Simple Summary
Medical control of cancer pain is often unsatisfactory. Narcotic drugs (opioids) are effective pain killers, but they have important negative effects on the central nervous system and the are also highly addictive. A logical strategy to avoid central narcotic adverse effects is to target the peripheral nociceptors where cancer pain is generated. Sensory afferents that express the capsaicin receptor TRPV1 play a central role in cancer pain. In animal experiments, pharmacological blockade or chemical ablation of these nerves provide lasting cancer pain relief. High-dose capsaicin patches are already in clinical use in patients with chemotherapy-induced neuropathic pain. Site-specific resiniferatoxin (an ultrapotent capsaicin analog) injections are currectly undergoing clinical trials in patients with chronic intractable cancer pain caused by metastatic bone disease. This review explores the analgesic potential of small molecule TRPV1 antagonists and the sensory afferent desensitization in cancer patients.
Abstract
Chronic intractable pain affects a large proportion of cancer patients, especially those with metastatic bone disease. Blocking sensory afferents for cancer pain relief represents an attractive alternative to opioids and other drugs acting in the CNS in that sensory nerve blockers are not addictive and do not affect the mental state of the patient. A distinct subpopulation of sensory afferents expresses the capsaicin receptor TRPV1. Intrathecal resiniferatoxin, an ultrapotent capsaicin analog, ablates TRPV1-expressing nerve endings exposed to the cerebrospinal fluid, resulting in permanent analgesia in women with cervical cancer metastasis to the pelvic bone. High-dose capsaicin patches are effective pain killers in patients with chemotherapy-induced peripheral neuropathic pain. However, large gaps remain in our knowledge since the mechanisms by which cancer activates TRPV1 are essentially unknown. Most important, it is not clear whether or not sensory denervation mediated by TRPV1 agonists affects cancer progression. In a murine model of breast cancer, capsaicin desensitization was reported to accelerate progression. By contrast, desensitization mediated by resiniferatoxin was found to block melanoma growth. These observations imply that TRPV1 blockade for pain relief may be indicated for some cancers and contraindicated for others. In this review, we explore the current state of this field and compare the analgesic potential of TRPV1 antagonism and sensory afferent desensitization in cancer patients.
1. Introduction
Cancer pain is a general term for a broad range of pain conditions with different etiologies and molecular mechanisms [1,2,3]. It is a serious problem, affecting an estimated one third of cancer patients [4]. In the terminally ill population, the proportion of patients with chronic, intractable cancer pain may exceed 80% [5].
Cancer pain is complex and poorly understood [1,2,3,6], hindering drug development. The guidelines for the management of cancer pain were developed by the World Health Organization almost four decades ago [7]. Since cancer pain is not homogenous, satisfactory pain management should be tailored based on an individual assessment of the pain mechanisms. Yet, available treatment options (Table 1) remain symptomatic [8], and for prolonged treatment, patients may become refractory. At present, opioids constitute the mainstay of treatment with worrisome side-effects, like a sedated mental state and constipation, which negatively affect the quality of life of the patients [9]. Even worse, overdosing with opioids can be lethal. Consequently, cancer pain is often undertreated [10]. Clearly, there is a dire need for new treatment modalities with more tolerable side effects.

Table 1.
Cancer pain; cancer pain treatments in adult patients—briefly.
Recently, chronic pain has been divided into primary (unknown underlying disease) and secondary pain. Chronic cancer pain is a form of secondary pain; that is, pain linked to underlying disease [11]. The classification of cancer pain is different for solid tumors and hematological malignancies (Table 2 and Table 3) [12,13,14]. In a much simplified manner, pain caused by solid tumors can be divided into three major mechanisms. One, tumor cells can secrete substances that, in turn, activate the tumor-infiltrating sensory nerve ending [15]. Two, cancers can press, infiltrate, or destroy tissues, including sensory nerves [16,17]. And three, metastatic tumors (especially bone metastasis) can create their acidic microenvironment, rich in sensory innervation [18]. Of course, the line between these groups is often blurred. For example, metastatic bone tumors can cause pathological fractures; in this case, generalized bone pain (due to bone marrow infiltration) and bone fracture pain may combine with pain due to activation by the protons of sensory afferents.

Table 2.
Representative examples of chronic pain syndromes caused by solid tumors. GI, gastrointestinal.

Table 3.
Types of chronic pain caused by hematological malignancies with representative examples.
Hematological malignancies can cause nociceptive, neuropathic, and mixed pain (Table 3) [14]. The nociceptive group can be further subdivided into superficial somatic, deep somatic, and visceral pain. Neuropathic pain can be central or peripheral. Again, the pain is often mixed. For example, plasma cell myeloma may induce bone pain via osteolysis and fracture, with peripheral neuropathic pain via paraprotein/amyloid production [19].
Of note, chemotherapy for cancers can cause neuropathic pain. In fact, the incidence of chemotherapy-induced neuropathic pain (CINP) can be as high as 80% [20]. Sadly, CIPN persists in a large subset of patients even after the discontinuation of the chemotherapy.
Sensory nerves are attractive candidates to ameliorate cancer pain since they are involved in all major mechanisms (nociceptive, inflammatory, and neuropathic) of cancer pain generation [15,16,17,18]. A major subdivision of primary sensory neurons is characterized by its unique sensitivity to capsaicin [21,22]. These nerves express the capsaicin receptor Transient Receptor Potential Vanilloid 1 (TRPV1) [23], but also carry other TRP channels, like TRPA1 [24,25], as well as non-TRP channels, for example, acid-sensing ion channels (ASICs) [26] implicated in pain perception (Figure 1). This redundancy in pain targets begs the question of whether or not blocking a single receptor, like TRPV1, can provide meaningful pain relief or the whole neuron must be silenced (Figure 2). There are arguments pro and contra for both approaches. In this review, we provide an overview of the current state of this exciting and rapidly changing field, from basic research to clinical trials.
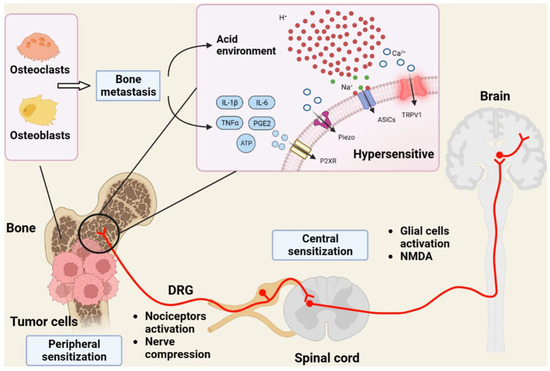
Figure 1.
The complex etiology of bone cancer pain. Tumor cells that metastasize to the bone create an acidic environment that stimulates TRPV1 and other proton-sensitive channels in sensory afferents, like acid-sensitive ASICs. In turn (not shown), TRPV1-expressing nerve terminals release CGRP, a known facilitator of tumor cell growth. DRG, dorsal root ganglion; IL-6, interleukin-6; TNFα, tumor necrosis factor-α; PGE2, prostaglandin E2; P2XR, purinergic P2X receptor; Piezo, mechanosensitive ion channel. Figure reproduced with permission from [27].
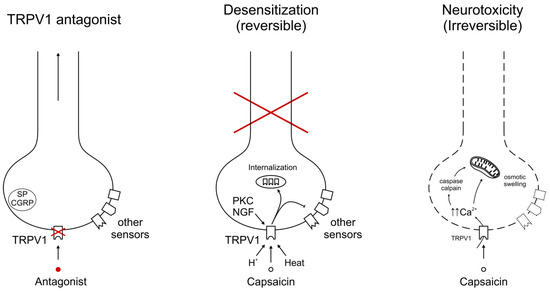
Figure 2.
Molecular mechanisms of cancer pain relief, targeting TRPV1. Small-molecule TRPV1 antagonists block the channel protein only, leaving other pain-sensing targets functional. This approach will work only if cancer pain is mediated by an algesic compound that acts directly via TRPV1. By contrast, the chemical defunctionalization of TRPV1-expressing sensory afferents silences the whole neuron. This action can be either reversible (traditionally termed “desensitization”) or irreversible. A high-dose (8%) capsaicin patch is a representative example of desensitization. Intrathecal RTX (a “molecular scalpel”) may irreversibly ablate the central terminals of capsaicin-sensitive afferents for permanent pain relief. The cross in red indicates disruption by capsaicin of sensory information from the periphery (“capsaicin desensitization”).
Please note that other TRP channels relevant to cancer pain (e.g., TRPA1 and TRPM8) will be discussed elsewhere in this thematic issue.
2. TRPV1 in Cancer Pain: Molecular Mechanisms
TRPV1 is best known as the receptor for capsaicin, the pungent substance in hot pepper [23]. However, TRPV1 is also directly activated by noxious heat [23], a discovery (molecular mechanism of temperature sensation) that earned a shared (with Ardem Patapoutian for the discovery of touch receptors) Nobel prize in Physiology and Medicine for David Julius in 2021. In accord, TRPV1-null mice [28,29], as well as men with non-functioning TRPV1 [30], exhibit deficits in noxious heat sensation. Furthermore, TRPV1 is activated by changes in pH (in particular, by protons) [31,32] and represents a downstream target for various pain-generating substances, as exemplified by bradykinin [33,34].
The cross-talk between cancer cells and sensory afferents in the tumor microenvironment is subject to intensive research [35,36,37,38]. Tumors can actively recruit nerves, and extensive tumor innervation was suggested to herald aggressive disease [39,40,41]. Conversely, the ablation of tumor-infiltrating afferents may ameliorate cancer progression [39,40,41]. Interestingly, cancers display higher electrical activity than normal tissues, and tumors implanted into transgenic mice lacking TRPV1-positive nociceptors neurons show reduced electrical activity [42].
There is preliminary evidence that cancer cells can synthesize substances capable of activating TRPV1. For example, sarcoma cells were shown to produce a lipophilic TRPV1-targeting molecule that is yet to be identified [43]. Tumor cells can also generate formaldehyde, which, in concert with the acidic microenvironment, can synergistically activate TRPV1 [44]. Oral squamous cell carcinoma cells secrete nerve growth factor (NGF) [45], a known regulator of TRPV1 expression [46,47]. NGF can increase TRPV1 protein synthesis, whereas the proto-oncogen Src kinase may promote TRPV1 trafficking into the cell membrane [48,49]. Macrophages in the tumor microenvironment can also activate sensory nerve endings via the interleukin-23 (IL23)/IL17A/TRPV1 axis [50].
The phosphorylation status of the TRPV1 channel protein is, in part, regulated by calcineurin [51], a Ca2+ and calmodulin-dependent serine/threonine protein phosphatase. Decreased calcineurin activity is thought to facilitate the transition of acute pain to chronic pain [52,53]. Indeed, tacrolimus (a calcineurin inhibitor) was found to cause severe pain in some transplant patients [53], though it may be mediated by TRPA1 [54], rather than TRPV1 channels. Conversely, restoring calcineurin activity provides pain relief.
In a rodent orthotopic model of breast cancer, dense sensory afferent innervation of the tumor was observed [55]. Co-cultured with sensory neurons, breast cancer cells stimulated neurite outgrowth [56]. This breast cancer-induced aberrant sensory branching may be a major player in breast cancer pain. Of note, melanoma cells can also interact with nociceptive neurons to facilitate neurite growth [57]. The mechanisms by which tumor cells facilitate neurite growth are unknown. One possible candidate is cyclin-dependent like kinase-5 (CDKL5), an enzyme highly expressed in nociceptive neurons [58]. In fact, CDKL5-null mice exhibit defective epidermal innervation and impaired nociception [58]. One may argue that tumor cells promote neurite growth by stimulating CDKL5.
Most patients with metastatic bone cancer experience severe pain. The molecular mechanisms by which bone metastasis generates pain are only beginning to be understood (Figure 1) [59,60,61,62]. Cancer cells create their own acidic microenvironment [62,63,64]. Protons are known activators of TRPV1 [32,65]. In fact, bafilomycin A1, a selective blocker of proton secretion [66], alleviates bone cancer pain [67]. Cancers need a blood supply to grow; therefore, they promote vascular neogenesis (Figure 3) [68,69,70]. Furthermore, osteoblasts can produce insulin-like growth factor-1 (IGF-1) [71]. In a rat model of bone cancer pain, IGF1 was found to up-regulate TRPV1 expression in sensory afferents [72].
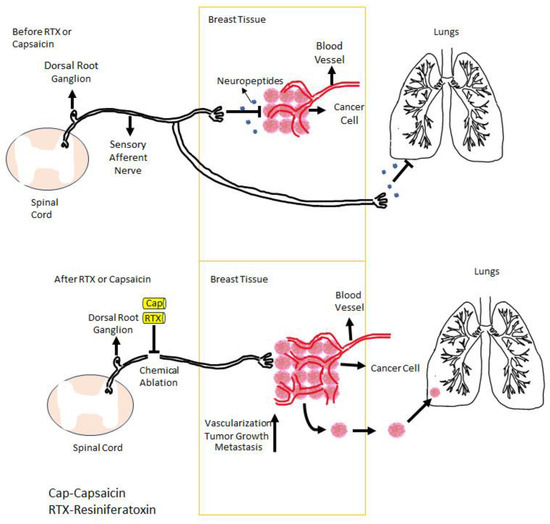
Figure 3.
In breast cancer, TRPV1-expressing sensory afferents negatively control the vascular supply (neovascularization) of the tumor through the neuropeptides that they release. If these afferents are ablated either by capsaicin or RTX, this negative control is lost, and the tumor is starting to grow and metastasize. Figure courtesy of Mertay Şimşek and Nuray Erin (Akdeniz University, Turkey).
TRPV1-expressing nerve endings release calcitonin gene-related peptide (CGRP) that, in turn, can stimulate cancer growth [73,74,75]. In a murine cancer model (Lewis carcinoma inoculated into the paw), tumor growth was attenuated in both TRPV1-null and αCGRP-null mice compared to in wild-type littermates [76]. Thus, a vicious circle is generated in which cancer cells create their own aberrant sensory innervation, and these nerves promote cancer growth.
Most recently, cancer-derived small extracellular vesicles have been implicated in the cross-talk between head-and-neck carcinoma and TRPV1-expressing afferents [76]. The injection of purified cancer-derived vesicles into naïve mice induces hypersensitivity that was absent in TRPV1-null animals [77]. Cancer-derived vesicles also evoke Ca2+ uptake in nociceptors, presumably by opening the TRPV1 channel [77].
There is increasing evidence that visceral pain differs from somatic pain. CINP is also fundamentally different from other forms of cancer pain [78,79,80,81]. For example, paclitaxel affects lipid raft formation in sensory neurons [82]. In addition, paclitaxel sensitizes TRPV1 through phosphorylation and increases the number of TRPV1 channels in the lipid rafts [82]. This enhances the chance of an interaction between TRPV1 and toll-like receptor-4 (TLR-4) [82]. Cisplatin [83] and oxaliplatin also stimulate TRPV1 expression [84] (along with TRPA1 [84] and TRPM8 [85]) in sensory afferents.
3. Can Selective TRPV1 Antagonism Ameliorate Cancer Pain?
There is increasing evidence that TRPV1 plays a major role in cancer pain [86]. In preclinical studies, selective TRPV1 inactivation through genetic manipulation [87,88] or pharmacological blockade [89] was shown to ameliorate cancer pain. Ehrlich tumor cells injected into the paw of mice cause nociception [90]. The intrathecal administration of AMG9810, a potent small-molecule TRPV1 antagonist, blocks both mechanical and thermal hyperalgesia [90]. The genetic inactivation of Trpv1 (TRPV1-null mice) also prevents thermal hyperalgesia but has no effect on tumor growth [90]. In a rat model of bone cancer pain (SCC158 carcinoma cells injected into the hind paw), the first generation TRPV1 antagonist capsazepine blocked both mechanical and thermal hyperalgesia [91]. In a follow-up experiment, TRPV1 knock-down via siRNA also ameliorated both mechanical and thermal hyperalgesia [87]. Mice injected with Lewis lung cancer cells experience progressive bone cancer pain, which is markedly reduced in TRPV1-null animals [92].
Taken together, these experiments imply the clinical value of selective TRPV1 blockade in patients with cancer pain.
4. Topical Capsaicin Patch for Chemotherapy-Induced Peripheral Neuropathy (CIPN)
Capsaicin is unique among natural compounds in that the initial burning sensation that it evokes is followed by a lasting refractory state (traditionally termed “desensitization”) in which the previously excited sensory neurons are refractory to not only capsaicin but various unrelated chemical and physical stimuli [21,22,93,94,95]. The molecular mechanisms of capsaicin desensitization are only beginning to be understood [22,95,96,97]. Reversible desensitization (sometimes referred to as “functionalization”) should be distinguished from the irreversible loss (chemical ablation) of TRPV1-expressing sensory afferents [95,96].
Strictly speaking, capsaicin desensitization is mediated by the TRPV1 protein. However, at high concentrations, the selectivity of capsaicin for TRPV1 is lost, and capsaicin may start interacting with other targets, including voltage-gated Na+ channels [98,99]. In fact, voltage-gated Na+ channels are expressed in capsaicin-sensitive dorsal root ganglion (DRG) neurons [100], and capsaicin can be used to deliver the impermeant (permanently charged) sodium channel blocker QX-314 to achieve long-lasting nociceptive blockade [101,102]. However, at present, it is not clear what (if any) role the off-target capsaicin blockade of voltage-gated Na+ channels may play in capsaicin-induced analgesia. Regardless of its molecular underpinnings, the analgesic potential of capsaicin desensitization has been confirmed in clinical studies [103,104,105].
High-dose capsaicin patches are well suited for patients with localized pain, such as peripheral neuropathy affecting the feet (Figure 4). In 16 patients with chronic CIPN (mean duration of 2.5 years), a high-dose capsaicin patch (8% capsaicin, Qutenza) applied for 30 min to the affected foot provided the significant reduction of both spontaneous (mean Numeric Pain Rating Scale, −1.27) and evoked pain [106]. The Short-Form McGill questionnaire revealed a reduction if neuropathic pain and the Patient Global Impression of Change showed a significant improvement [106]. Importantly, the treatment restored epidermal nerve fibers in skin biopsies, potentially a “disease-modifying” action [106]. No systemic side-effects were reported.
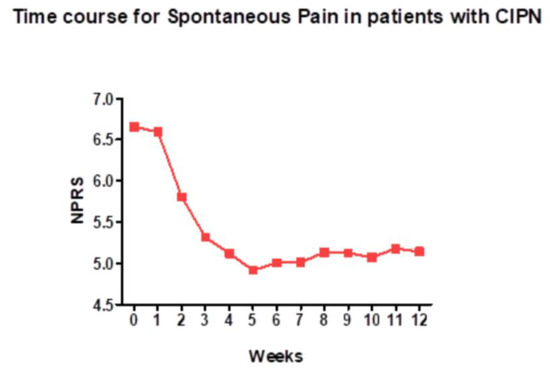
Figure 4.
A high-dose (8%) capsaicin patch relieves spontaneous pain in patients with chemotherapy-induced peripheral neuropathy. NPRS (Numeric Pain Rating Scale) is a numeric version of the visual analog scale, in which patients select a number between 0 and 10 that best reflects the reduction in pain (10, no change; 0, complete loss of pain). Figure courtesy of Dr. Praveen Anand, Imperial College, London, UK.
Post-mastectomy pain affects 25 to 60% of breast cancer patients. It is defined as pain that persists for at least 3 months after surgery. In a pilot study, 12 of the 14 study participants completed a 4-week trial with topical 0.025% capsaicin: eight patients reported satisfactory pain relief [107]. In a follow-up randomized, placebo-controlled trial with 0.075% capsaicin, 5 of the 13 patients were categorized as good responders based on the visual analogue scale for steady pain [108]. An open-label clinical trial with 0.025% capsaicin in Italy reported similar results: out of the 19 study participants, two reported complete pain relief, and an additional 11 patients described a significant reduction in pain [109]. The treatment was well-tolerated with no drop-out due to side-effects. With the 0.075% topical capsaicin cream, clinically meaningful pain relief (53% compared to 17% in the placebo group) was reported in a cohort of 99 cancer patients with post-surgical pain [110]. A recent retrospective analysis of the post-mastectomy pain studies with low-concentration capsaicin creams has identified several problems [111]; therefore, this treatment modality is no longer be recommended.
A high-dose (8%) capsaicin patch is an effective intervention to ameliorate post-surgical pain in general [112] and also in cancer patients [113]. Two case reports described the relief of post-mastectomy pain with Qutenza [114,115]. A multicentric, open, randomized clinical trial is ongoing to compare an 8% capsaicin patch (Qutenza) to per os pregabalin (recommended as an adjuvant analgesic for neuropathic cancer pain [116]) in the early treatment of neuropathic pain after breast surgery [117].
In a French study involving 279 breast cancer patients with peripheral neuropathy caused by surgery, chemotherapy, or radiation therapy, repeated Qutenza applications (on average, 4) resulted in a significant analgesic effect in 82% of the study participants, including those with post-mastectomy pain [118].
In a monocentric observational retrospective real-world data study, in which independent pain physicians completed a Clinician Global Impression of Change survey, significant or complete pain relief was noted in 44% of the 57 participating patients after a total of 184 capsaicin applications [119]. Pain relief was observed after at least three capsaicin applications, and the efficacy increased with repeated treatments. This study also pointed out that capsaicin is not effective in patients whose CIPN was platinum-induced [119]. The most significant side-effect of the capsaicin patch was an initial burning sensation that could be minimized by topical analgesics or cooling of the treated area.
Based on this study, ESMO (European Society for Medical Oncology) has recommended high-dose capsaicin patches as 2nd line therapy for CIPN [120].
The on-going TEC-ORL phase-2 clinical trial (NCT04704453) wishes to compare the analgesic potential of the high-dose (8%) capsaicin patch, Qutenza, and amitryptiline (Laroxyl, the most common analgesic adjuvant used for cancer patients with neuropathic pain [121]) in 130 patients with head-and-neck squamous cell carcinoma [122]. The primary outcome is pain reduction by 2 points during a 9-month trial.
As mentioned above, high-dose capsaicin patches are appropriate for patients with localized, but not generalized, pain. Cancer pain usually affects several dermatomes. Of note, oral cancer patients often experience severe pain at the site of cancer with new sensitivity to spicy food [123]. These patients are very sensitive to local capsaicin challenge [124], indicating increased TRPV1 expression and/or sensitization. It would be interesting to see if desensitization to topical capsaicin could provide pain relief in this patient population.
5. Resiniferatoxin for Permanent Cancer Pain Relief: Preclinical Studies
Resiniferatoxin (RTX), isolated from the latex of Euphorbia resinifera Berg [125], is an ultrapotent analog of capsaicin with some important differences in pharmacological actions [94]. For example, RTX does not provoke the pulmonary chemoreflex [126], a dose-limiting side-effect of capsaicin administration. Consequently, in the rat full, desensitization of the neurogenic inflammatory response can be achieved by means of a single s.c. RTX administration [94]. The same response can only be replicated with repeated capsaicin administrations over several days.
In the human urinary bladder, RTX evokes a long-lasting (several weeks) but fully reversible desensitization [127,128,129]. This contrasts the irreversible “silencing” action of intrathecal RTX on TRPV1-expressing axons exposed to the cerebrospinal fluid [130,131,132]. This action earned the name “molecular scalpel” for RTX [133].
In the rat, intrathecal RTX (10 to 200 ng administered via lumbar puncture) ablated most TRPV1-positive nerve endings in the dorsal horn of the spinal cord [134,135] and, at the same time, increased the withdrawal latency to radiant noxious heat [134]. In a mouse model of bone cancer pain, intrathecal RTX achieved lasting pain relief [136].
Cancer pain in companion dogs is an important issue in veterinary medicine [137]. Large dogs are prone to develop osteosarcoma in their limbs. These animals experience severe pain and keep the affected, painful limb in an elevated, guarding position. Inspired by the rodent experiments, intrathecal RTX (1.2 μg/kg) was administered to twenty companion dogs with intractable cancer pain under general anesthesia [138]. One hour later the animals were awakened and their vital signs were tested. A transient increase in blood pressure (79 to 131 mmHg) and the heart rate (123 to 161 beats per minute) was noted, which peaked 1–2 h after RTX administration and disappeared by 4 h. The animals also showed a reduced rectal temperature. None of this was really unexpected. Hypothermia is a well-known effect of capsaicin and RTX administration [139]. Furthermore, TRPV1 is expressed in resistance arteries where their activation leads to vasoconstriction, elevating the blood pressure [140]. Importantly, the day after intrathecal RTX administration, the dogs became ambulatory and their owners reported increased comforts levels [138]. RTX did not slow down the progression of the cancer, but the analgesic action persisted until the animals perished [138].
A prospective, randomized, and blinded trial comparing intrathecal RTX (1.2 μg/kg injected into the cisterna magna) with the standard-of-care was carried out on 72 dogs with bone cancer pain [141]. The animal was removed from the trial (“unblinded”) when the owner reported too much pain. More animals in the standard-of-care group (78%) were “unblinded” sooner than those in the RTX group (50%), indicating significant pain relief mediated by RTX [141].
6. Resiniferatoxin for Permanent Cancer Pain Relief: Clinical Trials
The favorable experience with intrathecal RTX in companion dogs with bone cancer pain has incentivized the transition to human clinical trials [133,142,143,144]. The first clinical trial with intrathecal RTX (NCT 00804154), by and large, followed the design of the veterinary trial. Patients with intractable pain due to metastatic bone disease (for example, women with cervical carcinoma metastasis to the pelvic bone) were recruited, and RTX was administered intrathecally under general anesthesia (Figure 5) [145]. This open-label, single-site, phase-1 clinical trial (NCT 00804154) involved nine patients. RTX was injected manually at a starting dose of 3 μg in a volume of 1 mL. The first patient reported pain relief at the starting dose, whereas the three other study participants needed a second dose of 13 μg to achieve pain relief. An additional five patients were given a higher RTX dose of 26 μg. Whereas the starting dose was well tolerated, the higher RTX dose resulted in impaired noxious heat sensation as an on-target adverse effect [146]. For example, a few patients suffered scalding injuries by the imbibition of hot coffee. Most of these episodes could be prevented by warning the patients of the danger of hot food and fluids. At present, this study is recruiting patients to test the analgesic potential of an even higher RTX dose (44 μg).
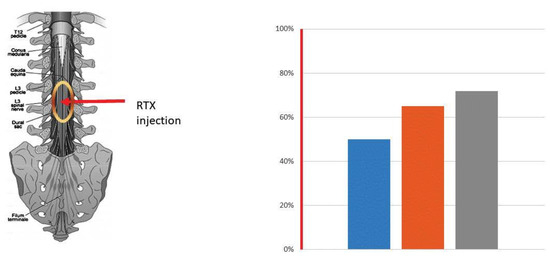
Figure 5.
Cancer pain relief mediated by intrathecal RTX, 13 µg. The red arrow indicates the site of RTX injection between L3 and L4. The oval sign shows the spread of RTX in the spinal cord. RTX was injected under general anesthesia (1.5 h). An i.v. opioid was administered for the residual acute burning pain evoked by the RTX injection. The bars represent a reduction (in %) in the average NPR Score (50%, blue column), the 7 brief pain inventory (65%, brown column), and daily oxycodone use (72%, grey column) after RTX injection. Data are from a middle-age male patient with supraglottic squamous cell carcinoma metastasizing to the pelvic bone [145].
Neuroaxial analgesia includes intrathecal and epidural drug delivery. Since intrathecal (subarachnoid) analgesia is the administration of analgesics directly into the cerebrospinal fluid, drugs given via this route are both much faster and more potent in action. Therefore, it is not unexpected that most opioid side effects are more common and severe if the opioid is administered intrathecally [147]. Similar considerations may apply to neuroaxial RTX treatment [148].
Intrathecal RTX is an effective but problematic means to achieve analgesia. It must be given under general anesthesia because of the transient pain that it evokes, and it puts significant burden on the heart by elevating blood pressure and accelerating the heart rate [114]. Based on the experience with opioids, epidural RTX is expected to lack these complications.
The first clinical trial with epidural RTX (0.4 μg to 25 μg given under mild sedation) enrolled 17 patients (Table 4) [149]. RTX was injected either directly into the epidural space or administered via a catheter placed under fluoroscopic guidance. Three patients reported significant (30%, 50%, and 70%, respectively) reductions in pain scores that lasted until the very end of the study (12 weeks) [149]. Four study participants withdrew from the study or were lost to follow-up, whereas the remaining ten patients died due to the progression of their metastatic disease. At present, Sorrento Therapeutics (San Diego, CA, USA) is recruiting patients for a multicenter, randomized, Phase 2 study to assess the efficacy and safety of a single epidural administration of RTX (15, 20, or 25 μg in 2 mL) versus the placebo for the treatment of intractable pain associated with cancer [150]. This trial was supposed to start in September 2023 with 120 patients.

Table 4.
Clinical analgesic efficacy of epidural RTX (15 µg or 25 µg) assessed at a 30%, 50%, and 70% decrease in pain (average and worst pain) from the baseline NPRS score. Data are from [142].
7. Conclusions and Future Research Directions
The complex and poorly understood nature of cancer pain represents a large barrier to drug development [1,2,3,6,151,152]. Available treatment options (Table 1) are symptomatic and cause significant side-effects [8,9]. Sensory afferents represent an attractive alternative to analgesic drugs targeting the CNS, in that drugs that block these nerves are not addictive and do not affect cognition.
A distinct subpopulation of sensory afferents expresses the capsaicin receptor TRPV1 [21,22,153]. There is good evidence both in preclinical [136,138,141] and clinical studies [145,146,149] that TRPV1-positive afferents play an important role in cancer pain. Cancer pain is reduced in TRPV1-null mice compared to in wild-types [76,92]. Moreover, the ablation of TRPV1-positive nerve endings mediated by intrathecal [138,145] or epidural [149] RTX results in lasting pain relief (Figure 5). Intrathecal RTX, however, has to be administered under general anesthesia because of the initial transient pain reaction that it evokes [138,148]. Moreover, intrathecal RTX is associated with significant on-target side-effects, including a spike in blood pressure, increased heart rate, urinary retention, and impaired noxious heat sensation [138]. The optimal dose at which intrathecal RTX provides meaningful analgesia with easily manageable side-effects is yet to be determined.
Epidural RTX can be administered under mild sedation. Furthermore, based on the clinical experience with opioids [147], epidural RTX should be devoid of the adverse effects that complicate the use of intrathecal RTX. This theory is currently being tested in an ongoing clinical trial [150].
In preclinical models of cancer pain, pharmacological blockade [91] or the knock-down of TRPV1 [86,87] also showed analgesic potential. The biggest challenge in analgesic drug development is to determine if a drug that showed promise in animal experiments will also work in human patients. Generally speaking, rodent models are good for acute pain but not so good for chronic pain, like cancer pain [154]. The limited success of translation from preclinical studies to the clinic may reflect our rudimentary understanding of the molecular mechanisms that drive chronic cancer pain [17]. Cancer pain is broadly described as nociceptive, inflammatory, and neuropathic [17], and the animal models were designed accordingly. However, self-perceived pain in cancer patients shows individual differences [155], depending on both the ethnic background [156] and gender [157]. (Parenthetically, RNA sequencing of the trigeminal sensory neurons revealed distinct transcriptomic profiles between male and female mice under tongue-tumor bearing conditions [158].) Furthermore, cancer pain is often associated with anxiety and depression [159], and patients may even experience referred pain at sites not affected by the disease [13]. Therefore, the observation that TRPV1 antagonists ameliorate cancer pain in rodents should be considered with reservations. It has to be kept in mind that the mechanisms by which cancer activates TRPV1 are unknown, therefore it is not clear either whether cancer models mimic human bone cancer pain. As a word of caution, small-molecule TRPV1 antagonists were effective analgesic agents in animal models of inflammatory and neuropathic pain, yet, they failed in clinical trials to relieve migraine or osteoarthritis pain [160,161].
A special situation of cancer pain is oropharyngeal carcinoma, which is amenable to topical treatment. Many patients with tongue or oral carcinoma exhibit increased sensitivity to capsaicin challenge [124]. In the mouse, radiotherapy (20 Gy) evokes mechanical and thermal allodynia via TRPA1 and TRPV1 activation [162]. Furthermore, in animal experiments, radiotherapy-associated oral pain was found to facilitate tumor growth [163]. Therefore, one may argue that the relief of oral pain mediated by capsaicin may also prevent early cancer relapse.
High-dose (8%) capsaicin patches represent a 2nd line of treatment for chemotherapy-induced neuropathic pain [120]. They also show promise for cancer patients with post-operative pain, including post-mastectomy pain [114] and pain that develops after melanoma surgery [113]. The injectable RTX [164] and capsaicin [165] preparations are yet to be tested in this patient population.
One third of healthy individuals reports no pain response to topical capsaicin (1%) challenge [166]. There is increasing evidence that a TRPV1 gene polymorphism is responsible for individual differences in human sensitivity to capsaicin [167,168]. Therefore, the one-size-fits all approach may not be applicable to capsaicin desensitization: depending on their genotype, some patients may show excellent therapeutic response to capsaicin, whereas others (the “non-responders” [166]) may not. Furthermore, animal experiments suggest that TRPV1 expression may be decreased in some patients, for example those with diabetic polyneuropathy [169]. Therefore, testing the response of TRPV1 channels to capsaicin may help to select the patients who may benefit from the capsaicin therapy.
Last, there are conflicting preclinical results regarding the effect of TRPV1 blockade on cancer progression that still baffle researchers. For example, in preclinical experiments, the ablation of TRPV1-expressing afferents mediated by capsaicin or RTX blocked the growth of melanoma [57], had no effect on canine osteosarcoma [138], and accelerated the progression of 4T1 breast cancer metastasis to the lung (Figure 6, high-resolution version) [170,171,172]. Confusingly, in mice, the genetic inactivation of Trpv1 prevented both bone colonization and lung metastasis formation by 4T1 breast cancer cells [173]. Adding to the confusion, the repeated activation of TRPV1-expressing sensory neurons was reported to promote tumor growth [174].
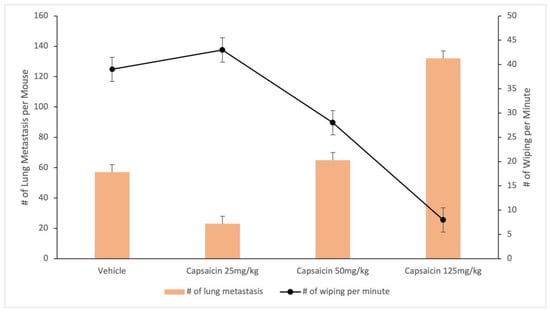
Figure 6.
Desensitization of TRPV1-expressing afferents via s.c. capsaicin facilitates lung metastasis spread in a murine model of breast carcinoma. Data are from [124].
Whether these differences are cancer-pain-model-, species-, or cancer-related has far-reaching implications for drug development.
Outstanding questions
- Is there a role for per os TRPV1 antagonists in cancer pain relief?
- Can site-specific (into the surgical wound) capsaicin or resiniferatoxin injections prevent the development of post-surgical pain in cancer patients?
- Can high-dose (8%) capsaicin patches relieve post-surgical (specifically, post-mastectomy) pain in cancer patients?
- Can topical capsaicin ameliorate localized pain in patients with oropharyngeal squamous cell carcinoma?
- What is the optimal dose of intrathecal resiniferatoxin at which it provides adequate pain relief with acceptable side-effects?
- Is epidural resiniferatoxin devoid of the side-effects of intrathecal administration?
- For TRPV1 knock-down mediated by siRNA given intrathecally, how does it compare to intrathecal resiniferatoxin?
- Does capsaicin/resiniferatoxin desensitization affect cancer growth?
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Schmidt, B.L. The neurobiology of cancer pain. J. Oral Maxillofac. Surg. 2015, 73, S132–S135. [Google Scholar] [CrossRef]
- Caraceni, A.; Shkodra, M. Cancer pain assessment and classification. Cancers 2019, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Haroun, R.; Wood, J.N.; Sikandar, S. Mechanisms of cancer pain. Front. Pain Res. 2022, 3, 1030899. [Google Scholar] [CrossRef]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on prevalence of pain in patients with cancer 2022: A systematic literature review and meta-analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef] [PubMed]
- Elmstedt, S.; Mogensen, H.; Hallmans, D.E.; Tavelin, B.; Lundström, S.; Lindskog, M. Cancer patients hospitalized in the last week of life risk insufficient care quality—A population-based study from the Swedish Register of Palliative Care. Acta Oncol. 2019, 4, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. The patient with difficult cancer pain. Cancers 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Pain Relief; World Health Organization: Geneva, Switzerland, 1986. [Google Scholar]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Opioid for Cancer Pain. American Cancer Society. Available online: https://cancer.org/content/dam/CRC/PDF/Public/8325.00.pdf (accessed on 22 January 2024).
- Deandrea, S.; Montanari, M.; Apolone, G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008, 19, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Bonoliel, R.; Cohen, M.; Evers, S.; Giamberandino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 060, 28–37. [Google Scholar] [CrossRef]
- Knudsen, A.K.; Aass, N.; Fainsinger, R.; Caraceni, A.; Klepstad, P.; Jordhoy, M.; Hjermstad, M.; Kaasa, S. Classification of pain in cancer patients—A systematic literature review. Palliat. Med. 2009, 23, 992–1022. [Google Scholar] [CrossRef]
- Potenoy, R.K.; Ahmed, E. Cancer pain syndromes. Hematol. Oncol. Clin. N. Am. 2018, 32, 371–386. [Google Scholar] [CrossRef]
- Niscola, P.; Tendas, A.; Scaramucci, L.; Giovaninni, M.; Cupelli, L.; De Sanctis, V.; Brunetti, G.A.; Bondanini, F.; Palumbo, R.; Lamanda, M. Pain in malignant hematology. Expert Rev. Hematol. 2011, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Stucky, C.L.; Harding-Rose, C.; Eikmeier, L.; Beitz, L.; Coicou, L.G.; Hanson, A.E.; Simone, D.A.; Seybold, V.S. Chemical interaction between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J. Neurosci. 2007, 27, 10289–10298. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Ghilardi, J.R.; Keyser, C.P.; Kubota, K.; Lindsay, T.H.; Luger, N.M.; Mach, D.B.; Schwei, M.J.; Sevcik, M.A.; Mantyh, P.W. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp. Neurol. 2005, 193, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Dickenson, A.H. Pain and nociception: Mechanisms of cancer-induced bone pain. J. Clin. Oncol. 2014, 32, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hiasa, M.; Okui, T.; Hata, K. Sensory nerves: A driver of the vicious cycle in bone metastasis? J. Bone Oncol. 2021, 30, 100387. [Google Scholar] [CrossRef] [PubMed]
- Diaz-delCastillo, M.; Chantry, A.D.; Lawson, M.A.; Heegaard, A.-M. Multiple myeloma—A painful disease of the bone marrow. Semin. Cell Develop. Biol. 2021, 112, 49–58. [Google Scholar]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J. Capsaicin and sensory neurones: A historical perspective. Prog. Drug Res. 2014, 68, 1–37. [Google Scholar]
- Fischer, M.J.; Ciotu, C.I.; Szallasi, A. The mysteries of capsaicin-sensitive afferents. Front. Physiol. 2020, 11, 554195. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interactions between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef]
- Patil, M.J.; Kim, S.H.; Bahia, P.K.; Nair, S.S.; Darcey, T.S.; Fiallo, J.; Zhu, X.X.; Frisina, R.D.; Hadley, S.H.; Taylor-Clark, T.E. A novel Flp reporter mouse shows that TRPA1 expression in largely limited to sensory neuron subsets. Eneuro 2023, 10. [Google Scholar] [CrossRef]
- Leffler, A.; Mönter, B.; Koltzenburg, M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 2006, 139, 699–709. [Google Scholar] [CrossRef]
- Lu, H.-J.; Wu, X.-B.; Wei, Q.-Q. Ion channels in cancer-induced bone pain: From molecular mechanisms to clinical applications. Front. Mol. Neurosci. 2023, 16, 1239599. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Zaguri, R.; Edvardson, S.; Maayan, C.; Elpeleg, O.; Lev, S.; Davidson, E.; Peters, M.; Kfir-Erenfeld, S.; Brger, E.; et al. Nociception and pain in humans lacking a functional TRPV1 channel. J. Clin. Investig. 2023, 133, e153558. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Tominaga, M.; Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef] [PubMed]
- Dray, A.; Perkins, M.N. Bradykinin activates peripheral capsaicin-sensitive fibres via a second messenger system. Agents Actions 1988, 25, 214–215. [Google Scholar] [CrossRef]
- Chuang, H.H.; Prescott, E.D.; Kong, H.; Shields, S.; Jordt, S.E.; Basbaum, A.I.; Chao, M.V.; Julius, D. Bradykinin and nerve growth factor release the capsaicin receptor from Ptdlns(4,5)P2-mediated inhibition. Nature 2001, 411, 957–962. [Google Scholar] [CrossRef]
- Hutchings, C.; Phillips, J.A.; Djamgoz, M.B.A. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188411. [Google Scholar] [CrossRef]
- Erin, N.; Shurin, G.V.; Baraldi, J.H.; Shurin, M.R. Regulation of carcinogenesis by sensory neurons and neuromediators. Cancers 2022, 14, 2333. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, T.; Amit, M. Targeting the nerve-cancer circuit. Cancer Res. 2023, 83, 2445–2447. [Google Scholar] [CrossRef]
- Erin, N.; Szallasi, A. Carcinogenesis and metastasis: Focus on TRPV1-positive neurons and immune cells. Biomolecules 2023, 13, 983. [Google Scholar] [CrossRef]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor innervation: Cancer has some nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef] [PubMed]
- Restaino, A.C.; Vermeer, P.D. Neural regulation of the tumor microenvironment. FASEB Bioadv. 2022, 4, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Restaino, A.C.; Walz, A.; Vermeer, S.J.; Barr, K.; Kovács, A.; Fettig, R.R.; Vermeer, D.W.; Reavis, H.; Williamson, C.S.; Lucido, C.T.; et al. Functional neuronal circuits promote disease progression in cancer. Sci. Adv. 2023, 9, eade4443. [Google Scholar] [CrossRef] [PubMed]
- Lautner, M.A.; Ruparel, S.B.; Patil, M.J.; Hargreaves, K.M. In vitro sarcoma cells release a lipophilic substance that activates the pain transduction system via TRPV1. Ann. Surg. Oncol. 2011, 18, 866–871. [Google Scholar] [CrossRef]
- Tong, Z.; Luo, W.; Wang, Y.; Yang, F.; Han, Y.; Li, H.; Luo, H.; Duan, B.; Xu, T.; Maoying, Q.; et al. Tumor tissue-derived formaldehyde and acidic microenvironment synergistically induce bone cancer pain. PLoS ONE 2010, 5, e10234. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Dang, D.; Zhang, J.; Viet, C.T.; Lam, D.K.; Dolan, J.C.; Gibbs, J.L.; Schmidt, B.L. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol. Cancer Ther. 2011, 10, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Shimosato, G.; Nagano, M.; Ueda, M.; Hashimoto, S.; Tanaka, Y.; Suzuki, H.; Tanaka, M. NGF and GDNF differentially regulates TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur. J. Neurosci. 2004, 20, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Morsy, N.; Winston, J.; Pasricha, P.J.; Garrett, K.; Akbarali, H.I. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am. J. Physiol. Cell Physiol. 2004, 287, C558–C563. [Google Scholar] [CrossRef] [PubMed]
- Robilotto, G.L.; Mohapatra, D.P.; Shepherd, A.J.; Mickle, A.D. Role of Src kinase in regulating protein kinase C mediated phosphorylation of TRPV1. Eur. J. Pain 2022, 26, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, O.; Wang, Z.; Bang, S.; Li, J.; Lee, S.H.; Huh, Y.; Furutani, K.; He, Q.; Tao, X.; et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 2021, 109, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.R.; Pan, H.L. Calcineurin regulates synaptic plasticity and nociceptive transmission at the spinal cord level. Neuroscientist 2022, 28, 628–638. [Google Scholar] [CrossRef]
- Prommer, E. Calcineurin-inhibitor pain syndrome. Clin. J. Pain 2012, 28, 556–559. [Google Scholar] [CrossRef]
- Kita, T.; Uchia, K.; Kato, K.; Suzuki, Y.; Tominaga, M.; Yamazaki, J. FK506 (tacrolimus) causes pain sensation through the activation of transient receptor ankyrin 1 (TRPA1) channels. J. Physiol. Sci. 2018, 69, 305–316. [Google Scholar] [CrossRef]
- Cui, Q.; Jiang, D.; Zhang, Y.; Chen, C. The tumor-nerve circuit in breast cancer. Cancer Metastasis Rev. 2023, 42, 543–574. [Google Scholar] [CrossRef]
- Jerard, C.; Madhusudanan, P.; Swamy, A.; Ravikumar, K. Secretome mediated interactions between sensory neurons and breast cancer cells. Int. J. Cancer 2023, 153, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Balood, M.; Ahmadi, M.; Eichwald, T.; Ahmadi, A.; Majdoubi, R.; Roversi, K.; Lucido, C.T.; Restaino, A.C.; Huang, S.; Li, L.; et al. Nociceptor neurons affect cancer immunosurveillance. Nature 2022, 611, 405–412. [Google Scholar] [CrossRef] [PubMed]
- La Montanara, P.; Hervera, A.; Baltussen, L.L.; Hutson, T.H.; Palmisano, I.; De Virgillis, F.; Kong, G.; Chadwick, J.; Gao, Y.; Bartus, K.; et al. Cyclin-dependent-like kinase 5 is required for pain signaling in human sensory neurons and mouse models. Sci. Transl. Med. 2020, 12, eaax4846. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain 2013, 154, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Aielli, F.; Ponzetti, M.; Rucci, N. Bone metastasis pain, from the bench to the bedside. Int. J. Mol. Sci. 2019, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, W.; Zhang, R.; Wang, Y. Peripheral mechanism of cancer-induced bone pain. Neurosci. Bull. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hata, K.; Nakanishi, M.; Nagae, M.; Nagayama, T.; Wakabayashi, H.; Nishisho, T.; Sakurai, T.; Hiraga, T. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone 2011, 48, 100–105. [Google Scholar] [CrossRef]
- Di Pompo, G.; Cortini, M.; Baldini, N.; Avnet, S. Acid microenvironment in bone sarcomas. Cancers 2021, 13, 3848. [Google Scholar] [CrossRef]
- Swietach, P.; Boedtkjer, E.; Pedersen, S.F. How protons pave the way to aggressive cancers. Nature Rev. Cancer 2023, 23, 825–841. [Google Scholar] [CrossRef]
- Fischer, M.J.; Reeh, P.W.; Sauer, S.K. Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur. J. Neurosci. 2003, 18, 803–810. [Google Scholar] [CrossRef]
- Crider, B.P.; Xie, X.S.; Stone, D.K. Bafilomycin inhibits proton flow through the H+ channel of vacuolar proton pumps. J. Biol. Chem. 1994, 269, 17379–17381. [Google Scholar] [CrossRef]
- Hiasa, M.; Okui, T.; Allette, Y.M.; Ripsch, M.S.; Sun-Wada, G.H.; Wakabayashi, H.; Roodman, G.D.; White, F.A.; Yoneda, T. Bone pain-induced by multiple myeloma is reduced by targeting V-ATPase and ASIC3. Cancer Res. 2017, 77, 1283–1295. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.H.; Dvoark, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Roda, N.; Blandano, G.; Pelicci, P.G. Blood vessels and peripheral nerves as key players in cancer progression and therapy resistance. Cancers 2021, 13, 4471. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Yuan, Y. The role of tumor neogenesis pipelines in tumor progression and their therapeutic potential. Cancer Med. 2023, 12, 1558–1571. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Werner, H.; Rosen, C.J. Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 2018, 61, T115–T137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, J.; Han, Y.; Xiao, X.; Meng, X.L.; Su, L.; Liu, F.Y.; Xing, G.G.; Wan, Y. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur. J. Pain 2014, 18, 774–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.; Wang, X.; Tong, J. Calcitonin gene-related peptide: A promising bridge between cancer development and cancer-associated pain in oral squamous cell carcinoma. Oncol. Lett. 2020, 20, 253. [Google Scholar] [CrossRef]
- Zhu, W.; Sheng, D.; Shao, Y.; Zhang, Q.; Peng, Y. Neuronal calcitonin gene-related peptide promotes prostate tumor growth in the bone microenvironment. Peptides 2021, 135, 170423. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Rodriguez, F.D.; Covenas, R. Peptidergic systems and cancer: Focus on tachykinin and calcitonin/calcitonin gene-related peptide families. Cancers 2023, 15, 1694. [Google Scholar] [CrossRef]
- Inyang, K.; Evans, C.M.; Heussner, M.; Petroff, M.; Reimers, M.; Vermeer, P.D.; Tykocki, N.; Folger, J.K.; Leumet, G. HPV+ head and neck cancer-derived small extracellular vesicles communicate with TRPV1+ neurons to mediate cancer pain. Pain 2023. [Google Scholar] [CrossRef]
- Quasthoff, S.; Hartung, H.P. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002, 249, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, P. Chemotherapy-induced neuropathic pain. Curr. Opin. Support. Palliat. Care 2011, 5, 1–7. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef]
- Yoshida, A.; Nishibata, M.; Mruyama, T.; Sunami, S.; Isono, K.; Kawamata, T. Activation of transient receptor potential vanilloid 1 is involved in both pain and tumor growth in a mouse model of cancer pain. Neuroscience 2023, 538, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Avallone, A.; Bimonte, S.; Cardone, C.; Cascella, M.; Cuomo, A. Pathophysiology and therapeutic perspectives for chemotherapy-induced peripheral neuropathy. Anticancer Res. 2022, 42, 4667–4678. [Google Scholar] [CrossRef] [PubMed]
- Navia-Pelaez, J.M.; Borges Paes Lemes, J.; Gonzalez, L.; Delay, L.; Dos Santos, A.C.L.; Lu, J.W.; Dos Santos, G.G.; Gregus, A.M.; Doughery, P.M.; Yaksh, T.; et al. AIBP regulates TRPV1 activation in chemotherapy-induced peripheral neuropathy by controlling lipid raft dynamics and proximity to TLR4 in dorsal root ganglion neurons. Pain 2023, 64, e274–e285. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Bieber, A.J.; Carlton, S.M.; Loprinzi, C.L.; Low, P.A.; Windebank, A.J. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol. Pain 2010, 6, 15. [Google Scholar] [CrossRef]
- Zhao, M.; Isami, K.; Nakamura, S.; Shiakawa, H.; Nakagawa, T.; Kaneko, S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by enhanced responsiveness of TRPA1 in mice. Mol. Pain 2012, 8, 55. [Google Scholar] [CrossRef]
- Gauchan, P.; Andoh, T.; Kato, A.; Kuraishi, Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 2009, 458, 93–95. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Hao, X.; Liu, C. TRPV1 in dorsal root ganglion contributed to bone cancer pain. Front. Pain Res. 2022, 3, 1022022. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Meng, Q. AAV-mediated siRNA against TRPV1 reduced nociception in a rat model of bone cancer pain. Neurol. Res. 2019, 41, 972–979. [Google Scholar] [CrossRef]
- Akhilesh; Uniyal, A.; Gadepalli, A.; Tiwari, V.; Allani, M.; Chouhan, D.; Ummadisetty, O.; Verma, N.; Tiwari, V. Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. 2022, 288, 120187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, L.; Liao, T.T.; Li, W.; Zhou, J.; You, Y.; Shi, J. Progress in the development of TRPV1 small-molecule antagonists: Novel strategies for pain management. Eur. J. Med. Chem. 2023, 261, 115806. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, M.M.; Saraiva-Santos, T.; Zaninelli, T.H.; Pinho-Ribeiro, F.A.; Fattori, V.; Staurengo-Ferrari, L.; Ferraz, C.R.; Domiciano, T.P.; Calixto-Campos, C.; Borghi, S.M.; et al. Ehlich tumor induces TRPV1-dependent evoked and non-evoked pain-like behavior in mice. Brain Sci. 2022, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Ogino, A.; Ozaki, N.; Urano, H.; Hironaka, K.; Yasui, M.; Sugiura, Y. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J. Pain 2008, 9, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Wakisaka, S.; Hiraga, T.; Hata, K.; Nishimura, R.; Tominaga, M.; Yoneda, T. Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J. Bone Miner. Metab. 2018, 36, 274–285. [Google Scholar] [CrossRef]
- Maggi, C.A.; Meli, A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988, 19, 1–43. [Google Scholar] [CrossRef]
- Buck, S.H.; Burks, T. F The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar]
- Szallasi, A.; Blumberg, P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [PubMed]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Nolden, A.A.; Lenart, G.; Spielman, A.I.; Hayes, J.E. Inducible desensitization to capsaicin with repeated low-dose exposure in human volunteers. Physiol. Behav. 2023, 275, 114447. [Google Scholar] [CrossRef] [PubMed]
- Tomohiro, D.; Mizuta, K.; Fujita, T.; Nishikubo, Y.; Kumamoto, E. Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves. Life Sci. 2013, 92, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Pierau, F.K.; Weyrich, M. The influence of capsaicin on membrane currents in dorsal root ganglion neurons of guinea-pig and chicken. Pflügers Arch. 1987, 409, 403–410. [Google Scholar] [CrossRef]
- Arbuckle, J.B.; Docherty, R.J. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci. Lett. 1995, 185, 70–73. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Bean, B.P.; Woolf, C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 2007, 449, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.R.; Pillai, R.; Chung, C.C.W.; Wang, J.T.C.; MacLeod, B.A.; Schwarz, S.K.W. QX-314 produces long-lasting local anesthesia modulated by transient receptor potential vanilloid receptors in mice. Anesthesiology 2009, 111, 122–126. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Li Wan Po, A. The effectiveness of topically applied capsaicin. A meta-analysis. Eur. J. Clin. Pharmacol. 1994, 46, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Sven-Rice, A.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2013, 28, CD007393. [Google Scholar]
- Mou, J.; Paillard, F.; Turnbull, B.; Trudeau, J.; Stoker, M.; Katz, N.P. Effiacy of Qutenza (capsaicin) 8% patch for neuropathic pain: A meta-analysis of the Qutenza Clinical Trials Database. Pain 2013, 154, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Elsafa, E.; Privitera, R.; Naidoo, K.; Yiangou, Y.; Donatien, P.; Gabram, H.; Wasan, H.; Kenny, L.; Rahemtulla, A.; et al. Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8& patch: From pain relief towards disease modification. J. Pain Res. 2019, 12, 2039–2052. [Google Scholar] [PubMed]
- Watson, C.P.; Evans, R.J.; Watt, V.R. The post-mastectomy pain syndrome and the effect of topical capsaicin. Pain 1989, 38, 177–186. [Google Scholar] [CrossRef]
- Watson, P.N.C.; Evans, R.J. The post-mastectomy pain syndrome and topical capsaicin: A randomized trial. Pain 1992, 51, 375–379. [Google Scholar] [CrossRef]
- Dini, D.; Bertelli, G.; Gozza, A.; Forno, G.G. Treatment of the post-mastectomy pain syndrome with topical capsaicin. Pain 1993, 54, 223–226. [Google Scholar] [CrossRef]
- Ellison, N.; Loprinzi, C.L.; Kugler, J.; Hatfield, A.K.; Miser, A.; Sloan, J.A.; Wender, D.B.; Rowland, K.M.; Molina, R.; Cascino, T.L.; et al. Phase-III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J. Clin. Oncol. 1997, 15, 2974–2980. [Google Scholar] [CrossRef]
- Larsson, I.M.; Sorensen, J.A.; Bilel, C. The post-mastectomy pain syndrome—A systematic review of the treatment modalities. Breast J. 2017, 23, 338–343. [Google Scholar] [CrossRef]
- Casale, R. Capsaicin 179 mg cutaneous patch in the treatment of post-surgical neuropathic pain: A scoping review of current evidence and place in therapy. Expert Rev. Neurother. 2021, 21, 1147–1158. [Google Scholar] [CrossRef]
- Laude-Pagniez, E.; Leclerc, J.; Lok, C.; Chanz, G.; Arnault, J.P. Capsaicin 8% patch as therapy for neuropathic chronic postsurgical pain after melanoma excision surgery: A single center case series. JAAD Case Rep. 2022, 30, 70–75. [Google Scholar] [CrossRef]
- O’Brien, J.; Murphy, K.; Weekes, G.; Keaveny, J. Management of post mastectomy neuropathic pain with capsaicin (8%) topical patch (Qutenza)—Case study. Eur. J. Oncol. Nurs. 2012, 16, S26. [Google Scholar] [CrossRef]
- Atreya, S. Pregabalin in chemotherapy-induced neuropathic pain. Ind. J. Palliat. Care 2016, 22, 101–103. [Google Scholar] [CrossRef]
- Evaluation in the Treatment of Neuropathic Pain Post Breast Surgery (CAPTRANE). Available online: https://ctv.veeva.com/study/evaluation-in-the-treatment-of-neuropathic-pain-post-breast-surgery (accessed on 22 January 2024).
- Dupoiron, D.; Jubier-Hamon, S.; Seegers, V.; Bienfalt, F.; Luchon, Y.M.; Lebrec, N.; Jaoul, V.; Delorme, T. Peripheral neuropathic pain following breast cancer: Effectiveness and tolerability of high-concentration capsaicin patch. J. Pain Res. 2022, 15, 241–255. [Google Scholar] [CrossRef]
- Bienfait, F.; Arther, J.; Jubier-hamon, S.; Seegers, V.; Delorme, T.; Jaoul, V.; Pluchon, Y.-M.; Lebrec, N.; Dupoiron, D. Evaluation of 8% capsaicin patches in chemotherapy-induced peripheral neuropathy: A retrospective study in a comprehensive cancer center. Cancers 2023, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Flöther, L.; Avila-Castillo, D.; Burgdorff, A.-M.; Benndorf, R. Capsaicin in the treatment of refractory neuropatic pain after mastectomy surgery: A case report. Case Rep. Oncol. 2020, 13, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotte, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Kautio, A.-L.; Haanpaa, M.; Saarto, T.; Kalso, E. Mitryptiline in the treatment of chemotherapy-induced neuropathic symptoms. J. Pain Sympt. Manag. 2008, 35, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Boden, A.; Lusque, A.; Lodin, S.; Bourgouin, M.; Mauries, V.; Moreau, C.; Fabre, A.; Mounier, M.; Poublanc, M.; Caunes-Hilary, N.; et al. Study protocol of the TEC-ORL clinical trial: A randomized comparative phase II trial investigating the analgesic activity of capsaicin vs Laroxyl in head and neck cancer survivors presenting with neuropathic pain sequelae. BMC Cancer 2022, 22, 1260. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; de Melo Cardoso, D.; Mitsuy Kayahara, G.; Gelera Benabe, D. A pilot study to improve pain phenotyping in head and neck cancer patients. Front. Pain Res. 2023, 4, 1146667. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Janal, M.N.; Nicholson, S.J.; Wu, A.K.; Schmidt, B.L.; Albertson, D.G. Oral cancer patients experience mechanical and chemical sensitivity at the site of the cancer. BMC Cancer 2022, 22, 1165. [Google Scholar] [CrossRef]
- Hergenhahn, M.; Kusumoto, M.; Hecker, E. On the active principles of the spurge family (Euphorbiaceae). V. Extremely skin-irritant and moderate tumor-promoting diterpene esters from Euphorbia resinifera Berg. J. Cancer Res. Clin. Oncol. 1984, 108, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Szallasi, A.; Szallasi, Z.; Joó, F.; Blumberg, P.M. Resiniferatoxin: An ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 1990, 255, 923–928. [Google Scholar] [PubMed]
- Cruz, F.; Guimaraes, M.; Silva, C.; Reis, M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet 1997, 350, 640–641. [Google Scholar] [CrossRef]
- Lazzeri, M.; Spinelli, M.; Beneforti, P.; Zanollo, A.; Turini, D. Intravesical resiniferatoxin for the treatment of hyperreflexia refractory to capsaicin in patients with chronic spinal cord disease. Scand. J. Urol. Nephrol. 1998, 32, 331–334. [Google Scholar]
- Rios, L.A.S.; Panhoca, R.; Mattos, D.; Srugi, M.; Bruschini, H. Intravesical resiniferatoxin for the treatment of women with idiopathic detrusor overactivity and urgency incontinence. A single dose, 4 weeks, double-blind, randomized, placebo-controlled trial. Neurourol. Urodyn. 2007, 26, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Tender, G.C.; Walbridge, S.; Oláh, Z.; Karai, L.; Iadarola, M.J.; Oldfield, E.H.; Lonser, R.R. Selective ablation of nociceptive neurons for elimination of hyperalgesia and neurogenic inflammation. J. Neurosurg. 2005, 102, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Sapio, M.R.; Neubert, J.K.; LaPaglia, D.M.; Maric, D.; Keller, J.M.; Raithel, S.J.; Rohrs, E.L.; Anderson, E.M.; Butman, J.A.; Caudle, R.M.; et al. Pain control through selective chemo-ablation of centrally projecting TRPV1+ sensory neurons. J. Clin. Investig. 2018, 128, 1657–1670. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hoon, M.A. Ablation of Trpv1 neurons reveals their selective role in thermal pain sensation. Mol. Cell. Neurosci. 2010, 43, 157–163. [Google Scholar] [CrossRef]
- Cimino Brown, D. Resiniferatoxin: The evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals 2016, 9, 47. [Google Scholar] [CrossRef]
- Cruz, C.D.; Charrua, A.; Vieira, E.; Valente, J.; Avelino, A.; Cruz, F. Intrathecal delivery of resiniferatoxin (RTX) reduces detrusor overactivity and spinal expression of TRPV1 in spinal cord injured animals. Exp. Neurol. 2008, 214, 301–308. [Google Scholar] [CrossRef]
- Bishnoi, M.; Bosgraaf, C.A.; Premkumar, L.S. Preservation of acute pain and efferent functions following intrathecal resiniferatoxin-induced analgesia in rats. J. Pain 2011, 12, 991–1003. [Google Scholar] [CrossRef]
- Menéndez, L.; Juárez, L.; Garcia, E.; Garcia-Suárez, O.; Hidalgo, A.; Baamonde, A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci. Lett. 2006, 393, 70–73. [Google Scholar] [CrossRef]
- Rancilio, N.; Poulson, J.; Ko, J. Strategies for managing cancer pain in dogs and cats. Part 1. Pathophysiology and assessment of cancer pain. Today’s Vet. Med. 2015, 5, 60–68. [Google Scholar]
- Cimino Brown, D.; Iadarola, M.J.; Perkowski, S.Z.; Erin, H.; Shofer, F.; Karai, K.J.; Oláh, Z.; Mannes, A.J. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005, 103, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J. Effect of capsaicin on thermoregulation: An update with new aspects. Temperature 2015, 2, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.X.; Ton, H.T.; Gulyás, H.; Pórszász, R.; Tóth, A.; Russo, R.; Kay, M.W.; Sahibzada, A.; Ahern, G.P. TRPV1 expressed throughout the arterial circulation regulates vasoconstriction and blood pressure. J. Physiol. 2020, 598, 5639–5659. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Agnello, K.; Iadarola, M.J. Intrathecal resiniferatoxin in a dog model: Efficacy in bone cancer pain. Pain 2015, 156, 1018–1024. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Mannes, A.J. The vanilloid agonist resiniferatoxin for interventional-based pain control. Curr. Top. Med. Chem. 2011, 11, 2171–2179. [Google Scholar] [CrossRef]
- Parisi, J.R.; Martins de Andrade, A.L.; Torres Silva, J.R.; Silva, M.L. Antiallodynic effect of intrathecal resiniferatoxin on neuropathic pain model of chronic constriction injury. Acta Neurobiol. Exp. 2017, 77, 317–322. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Brown, D.C.; Nahama, A.; Sapio, M.R.; Mannes, A. Pain treatment in the companion canine model to validate rodent results and incentivize transition to human clinical trials. Front. Pharmacol. 2021, 12, 705743. [Google Scholar] [CrossRef]
- Heiss, J.; Iadarola, M.J.; Cantor, F.; Oughourli, R.; Smith, R.; Mannes, A. A Phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. J. Pain 2014, 15, S65. [Google Scholar] [CrossRef]
- Mannes, A.J.; Iadarola, M.J.; Jones, B.; Royal, M.A.; Heiss, J.D. Intrathecal resiniferatoxin for treating intractable cancer-related severe chronic pain. In Proceedings of the 15th World Congress on Pain, Buenos Aires, Argentina, 6–11 October 2014. [Google Scholar]
- Chaney, M.A. Side effects of intrathecal and epidural opioids. Can. J. Anaesth. 1995, 42, 891–903. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Gonella, G.L. Resiniferatoxin for pain treatment: An interventional approach to personalized pain medicine. Open Pain J. 2013, 6 (Suppl. I), 95–107. [Google Scholar] [CrossRef]
- Nedeljkovic, S.S.; Narang, S.; Rickerson, E.; Levitt, R.C.; Horn, D.B.; Patin, D.L.; Albores-Ibarra, N.; Nahama, A.; Zhao, T.; Bharati, P.; et al. A multicenter, open-label, phase 1b study to assess the safety and define the maximal tolerated dose of epidural resiniferatoxin (RTX) injection for treatment of intractable pain associated with cancer. In Proceedings of the 2020 Annual Meeting of the American Academy of Pain Medicine, National Harbor, MD, USA, 26 February–1 March 2020. [Google Scholar]
- Study to Assess Epidural Resiniferatoxin for the Treatment of Intractable Pain Associated with Advanced Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05067257 (accessed on 22 January 2024).
- Chwistek, M. Recent advances in understanding and managing cancer pain. F1000Research 2017, 6, 945. [Google Scholar] [CrossRef] [PubMed]
- Gadepalli, A.; Aklilesh; Uniyal, A.; Modi, A.; Chouhan, D.; Ummadisetty, O.; Khanna, S.; Solanki, S.; Allani, M.; Tiwari, V. Multifarious targets and recent developments in the therapeutics for the management of bone cancer pain. ACS Chem. Neurosci. 2021, 12, 4195–4208. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Vanilloid-sensitive neurons: A fundamental subdivision of the peripheral nervous system. J. Peripher. Nerv. Syst. 1996, 1, 6–18. [Google Scholar]
- Pineda-Farias, J.B.; Saloman, J.L.; Scheff, N.N. Animal models of cancer-related pain: Current perspectives in translation. Front. Pharmacol. 2020, 11, 610894. [Google Scholar] [CrossRef]
- Nielsen, C.S.; Staud, R.; Price, D.D. Individual differences in pain sensitivity: Measurement, causation, and consequences. J. Pain 2009, 10, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Im, E.-O. Ethnic differences in cancer pain experience. Nurs. Res. 2007, 56, 296–306. [Google Scholar] [CrossRef]
- Alodhayani, A.; Almitairi, K.M.; Vinluan, J.M.; Alsadhan, N.; Almigbal, T.H.; Alonazi, W.B.; Batais, M.A. Gender difference in pain management among adult cancer patients in Saudi Arabia: A cross-sectional assessment. Front. Psychol. 2021, 12, 628223. [Google Scholar] [CrossRef]
- Ibrahim, T.; Wu, P.; Wang, L.-J.; Chang, F.-M.; Murillo, J.; Merlo, J.; Shein, S.S.; Tumanov, A.V.; Lai, Z.; Weldon, K.; et al. Sex-dependent differences in the genomic profile of lingual sensory neurons in naïve and tongue-tumor bearing mice. Sci. Rep. 2023, 13, 13117. [Google Scholar] [CrossRef]
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef]
- Moran, M.M.; Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. Br. J. Pharmacol. 2018, 175, 2185–2203. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Voets, T.; Iadarola, M.J.; Szallasi, A. Targeting TRP channels for pain relief: A review of current evidence from bench to bedside. Curr. Opin. Pharmacol. 2023, in press. [Google Scholar]
- Su, C.-J.; Xu, J.-H.; Liu, X.; Zhao, F.-L.; Pan, J.; Zhang, Y.-S. X-ray induces mechanical and heat allodynia in mouse via TRPA1 and TRPV1 activation. Mol. Pain 2019, 15, 1–13. [Google Scholar]
- Meneses, C.S.; Gidcumb, E.M.; Marcus, K.L.; Gonzalez, Y.; Lai, Y.H.; Mishra, S.K.; Lascelles, B.D.X.; Nolan, M.W. Acute radiotherapy-associated oral pain may promote tumor growth at distant sites. Font. Oncol. 2023, 13, 1029108. [Google Scholar] [CrossRef] [PubMed]
- Grünenthal Starts Phase III Trials for Resiniferatoxin in Osteoarthritis-Related Pain. Available online: http://grunenthal.com/en/press-room/press-releases/2022/global-clinical-phae-iii-programme-for-resiniferatoxi-rtx (accessed on 22 January 2024).
- Available online: https://centrexion.com/science/pipeline/cntx-4975 (accessed on 22 January 2024).
- Wong, F.; Reddy, A.; Rho, Y.; Vollert, J.; Strutton, P.H.; Hughes, S.W. Responders and nonresponders to topical capsaicin display distinct temporal summation of pain profiles. Pain Rep. 2023, 8, e1071. [Google Scholar] [CrossRef]
- Forstenpointner, J.; Förster, M.; May, D.; Hofschulte, F.; Cascorbi, I.; Wasner, G.; Gierthmühlen, J.; Baron, R. TRPV1 polymorphism 1911 A>G alters capsaicin-induced sensory changes in healthy subjects. PLoS ONE 2017, 12, e0183322. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Okumura, M.; Tadokoro, O.; Sogawa, N.; Tomida, M.; Kondo, E. Effect of single-nucleotide polymorphisms in TRPV1 on burning pain and capsaicin sensitivity in Japanese adults. Mol. Pain 2018, 14, 1744806918804439. [Google Scholar] [CrossRef] [PubMed]
- Pabbidi, R.M.; Yu, S.-Q.; Peng, S.; Khardori, R.; Pauza, M.E.; Premkumar, L.S. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol. Pain 2008, 4, 9. [Google Scholar] [CrossRef]
- Erin, N.; Zhao, W.; Bylander, J.; Chase, G.; Clawson, G. Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res. Treat. 2006, 99, 351–364. [Google Scholar] [CrossRef]
- Erin, N.; Boyer, P.J.; Bonneau, R.H.; Clawson, G.A.; Welch, D.R. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res. 2004, 24, 1003–1009. [Google Scholar] [PubMed]
- Bencze, N.; Svarcz, C.; Danics, L.; Szőke, E.; Balogh, P.; Szallasi, A.; Hamar, P.; Helyes, Z.; Botz, B. Desensitization of capsaicin-sensitive afferents accelerates early tumor growth via increased vascular leakage in a murine model of triple negative breast cancer. Front. Oncol. 2012, 11, 685297. [Google Scholar] [CrossRef] [PubMed]
- Okui, T.; Hiasa, M.; Hatam, K.; Roodman, G.D.; Nakanishi, M.; Yoneda, T. The acid-sensing nociceptor TRPV1 controls breast cancer progression in bone via regulating HGF secretion from sensory neurons. Res. Sq. 2023, rs-3105966. [Google Scholar] [CrossRef]
- Tanaka, K.; Kondo, T.; Narita, M.; Muta, T.; Yoshida, S.; Sato, D.; Suda, Y.; Hamada, Y.; Tezuka, H.; Kuzumaki, N.; et al. Repeated activation of Trpv1-positive sensory neurons facilitates tumor growth associated with changes in tumor-infiltrating immune cells. Biochem. Biophys. Res. Commun. 2023, 648, 36–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).