Suspicious Ultrasound-Occult Non-Calcified Mammographic Masses, Asymmetries, and Architectural Distortions Are Moderate Probability for Malignancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
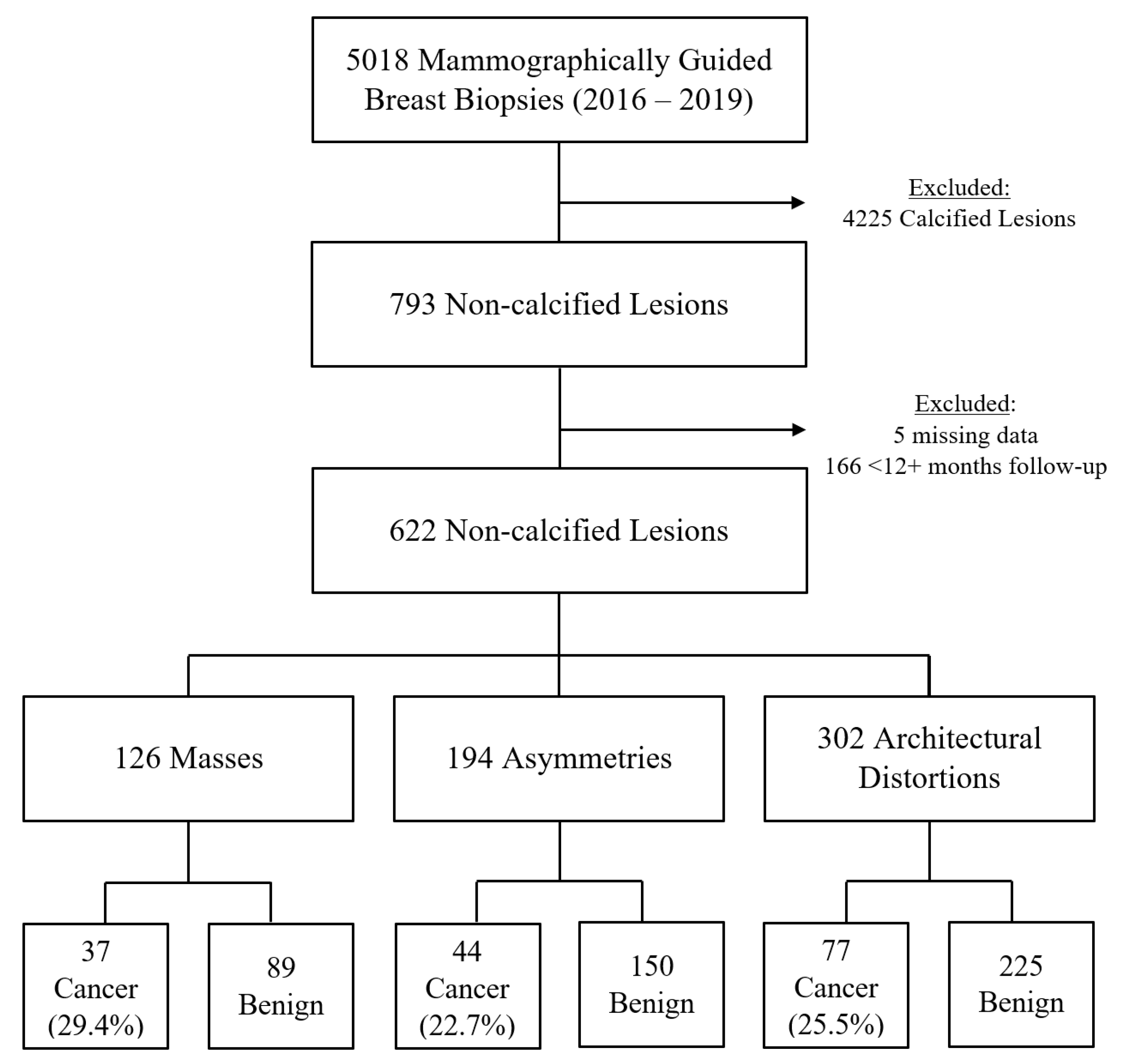
3.1. Study Population
3.2. Benign Versus Malignant Outcomes
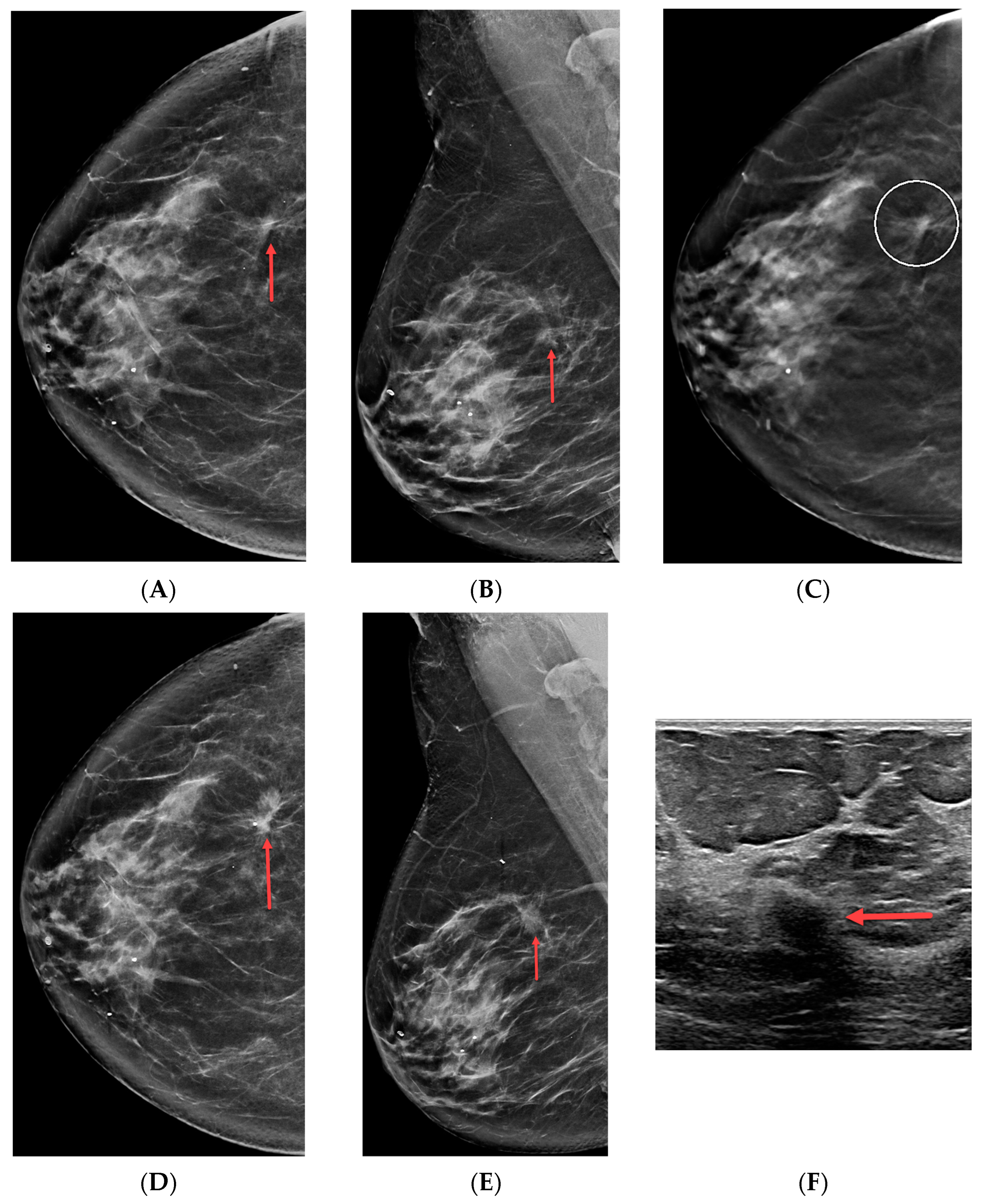
3.3. Architectural Distortions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Duffy, S.W.; Tabár, L.; Yen, A.M.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Törnberg, S.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979. [Google Scholar] [CrossRef]
- Tabár, L.; Dean, P.B.; Chen, T.H.; Yen, A.M.; Chen, S.L.; Fann, J.C.; Chiu, S.Y.; Ku, M.M.; Wu, W.Y.; Hsu, C.Y.; et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2019, 125, 515–523. [Google Scholar] [CrossRef]
- Duffy, S.W.; Tabár, L.; Chen, H.H.; Holmqvist, M.; Yen, M.F.; Abdsalah, S.; Epstein, B.; Frodis, E.; Ljungberg, E.; Hedborg-Melander, C.; et al. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 2002, 95, 458–469. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Bassett, L.W. Mammographic analysis of calcifications. Radiol. Clin. N. Am. 1992, 30, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.L.; Zhou, Z.H.; Wu, W.W.; Tian, J.; Xu, F.; Wu, S.C.; Tsui, P.H. A review of ultrasound detection methods for breast microcalcification. Math. Biosci. Eng. 2019, 16, 1761–1785. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Chu, P.; Kerlikowske, K.; Sickles, E.A.; Smith-Bindman, R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009, 250, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chang, T.H.; Chou, Y.C.; Hsu, H.H. Significance and positive predictive values of mammographic findings in the Asia-Pacific region: A single-centre study in Taiwan. Clin. Radiol. 2019, 74, 166.e1–166.e7. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.W.; Sickles, E.A. Developing asymmetry identified on mammography: Correlation with imaging outcome and pathologic findings. Am. J. Roentgenol. 2007, 188, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Sickles, E.A. The spectrum of breast asymmetries: Imaging features, work-up, management. Radiol. Clin. N. Am. 2007, 45, 765–771. [Google Scholar] [CrossRef]
- Durand, M.A.; Wang, S.; Hooley, R.J.; Raghu, M.; Philpotts, L.E. Tomosynthesis-detected architectural distortion: Management algorithm with radiologic-pathologic correlation. Radiographics 2016, 36, 311–321. [Google Scholar] [CrossRef]
- Choudhery, S.; Johnson, M.P.; Larson, N.B.; Anderson, T. Malignant Outcomes of Architectural Distortion on Tomosynthesis: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2021, 217, 295–303. [Google Scholar] [CrossRef]
- Patel, B.K.; Covington, M.; Pizzitola, V.J.; Lorans, R.; Giurescu, M.; Eversman, W.; Lewin, J. Initial Experience of Tomosynthesis-Guided Vacuum-Assisted Biopsies of Tomosynthesis-Detected (2D Mammography and Ultrasound Occult) Architectural Distortions. Am. J. Roentgenol. 2018, 210, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, G.R.; Newburg, A.; Vedantham, S. Positive Predictive Value of Tomosynthesis-guided Biopsies of Architectural Distortions Seen on Digital Breast Tomosynthesis and without an Ultrasound Correlate. J. Clin. Imaging Sci. 2019, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Walcott-Sapp, S.; Garreau, J.; Johnson, N.; Thomas, K.A. Pathology results of architectural distortion on detected with digital breast tomosynthesis without definite sonographic correlate. Am. J. Surg. 2019, 217, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Ambinder, E.B.; Plotkin, A.; Euhus, D.; Mullen, L.A.; Oluyemi, E.; Di Carlo, P.; Philip, M.; Panigrahi, B.; Cimino-Mathews, A.; Myers, K.S. Tomosynthesis-Guided Vacuum-Assisted Breast Biopsy of Architectural Distortion Without a Sonographic Correlate: A Retrospective Review. Am. J. Roentgenol. 2021, 217, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Linda, A.; Tarricone, R.; Londero, V.; Girometti, R.; Zuiani, C. Pathological outcome of sonographically occult architectural distortions (AD) visible only on digital breast tomosynthesis, and comparison with AD visible also on 2D mammography. Eur. J. Radiol. 2022, 146, 110075. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.O.; Korhonen, K.E.; Sun, J.; Leung, J.W.T. Comparison of prone and upright, stereotactic, and tomosynthesis-guided biopsies with secondary analysis of ultrasound-occult architectural distortions. Eur. Radiol. 2023, 33, 6189–6203. [Google Scholar] [CrossRef] [PubMed]
- Bahl, M.; Baker, J.A.; Kinsey, E.N.; Ghate, S.V. Architectural Distortion on Mammography: Correlation With Pathologic Outcomes and Predictors of Malignancy. Am. J. Roentgenol. 2015, 205, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Weaver, O.; Cohen, E.O.; Perry, R.E.; Tso, H.H.; Phalak, K.; Srinivasan, A.; Bassett, R.; Leung, J.W.T. Does lateral arm technique decrease the rate of clip migration in stereotactic and tomosynthesis-guided biopsies? Insights Imaging 2021, 12, 193. [Google Scholar] [CrossRef]
- Cohen, E.O.; Perry, R.E.; Tso, H.H.; Phalak, K.A.; Lesslie, M.D.; Gerlach, K.E.; Sun, J.; Srinivasan, A.; Leung, J.W.T. Breast cancer screening in women with and without implants: Retrospective study comparing digital mammography to digital mammography combined with digital breast tomosynthesis. Eur. Radiol. 2021, 31, 9499–9510. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, F.T.H.; van Asselt, A.A.; Dorrius, M.D.; Greuter, M.J.W.; de Bock, G.H. Mammographic breast density and the risk of breast cancer: A systematic review and meta-analysis. Breast 2022, 66, 62–68. [Google Scholar] [CrossRef]
- Price, E.R.; Joe, B.N.; Sickles, E.A. The developing asymmetry: Revisiting a perceptual and diagnostic challenge. Radiology 2015, 274, 642–651. [Google Scholar] [CrossRef]
- Youk, J.H.; Kim, E.K.; Ko, K.H.; Kim, M.J. Asymmetric mammographic findings based on the fourth edition of BI-RADS: Types, evaluation, and management. Radiographics 2009, 29, e33. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Samy, M.; Ali, A.M.; Hassan, R.A. Architectural distortion outcome: Digital breast tomosynthesis-detected versus digital mammography-detected. Radiol. Med. 2022, 127, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bahl, M.; Lamb, L.R.; Lehman, C.D. Pathologic Outcomes of Architectural Distortion on Digital 2D Versus Tomosynthesis Mammography. Am. J. Roentgenol. 2017, 209, 1162–1167. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Grady, D.; Barclay, J.; Sickles, E.A.; Eaton, A.; Ernster, V. Positive predictive value of screening mammography by age and family history of breast cancer. JAMA 1993, 270, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Sickles, E.A. Findings at mammographic screening on only one standard projection: Outcomes analysis. Radiology 1998, 208, 471–475. [Google Scholar] [CrossRef]
- Cohen, E.O.; Tso, H.H.; Phalak, K.A.; Mayo, R.C.; Leung, J.W.T. Screening Mammography Findings From One Standard Projection Only in the Era of Full-Field Digital Mammography and Digital Breast Tomosynthesis. Am. J. Roentgenol. 2018, 211, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Farshid, G.; Buckley, E. Meta-analysis of upgrade rates in 3163 radial scars excised after needle core biopsy diagnosis. Breast Cancer Res. Treat. 2019, 174, 165–177. [Google Scholar] [CrossRef] [PubMed]

| Masses (N = 126) | Asymmetries (N = 194) | Distortions (N = 302) | p Value | |
|---|---|---|---|---|
| Age (years) | 0.989 | |||
| Mean (SD) | 56.99 (10.75) | 57.12 (11.70) | 56.89 (10.84) | |
| Median (Range) | 57.76 (35.89, 80.97) | 56.18 (36.52, 86.20) | 56.30 (34.20, 88.00) | |
| Breast density | <0.001 | |||
| A | 11 (8.73%) | 15 (7.73%) | 3 (0.99%) | |
| B | 72 (57.14%) | 104 (53.61%) | 82 (27.15%) | |
| C | 43 (34.13%) | 71 (36.60%) | 197 (65.23%) | |
| D | 0 (0%) | 4 (2.06%) | 20 (6.62%) | |
| Personal history of breast cancer | 0.503 | |||
| No | 121 (96.03%) | 180 (92.78%) | 284 (94.04%) | |
| Yes | 5 (3.97%) | 14 (7.22%) | 18 (5.96%) | |
| Family history of breast cancer | 0.057 | |||
| No | 72 (57.14%) | 119 (61.34%) | 207 (68.54%) | |
| Yes | 54 (42.86%) | 75 (38.66%) | 95 (31.46%) | |
| Recently diagnosed with breast cancer † | 1 | |||
| No | 124 (98.41%) | 191 (98.45%) | 298 (98.68%) | |
| Yes | 2 (1.59%) | 3 (1.55%) | 4 (1.32%) | |
| Lesion size (millimeter) | <0.001 | |||
| N * | 115 | 165 | 256 | |
| Mean (SD) | 9.53 (6.42) | 19.11 (19.01) | 16.19 (10.16) | |
| Median (Range) | 8.00 (3.00, 46.00) | 13.00 (3.00, 132.00) | 14.00 (2.00, 63.00) | |
| Seen on 1 or 2 mammographic views | <0.001 | |||
| 1 | 2 (1.59%) | 29 (14.95%) | 30 (9.93%) | |
| 2 | 124 (98.41%) | 165 (85.05%) | 272 (90.07%) | |
| Possible ultrasound correlate | <0.001 | |||
| No | 74 (58.73%) | 158 (81.44%) | 204 (67.55%) | |
| Yes | 52 (41.27%) | 36 (18.56%) | 98 (32.45%) | |
| Reason for diagnostic mammogram | 0.087 | |||
| Screening recall | 104 (82.54%) | 151 (77.84%) | 246 (81.46%) | |
| BI-RADS 3 follow-up | 5 (3.97%) | 13 (6.70%) | 3 (0.99%) | |
| Palpable lump | 8 (6.35%) | 10 (5.15%) | 20 (6.62%) | |
| Prior history of breast cancer | 2 (1.59%) | 5 (2.58%) | 8 (2.65%) | |
| Other | 7 (5.56%) | 15 (7.73%) | 25 (8.28%) |
| Masses | Asymmetry | Architectural Distortion | Combined | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benign (N = 89) | Cancer (N = 37) | Total (N = 126) | p Value | Benign (N = 150) | Cancer (N = 44) | Total (N = 194) | p Value | Benign (N = 225) | Cancer (N = 77) | Total (N = 302) | p Value | Benign (N = 464) | Cancer (N = 158) | Total (N = 622) | p Value | |
| Age (years) | <0.001 | <0.001 | 0.006 | <0.001 | ||||||||||||
| Mean (SD) | 54.60 (10.38) | 62.74 (9.47) | 56.99 (10.75) | 54.43 (10.68) | 66.26 (10.42) | 57.12 (11.70) | 55.92 (11.04) | 59.72 (9.76) | 56.89 (10.84) | 55.18 (10.80) | 62.25 (10.20) | 56.98 (11.08) | ||||
| Median (Range) | 53.02 (35.89, 80.97) | 63.90 (40.06, 76.07) | 57.76 (35.89, 80.97) | 51.88 (36.52, 80.81) | 66.22 (44.96, 86.20) | 56.18 (36.52, 86.20) | 54.72 (34.20, 88.00) | 59.48 (41.00, 80.63) | 56.30 (34.20, 88.00) | 53.47 (34.20, 88.00) | 62.63 (40.06, 86.20) | 56.41 (34.20, 88.00) | ||||
| Breast density | 0.55 | 0.28 | 0.67 | 0.86 | ||||||||||||
| A | 9 (10.11%) | 2 (5.41%) | 11 (8.73%) | 9 (6.00%) | 6 (13.64%) | 15 (7.73%) | 2 (0.89%) | 1 (1.30%) | 3 (0.99%) | 20 (4.31%) | 9 (5.70%) | 29 (4.66%) | ||||
| B | 48 (53.93%) | 24 (64.86%) | 72 (57.14%) | 80 (53.33%) | 24 (54.55%) | 104 (53.61%) | 64 (28.44%) | 18 (23.38%) | 82 (27.15%) | 192 (41.38%) | 66 (41.77%) | 258 (41.48%) | ||||
| C | 32 (35.96%) | 11 (29.73%) | 43 (34.13%) | 58 (38.67%) | 13 (29.55%) | 71 (36.60%) | 143 (63.56%) | 54 (70.13%) | 197 (65.23%) | 233 (50.22%) | 78 (49.37%) | 311 (50.00%) | ||||
| D | 0 (0%) | 0 (0%) | 0 (0%) | 3 (2.00%) | 1 (2.27%) | 4 (2.06%) | 16 (7.11%) | 4 (5.19%) | 20 (6.62%) | 19 (4.09%) | 5 (3.16%) | 24 (3.86%) | ||||
| Personal history of breast cancer | 0.150 | 1.0 | 0.58 | 1 | ||||||||||||
| No | 87 (97.75%) | 34 (91.89%) | 121 (96.03%) | 139 (92.67%) | 41 (93.18%) | 180 (92.78%) | 210 (93.33%) | 74 (96.10%) | 284 (94.04%) | 436 (93.97%) | 149 (94.30%) | 585 (94.05%) | ||||
| Yes | 2 (2.25%) | 3 (8.11%) | 5 (3.97%) | 11 (7.33%) | 3 (6.82%) | 14 (7.22%) | 15 (6.67%) | 3 (3.90%) | 18 (5.96%) | 28 (6.03%) | 9 (5.70%) | 37 (5.95%) | ||||
| Family history of breast cancer | 0.70 | 0.73 | 0.20 | 0.180 | ||||||||||||
| No | 52 (58.43%) | 20 (54.05%) | 72 (57.14%) | 93 (62.00%) | 26 (59.09%) | 119 (61.34%) | 159 (70.67%) | 48 (62.34%) | 207 (68.54%) | 304 (65.52%) | 94 (59.49%) | 398 (63.99%) | ||||
| Yes | 37 (41.57%) | 17 (45.95%) | 54 (42.86%) | 57 (38.00%) | 18 (40.91%) | 75 (38.66%) | 66 (29.33%) | 29 (37.66%) | 95 (31.46%) | 160 (34.48%) | 64 (40.51%) | 224 (36.01%) | ||||
| Recently diagnosed with breast cancer † | 0.50 | 0.129 | 1.0 | 0.24 | ||||||||||||
| No | 88 (98.88%) | 36 (97.30%) | 124 (98.41%) | 149 (99.33%) | 42 (95.45%) | 191 (98.45%) | 222 (98.67%) | 76 (98.70%) | 298 (98.68%) | 459 (98.92%) | 154 (97.47%) | 613 (98.55%) | ||||
| Yes | 1 (1.12%) | 1 (2.70%) | 2 (1.59%) | 1 (0.67%) | 2 (4.55%) | 3 (1.55%) | 3 (1.33%) | 1 (1.30%) | 4 (1.32%) | 5 (1.08%) | 4 (2.53%) | 9 (1.45%) | ||||
| Lesion size (millimeter) | 0.88 | 0.44 | 0.32 | 0.117 | ||||||||||||
| N * | 78 | 37 | 115 | 126 | 39 | 165 | 190 | 66 | 256 | 394 | 142 | 536 | ||||
| Mean (SD) | 9.32 (5.71) | 9.97 (7.79) | 9.53 (6.42) | 18.19 (15.13) | 22.08 (28.19) | 19.11 (19.01) | 16.70 (10.66) | 14.71 (8.48) | 16.19 (10.16) | 15.72 (12.01) | 15.50 (16.83) | 15.66 (13.44) | ||||
| Median (Range) | 8.00 (3.00, 37.00) | 7.00 (3.00, 46.00) | 8.00 (3.00, 46.00) | 13.50 (3.00, 100.00) | 10.00 (4.00, 132.00) | 13.00 (3.00, 132.00) | 15.00 (2.00, 63.00) | 14.00 (2.00, 54.00) | 14.00 (2.00, 63.00) | 12.00 (2.00, 100.00) | 10.50 (2.00, 132.00) | 12.00 (2.00, 132.00) | ||||
| Seen on 1 or 2 mammographic views | 0.085 | 0.03 | 1.0 | 0.22 | ||||||||||||
| 1 | 0 (0.00%) | 2 (5.41%) | 2 (1.59%) | 27 (18.00%) | 2 (4.55%) | 29 (14.95%) | 23 (10.22%) | 7 (9.09%) | 30 (9.93%) | 50 (10.78%) | 11 (6.96%) | 61 (9.81%) | ||||
| 2 | 89 (100.00%) | 35 (94.59%) | 124 (98.41%) | 123 (82.00%) | 42 (95.45%) | 165 (85.05%) | 202 (89.78%) | 70 (90.91%) | 272 (90.07%) | 414 (89.22%) | 147 (93.04%) | 561 (90.19%) | ||||
| Possible ultrasound correlate | 0.33 | 0.121 | 0.162 | 0.012 | ||||||||||||
| No | 55 (61.80%) | 19 (51.35%) | 74 (58.73%) | 126 (84.00%) | 32 (72.73%) | 158 (81.44%) | 157 (69.78%) | 47 (61.04%) | 204 (67.55%) | 338 (72.84%) | 98 (62.03%) | 436 (70.10%) | ||||
| Yes | 34 (38.20%) | 18 (48.65%) | 52 (41.27%) | 24 (16.00%) | 12 (27.27%) | 36 (18.56%) | 68 (30.22%) | 30 (38.96%) | 98 (32.45%) | 126 (27.16%) | 60 (37.97%) | 186 (29.90%) | ||||
| Reason for diagnostic mammogram | 0.74 | 0.151 | 0.49 | 0.188 | ||||||||||||
| Screening recall | 73 (82.02%) | 31 (83.78%) | 104 (82.54%) | 120 (80.00%) | 31 (70.45%) | 151 (77.84%) | 183 (81.33%) | 63 (81.82%) | 246 (81.46%) | 376 (81.03%) | 125 (79.11%) | 501 (80.55%) | ||||
| BI-RADS 3 follow-up | 3 (3.37%) | 2 (5.41%) | 5 (3.97%) | 10 (6.67%) | 3 (6.82%) | 13 (6.70%) | 2 (0.89%) | 1 (1.30%) | 3 (0.99%) | 15 (3.23%) | 6 (3.80%) | 21 (3.38%) | ||||
| Palpable lump | 7 (7.87%) | 1 (2.70%) | 8 (6.35%) | 7 (4.67%) | 3 (6.82%) | 10 (5.15%) | 17 (7.56%) | 3 (3.90%) | 20 (6.62%) | 31 (6.68%) | 7 (4.43%) | 38 (6.11%) | ||||
| Prior history of breast cancer | 1 (1.12%) | 1 (2.70%) | 2 (1.59%) | 5 (3.33%) | 0 (0.00%) | 5 (2.58%) | 7 (3.11%) | 1 (1.30%) | 8 (2.65%) | 13 (2.80%) | 2 (1.27%) | 15 (2.41%) | ||||
| Other | 5 (5.62%) | 2 (5.41%) | 7 (5.56%) | 8 (5.33%) | 7 (15.91%) | 15 (7.73%) | 16 (7.11%) | 9 (11.69%) | 25 (8.28%) | 29 (6.25%) | 18 (11.39%) | 47 (7.56%) | ||||
| Screening Mammogram | Diagnostic Mammogram | p Value | ||
|---|---|---|---|---|
| Mass | Benign (N = 89) | 73 (82.02%) | 16 (17.98%) | 1.0 |
| Cancer (N = 37) | 31 (83.78%) | 6 (17.22%) | ||
| Asymmetry | Benign (N = 150) | 120 (80.00%) | 30 (20.00%) | 0.22 |
| Cancer (N = 44) | 31 (70.45%) | 13 (29.55%) | ||
| Architectural Distortion | Benign (N = 225) | 183 (81.33%) | 42 (18.67%) | 1.0 |
| Cancer (N = 77) | 63 (81.82%) | 14 (18.18%) | ||
| Combined | Benign (N = 464) | 376 (81.03%) | 88 (18.97%) | 0.64 |
| Cancer (N = 158) | 125 (79.11%) | 33 (20.89%) |
| DM | DBT | p Value, Benign: Cancer | ||
|---|---|---|---|---|
| Mass | Benign (N = 89) | 44 (72.13%) | 45 (69.23%) | 0.85 |
| Cancer (N = 37) | 17 (27.87%) | 20 (30.77%) | ||
| Total (N = 126) | 61 (48.41%) | 65 (51.59%) | ||
| Asymmetry | Benign (N = 150) | 77 (80.20%) | 73 (74.49%) | 0.39 |
| Cancer (N = 44) | 19 (19.80%) | 25 (25.51%) | ||
| Total (N = 194) | 96 (49.48%) | 98 (50.52%) | ||
| Architectural Distortion | Benign (N = 225) | 40 (71.43%) | 185 (75.20%) | 0.61 |
| Cancer (N = 77) | 16 (28.57%) | 61 (24.80%) | ||
| Total (N = 302) | 56 (18.54%) | 246 (81.46%) | ||
| Combined | Benign (N = 464) | 161 (75.59%) | 303 (74.08%) | 0.70 |
| Cancer (N = 158) | 52 (24.41%) | 106 (25.92%) | ||
| Total (N = 622) | 213 (34.24%) | 409 (65.76%) | ||
| p value Total DM: Total DBT | Mass vs. Asymmetry | 0.91 | ||
| Distortion vs. Mass | <0.001 | |||
| Distortion vs. Asymmetry | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, E.O.; Perry, R.E.; Legha, R.S.; Tso, H.H.; Shin, K.; Speer, M.E.; Phalak, K.A.; Sun, J.; Leung, J.W.T. Suspicious Ultrasound-Occult Non-Calcified Mammographic Masses, Asymmetries, and Architectural Distortions Are Moderate Probability for Malignancy. Cancers 2024, 16, 655. https://doi.org/10.3390/cancers16030655
Cohen EO, Perry RE, Legha RS, Tso HH, Shin K, Speer ME, Phalak KA, Sun J, Leung JWT. Suspicious Ultrasound-Occult Non-Calcified Mammographic Masses, Asymmetries, and Architectural Distortions Are Moderate Probability for Malignancy. Cancers. 2024; 16(3):655. https://doi.org/10.3390/cancers16030655
Chicago/Turabian StyleCohen, Ethan O., Rachel E. Perry, Ravinder S. Legha, Hilda H. Tso, Kyungmin Shin, Megan E. Speer, Kanchan A. Phalak, Jia Sun, and Jessica W. T. Leung. 2024. "Suspicious Ultrasound-Occult Non-Calcified Mammographic Masses, Asymmetries, and Architectural Distortions Are Moderate Probability for Malignancy" Cancers 16, no. 3: 655. https://doi.org/10.3390/cancers16030655
APA StyleCohen, E. O., Perry, R. E., Legha, R. S., Tso, H. H., Shin, K., Speer, M. E., Phalak, K. A., Sun, J., & Leung, J. W. T. (2024). Suspicious Ultrasound-Occult Non-Calcified Mammographic Masses, Asymmetries, and Architectural Distortions Are Moderate Probability for Malignancy. Cancers, 16(3), 655. https://doi.org/10.3390/cancers16030655






