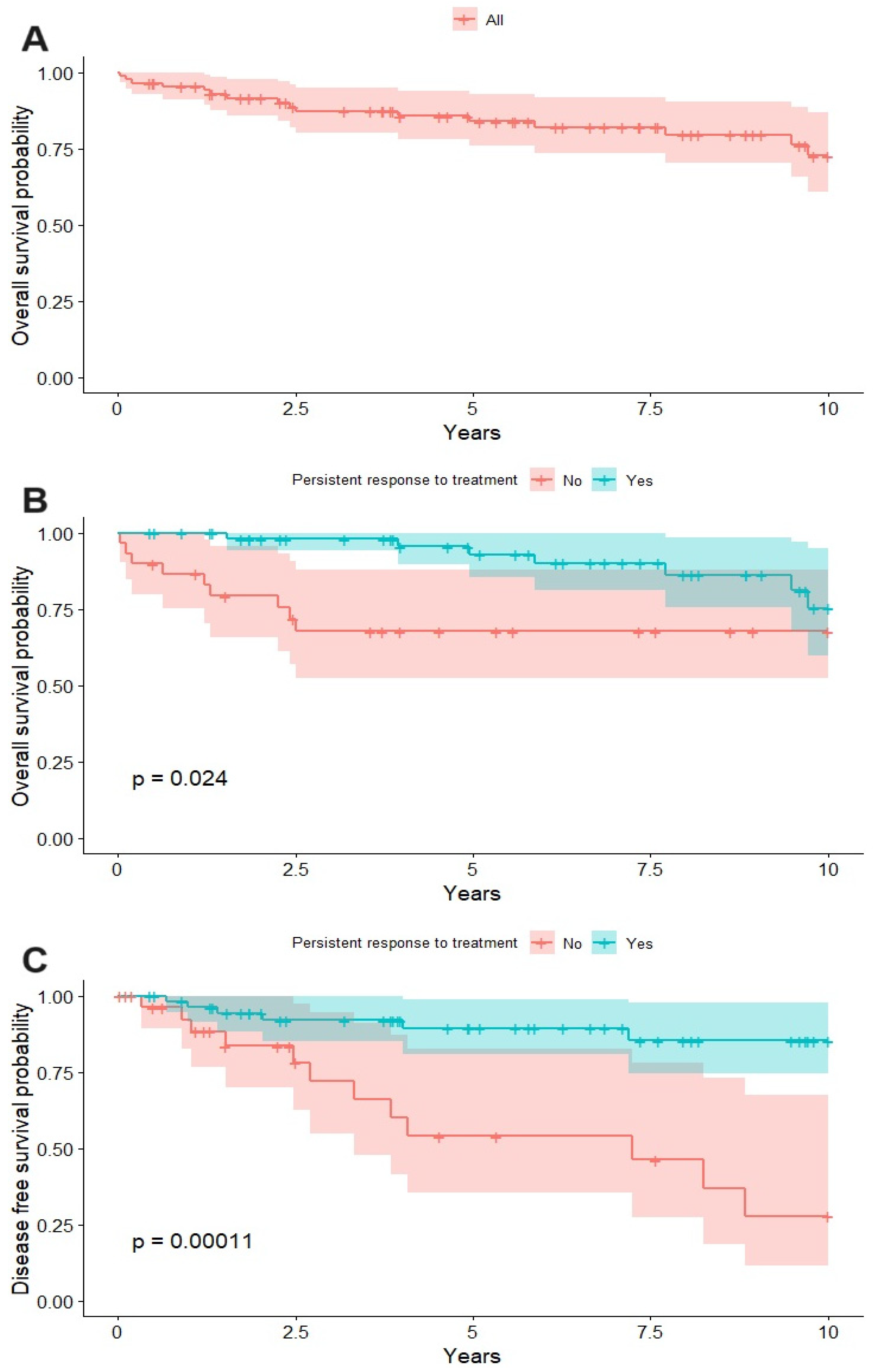
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaposi. Idiopathisches multiples Pigmentsarkom der Haut. Arch. Dermatol. Syph. 1872, 4, 265–273. [Google Scholar] [CrossRef]
- Esser, S.; Schöfer, H.; Hoffmann, C.; Claßen, J.; Kreuter, A.; Leiter, U.; Oette, M.; Becker, J.C.; Ziemer, M.; Mosthaf, F.; et al. S1 Guidelines for the Kaposi Sarcoma. J. Dtsch. Dermatol. Ges. 2022, 20, 892–904. [Google Scholar] [CrossRef]
- Friedman-Kien, A.E.; Saltzman, B.R. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J. Am. Acad. Dermatol. 1990, 22 Pt 2, 1237–1250. [Google Scholar] [CrossRef]
- Penn, I. Kaposi’s sarcoma in transplant recipients. Transplantation 1997, 64, 669–673. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. Morb. Mortal. Wkly. Rep. 1981, 30, 305–308. [Google Scholar]
- Hymes, K.; Greene, J.; Marcus, A.; William, D.; Cheung, T.; Prose, N.; Ballard, H.; Laubenstein, L. Kaposi’s sarcoma in homosexual men—A report of eight cases. Lancet 1981, 318, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Botta, L.; Perez, M.J.S.; López, M.D.C.; Marcos-Gragera, R.; Scuderi, T.; Huws, D.W.; Trama, A.; the RARECARENet WG. Kaposi sarcoma incidence, survival and trends: Data from the information network on rare cancers in Europe (RARECAREnet). Cancer Epidemiol. 2021, 70, 101877. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, Q.; Zuo, J.; Minhas, V.; Wood, C.; Zhang, T. The world-wide incidence of Kaposi’s sarcoma in the HIV/AIDS era. HIV Med. 2018, 19, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Etemad, S.A.; Dewan, A.K. Kaposi Sarcoma Updates. Dermatol. Clin. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 27 June 2023).
- Jackson, S.S.; Pfeiffer, R.M.; Hsieh, M.-C.; Li, J.; Madeleine, M.M.; Pawlish, K.S.; Zeng, Y.; Yu, K.J.; Engels, E.A. Sex differences in cancer incidence among solid organ transplant recipients. JNCI J. Natl. Cancer Inst. 2023, djad224. [Google Scholar] [CrossRef]
- Weskamp, P.; Ufton, D.; Drysch, M.; Wagner, J.M.; Dadras, M.; Lehnhardt, M.; Behr, B.; Wallner, C. Risk Factors for Occurrence and Relapse of Soft Tissue Sarcoma. Cancers 2022, 14, 1273. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nishimura, S.; Shinyashiki, Y.; Ito, T.; Akagi, M. Characterizing inflammatory markers in highly aggressive soft tissue sarcomas. Medicine 2022, 101, e30688. [Google Scholar] [CrossRef]
- Manuraj, S.; Steve, M.; Heinz, K.; Alistair, R. Negative HHV-8 immunoreactivity in HIV associated Kaposi’s sarcoma. J. Cutan. Pathol. 2016, 43, 626–628. [Google Scholar] [CrossRef]
- Martí, N.; Monteagudo, C.; Pinazo, I.; Jordá, E. Negative herpesvirus-8 immunoreactivity does not exclude a diagnosis of Kaposi sarcoma. Br. J. Dermatol. 2011, 164, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, L.; Genovese, G.; Berti, E.; Peris, K.; Rongioletti, F.; Micali, G.; Ayala, F.; DELLA Bella, S.; Mancuso, R.; Pinton, P.C.; et al. Diagnosis and treatment of classic and iatrogenic Kaposi’s sarcoma: Italian recommendations. Ital. J. Dermatol. Venereol. 2021, 156, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gallafent, J.H.; Buskin, S.E.; De Turk, P.B.; Aboulafia, D.M. Profile of Patients with Kaposi’s Sarcoma in the Era of Highly Active Antiretroviral Therapy. J. Clin. Oncol. 2005, 23, 1253–1260. [Google Scholar] [CrossRef]
- Holkova, B.; Takeshita, K.; Cheng, D.M.; Volm, M.; Wasserheit, C.; Demopoulos, R.; Chanan-Khan, A. Effect of Highly Active Antiretroviral Therapy on Survival in Patients With AIDS-Associated Pulmonary Kaposi’s Sarcoma Treated with Chemotherapy. J. Clin. Oncol. 2001, 19, 3848–3851. [Google Scholar] [CrossRef]
- Tam, H.K.; Zhang, Z.; Jacobson, L.P.; Margolick, J.B.; Chmiel, J.S.; Rinaldo, C.; Detels, R. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int. J. Cancer 2002, 98, 916–922. [Google Scholar] [CrossRef]
- Hleyhel, M.; Belot, A.; Bouvier, A.M.; Tattevin, P.; Pacanowski, J.; Genet, P.; De Castro, N.; Berger, J.L.; Dupont, C.; Lavolé, A.; et al. Risk of AIDS-Defining Cancers Among HIV-1–Infected Patients in France Between 1992 and 2009: Results From the FHDH-ANRS CO4 Cohort. Clin. Infect. Dis. 2013, 57, 1638–1647. [Google Scholar] [CrossRef]
- Gill, J.; Bourboulia, D.; Wilkinson, J.; Hayes, P.; Cope, A.; Marcelin, A.-G.; Calvez, V.; Gotch, F.; Boshoff, C.; Gazzard, B. Prospective Study of the Effects of Antiretroviral Therapy on Kaposi Sarcoma–Associated Herpesvirus Infection in Patients with and Without Kaposi Sarcoma. JAIDS J. Acquir. Immune Defic. Syndr. 2002, 31, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Paparizos, V.A.; Kyriakis, K.P.; Papastamopoulos, V.; Hadjivassiliou, M.; Stavrianeas, N.G. Response of AIDS-Associated Kaposi Sarcoma to Highly Active Antiretroviral Therapy Alone. J. Acquir. Immune Defic. Syndr. 2002, 30, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Lebbé, C.; Legendre, C.; Francès, C. Kaposi sarcoma in transplantation. Transplant. Rev. 2008, 22, 252–261. [Google Scholar] [CrossRef]
- Tessari, G.; Naldi, L.; Boschiero, L.; Cordiano, C.; Piaserico, S.; Fortina, A.B.; Cerimele, D.; La Parola, I.L.; Capuano, M.; Gotti, E.; et al. Incidence and clinical predictors of Kaposi’s sarcoma among 1721 Italian solid organ transplant recipients: A multicenter study. Eur. J. Dermatol. 2006, 16, 553–557. [Google Scholar] [PubMed]
- Cahoon, E.K.; Linet, M.S.; Clarke, C.A.; Pawlish, K.S.; Engels, E.A.; Pfeiffer, R.M. Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int. J. Cancer 2018, 143, 2741–2748. [Google Scholar] [CrossRef]
- Roy, D.; Sin, S.-H.; Lucas, A.; Venkataramanan, R.; Wang, L.; Eason, A.; Chavakula, V.; Hilton, I.B.; Tamburro, K.M.; Damania, B.; et al. mTOR Inhibitors Block Kaposi Sarcoma Growth by Inhibiting Essential Autocrine Growth Factors and Tumor Angiogenesis. Cancer Res. 2013, 73, 2235–2246. [Google Scholar] [CrossRef]
- Stallone, G.; Schena, A.; Infante, B.; Di Paolo, S.; Loverre, A.; Maggio, G.; Ranieri, E.; Gesualdo, L.; Schena, F.P.; Grandaliano, G. Sirolimus for Kaposi’s Sarcoma in Renal-Transplant Recipients. N. Engl. J. Med. 2005, 352, 1317–1323. [Google Scholar] [CrossRef]
- Nichols, L.A.; Adang, L.A.; Kedes, D.H. Rapamycin Blocks Production of KSHV/HHV8: Insights into the Anti-Tumor Activity of an Immunosuppressant Drug. PLoS ONE 2011, 6, e14535. [Google Scholar] [CrossRef]
- Mosam, A.; Shaik, F.; Uldrick, T.S.; Esterhuizen, T.; Friedland, G.H.; Scadden, D.T.; Aboobaker, J.; Coovadia, H.M. A Randomized Controlled Trial of Highly Active Antiretroviral Therapy Versus Highly Active Antiretroviral Therapy and Chemotherapy in Therapy-Naive Patients With HIV-Associated Kaposi Sarcoma in South Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 60, 150–157. [Google Scholar] [CrossRef]
- Martín-Carbonero, L.; Barrios, A.; Saballs, P.; Sirera, G.; Santos, J.; Palacios, R.; Valencia, M.E.; Alegre, M.; Podzamczer, D.; González-Lahoz, J.; et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi’s sarcoma. AIDS 2004, 18, 1737–1740. [Google Scholar] [CrossRef]
- Feller, L.; Anagnostopoulos, C.; Wood, N.H.; Bouckaert, M.; Raubenheimer, E.J.; Lemmer, J. Human Immunodeficiency Virus–Associated Kaposi Sarcoma as an Immune Reconstitution Inflammatory Syndrome: A Literature Review and Case Report. J. Periodontol. 2008, 79, 362–368. [Google Scholar] [CrossRef]
- Leidner, R.S.; Aboulafia, D.M. Recrudescent Kaposi’s Sarcoma After Initiation of HAART: A Manifestation of Immune Reconstitution Syndrome. AIDS Patient Care STDs 2005, 19, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Semeere, A.S.; Busakhala, N.; Martin, J.N. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Curr. Opin. Oncol. 2012, 24, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Borok, M.Z.; Campbell, T.B.; Casper, C.; Dittmer, D.P.; Hosseinipour, M.C.; Mitsuyasu, R.T.; Mosam, A.; Orem, J.; Phipps, W.T. Stage-Stratified Approach to AIDS-Related Kaposi’s Sarcoma: Implications for Resource-Limited Environments. J. Clin. Oncol. 2014, 32, 2512–2513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stebbing, J.; Sanitt, A.; Nelson, M.; Powles, T.; Gazzard, B.; Bower, M. A prognostic index for AIDS-associated Kaposi’s sarcoma in the era of highly active antiretroviral therapy. Lancet 2006, 367, 1495–1502. [Google Scholar] [CrossRef]
- Krell, J.; Stebbing, J. Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 2013, 32, 373–375. [Google Scholar] [CrossRef]
- Keegan, T.H.; Abrahão, R.; Alvarez, E.M. Survival Trends Among Adolescents and Young Adults Diagnosed with Cancer in the United States: Comparisons with Children and Older Adults. J. Clin. Oncol. 2023, JCO2301367. [Google Scholar] [CrossRef]




| Variable | Value | |
|---|---|---|
| Age | Min/max | 13.9/85.6 |
| Med [IQR] | 57.2 [45.1; 69.2] | |
| Mean (std) | 56.9 (14.7) | |
| Gender | Woman | 9 (10.47%) |
| Man | 77 (89.53%) | |
| Tumor Site | Single site | 53 (61.63%) |
| Lower limbs | 44 (51.16%) | |
| Head and neck | 4 (4.65%) | |
| Upper limbs | 3 (3.49%) | |
| Trunk | 2 (2.33%) | |
| Multiple sites | 33 (38.37%) | |
| Lower and upper limbs | 8 (9.30%) | |
| Lower limbs and trunk | 7 (8.14%) | |
| Lower and upper limbs, trunk, head, and neck | 4 (4.65%) | |
| Lower and upper limbs, head, and neck | 4 (4.65%) | |
| Lower limbs, trunk, head, and neck | 3 (3.49%) | |
| Lower and upper limbs and trunk | 3 (3.49%) | |
| Other combinations | 4 (4.65%) | |
| Type of KS | Classic | 37 (43.02%) |
| Endemic | 3 (3.49%) | |
| Epidemic | 29 (33.72%) | |
| Iatrogenic | 17 (19.77%) | |
| Involvement | Only cutaneous | 67 (77.91%) |
| Cutaneous and extracutaneous (node or mucosal) | 14 (12.28%) | |
| Only extracutaneous (node or mucosal) | 5 (5.81%) | |
| HIV | No | 56 (65.12%) |
| Yes | 30 (34.88%) | |
| Transplant | Yes | 13 (15.12%) |
| No | 73 (84.88%) | |
| Comorbidity | No | 12 (13.95%) |
| Yes | 74 (86.05%) | |
| HHV-8 Status | ND | 35 (39.53%) |
| No | 3 (3.37%) | |
| Yes | 48 (55.81%) | |
| Treatment | Single | 53 (61.63%) |
| Multiple | 33 (38.37%) | |
| Persistent Response | No | 30 (34.88%) |
| Yes | 56 (65.12%) | |
| Relapse | No | 67 (77.91%) |
| Yes | 19 (22.09%) | |
| Deceased | No | 69 (80.23%) |
| Yes | 17 (19.77%) | |
| Cause of Death | COPD or respiratory failure | 5 (%) |
| Cardiac | 1 (5.88%) | |
| Infection, pneumonia, or sepsis | 3 (%) | |
| Liver failure | 4 (23.53%) | |
| Chronic renal failure | 1 (5.88%) | |
| Neoplasm | 2 (11.76%) | |
| Of old age | 1 (5.88%) | |
| Follow-up Time (Years) | Min/max | 0.02/30.1 |
| Med [IQR] | 5.8 [2.4; 9.7] | |
| Mean (std) | 6.6 (5.1) | |
| Time to Relapse (Years) | Min/max | 0.3/15.0 |
| Med [IQR] | 2.7 [1.2; 5.6] | |
| Mean (std) | 4.0 (3.8) | |
| Time to Death (Years) | Min/max | 0.02/11.1 |
| Med [IQR] | 2.4 [1.2; 5.9] | |
| Mean (std) | 3.8 (3.7) | |
| Variable | Overall Survival | DFS | |||
|---|---|---|---|---|---|
| 1/HR | p-Value | 1/HR | p-Value | ||
| Age | 0.993 | 0.689 | 0.973 | 0.137 | |
| Gender | Man (ref woman) | 0 | 0.998 | 0 | 0.998 |
| Tumor Site | Lower limbs (present, ref absent) | 1.136 | 0.916 | 0.428 | 0.421 |
| Head and neck (present, ref absent) | 2.103 | 0.533 | 2.780 | 0.213 | |
| Upper limbs (present, ref absent) | 1.773 | 0.461 | 0.731 | 0.568 | |
| Trunk (present, ref absent) | 4.563 | 0.152 | 0.482 | 0.179 | |
| Type of KS | Endemic (ref classic) | 0.298 | 0.282 | 0.958 | 0.967 |
| Epidemic (ref classic) | 1.015 | 0.985 | 2.758 | 0.077 | |
| Iatrogenic (ref classic) | 0.211 | 0.011 | >>10 | 0.998 | |
| Involvement | Cutaneous and extracutaneous (ref only cutaneous) | 1.466 | 0.613 | 0.298 | 0.017 |
| Only extracutaneous (ref only cutaneous) | >>10 | 0.998 | >>10 | 0.998 | |
| HIV | Yes (ref no) | 2.308 | 0.192 | 1.977 | 0.23 |
| Transplant | Yes (ref no) | 0.254 | 0.008 | >>10 | 0.997 |
| Comorbidity | Yes (ref no) | 0 | 0.997 | 0.351 | 0.309 |
| Autoimmune/Immunologic | ND (ref no) | 3.401 | 0.244 | 0 | 0.998 |
| Yes (ref no) | 3.220 | 0.314 | 0 | 0.998 | |
| Neoplastic | ND (ref no) | 2.653 | 0.358 | 2.636 | 0.362 |
| Yes (ref no) | 1.478 | 0.719 | 5.022 | 0.172 | |
| Treatment | Surgery (undergone, ref not) | 3.630 | 0.052 | 0.759 | 0.649 |
| Antiretroviral drugs (undergone, ref not) | 2.747 | 0.171 | 1.942 | 0.429 | |
| Chemotherapy (undergone, ref not) | 1.092 | 0.897 | 0.480 | 0.226 | |
| Dose reduction of immunosuppressive therapy (undergone, ref not) | 0.930 | 0.918 | >>10 | 0.998 | |
| Radiotherapy (undergone, ref not) | 3.296 | 0.252 | 0.526 | 0.263 | |
| Electrochemotherapy (undergone, ref not) | 2.593 | 0.372 | 0.577 | 0.336 | |
| Persistent Response | Yes (ref no) | 2.958 | 0.032 | 5.616 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, I.; Marino, D.; Cozzolino, C.; Del Fiore, P.; Nerjaku, F.; Finotto, S.; Cattelan, A.; Calabrò, M.L.; Belloni Fortina, A.; Russano, F.; et al. Kaposi’s Sarcoma: Evaluation of Clinical Features, Treatment Outcomes, and Prognosis in a Single-Center Retrospective Case Series. Cancers 2024, 16, 691. https://doi.org/10.3390/cancers16040691
Russo I, Marino D, Cozzolino C, Del Fiore P, Nerjaku F, Finotto S, Cattelan A, Calabrò ML, Belloni Fortina A, Russano F, et al. Kaposi’s Sarcoma: Evaluation of Clinical Features, Treatment Outcomes, and Prognosis in a Single-Center Retrospective Case Series. Cancers. 2024; 16(4):691. https://doi.org/10.3390/cancers16040691
Chicago/Turabian StyleRusso, Irene, Dario Marino, Claudia Cozzolino, Paolo Del Fiore, Fitnete Nerjaku, Silvia Finotto, Annamaria Cattelan, Maria Luisa Calabrò, Anna Belloni Fortina, Francesco Russano, and et al. 2024. "Kaposi’s Sarcoma: Evaluation of Clinical Features, Treatment Outcomes, and Prognosis in a Single-Center Retrospective Case Series" Cancers 16, no. 4: 691. https://doi.org/10.3390/cancers16040691
APA StyleRusso, I., Marino, D., Cozzolino, C., Del Fiore, P., Nerjaku, F., Finotto, S., Cattelan, A., Calabrò, M. L., Belloni Fortina, A., Russano, F., Mazza, M., Galuppo, S., Bezzon, E., Sbaraglia, M., Krengli, M., Brunello, A., Mocellin, S., Piaserico, S., & Alaibac, M. (2024). Kaposi’s Sarcoma: Evaluation of Clinical Features, Treatment Outcomes, and Prognosis in a Single-Center Retrospective Case Series. Cancers, 16(4), 691. https://doi.org/10.3390/cancers16040691











