Evaluating the Impact of Bowel Gas Variations for Wilms’ Tumor in Pediatric Proton Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Image Data
2.2. Treatment Planning Scheme and Daily CBCT Analysis for Dose Accumulation
- GTV_P: Patients with unresected gross tumor volume (GTV) at the primary (P) site
- CTV_P: Patients with local spill or evidence of rupture contained to the flank, or patients with an involved surgical margin after upfront surgical resection or delayed surgical resection, or patients with tumor thrombus removed in more than one piece or incompletely resected including 5 mm margin over GTV_P
- GTV_LN: Patients with unresected gross lymph node disease
- CTV_LN: Patients with para-aortic, para caval, and renal hilar lymph node (LN) involvement including 5 mm margin over GTV_LN
- GTV_BWT: Patients with cut surgical surface within the residual kidney as involved with microscopic bilateral Wilms’ tumor (BWT)
- CTV_BWT: Patients with an involved surgical margin in the partial nephrectomy specimen including a 5 mm margin over GTV_BWT
- ITV_P, ITV_LN, ITV_BWT: Internal motion to expand CTV_P, CTV_LN, CTV_BWT based on 4D CT study
2.3. The Worst-Case Scenario Simulation
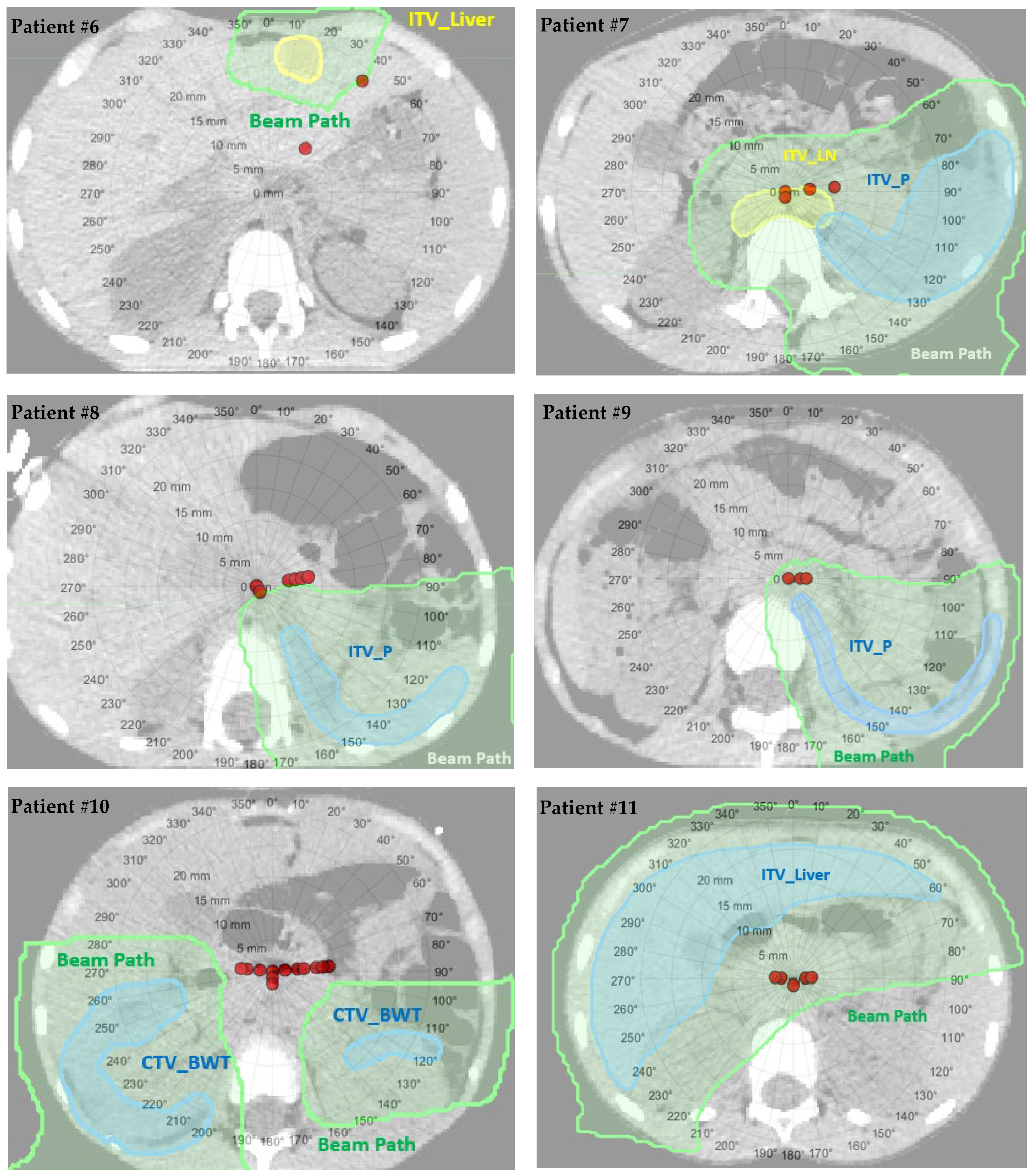
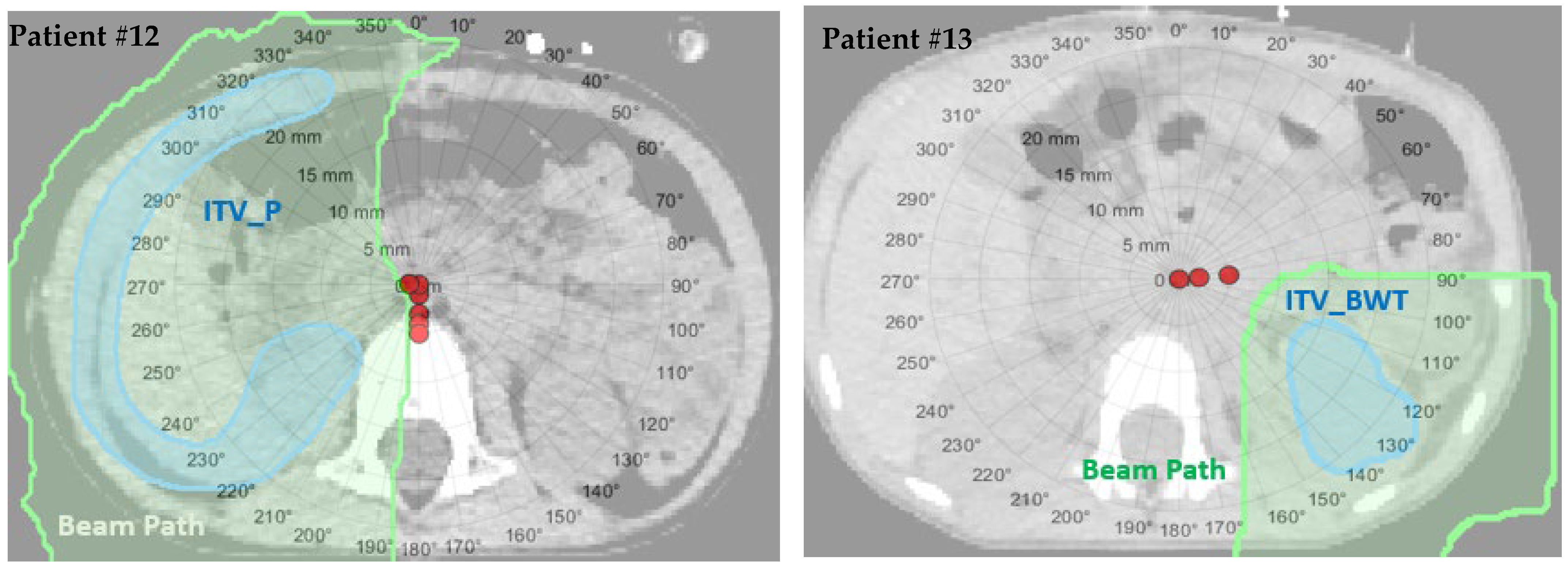
2.4. Water Equivalent Path Length Method
3. Results
3.1. Impact of Daily Bowel Gas Changes on Plan Quality
3.2. The Worst-Case Scenario Simulation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakayama, D.K.; Bonasso, P.C. The History of Multimodal Treatment of Wilms’ Tumor. Am. Surg. 2016, 82, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Dome, J.S.; Cotton, C.A.; Perlman, E.J.; Breslow, N.E.; Kalapurakal, J.A.; Ritchey, M.L.; Grundy, P.E.; Malogolowkin, M.; Beckwith, J.B.; Shamberger, R.C.; et al. Treatment of anaplastic histology Wilms’ tumor: Results from the fifth National Wilms’ Tumor Study. J. Clin. Oncol. 2006, 24, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Dome, J.S.; Graf, N.; Geller, J.I.; Fernandez, C.V.; Mullen, E.A.; Spreafico, F.; Van den Heuvel-Eibrink, M.; Pritchard-Jones, K. Advances in Wilms’ Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol. 2015, 33, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Wilimas, J.A.; Douglass, E.C.; Lewis, S.; Fairclough, D.; Fullen, G.; Parham, D.; Kumar, A.P.; Hustu, H.O.; Fleming, I. Reduced therapy for Wilms’ tumor: Analysis of treatment results from a single institution. J. Clin. Oncol. 1988, 6, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.R. Wilms’ Tumor: Changing Tole of Radiation Therapy. Semin. Radiat. Oncol. 1997, 7, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Mul, J.; van Grotel, M.; Seravalli, E.; Bosman, M.E.; van Tinteren, H.; Roy, P.; Dávila Fajardo, R.; Tytgat, G.A.M.; Mavinkurve-Groothuis, A.M.C.; van de Ven, C.P.; et al. Locoregional control using highly conformal flank target volumes and volumetric-modulated arc therapy in pediatric renal tumors: Results from the Dutch national cohort. Radiother. Oncol. 2021, 159, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Lin, H.; Both, S.; Tochner, Z.; Balis, F.; Hill-Kayser, C. Pencil beam scanning proton therapy for treatment of the retroperitoneum after nephrectomy for Wilms tumor: A dosimetric comparison study. Pediatr. Blood Cancer. 2017, 64, 39–45. [Google Scholar] [CrossRef]
- Foster, K.L.; Salehabadi, S.M.; Green, D.M.; Xing, M.; Ness, K.K.; Krull, K.R.; Brinkman, T.M.; Ehrhardt, M.J.; Chemaitilly, W.; Dixon, S.B.; et al. Clinical Assessment of Late Health Outcomes in Survivors of Wilms Tumor. Pediatrics 2022, 150, e2022056918. [Google Scholar] [CrossRef]
- Toramatsu, C.; Inaniwa, T. Beam angle selection incorporation of anatomical heterogeneities for pencil beam scanning charged-particle therapy. Phys. Med. Biol. 2016, 61, 8664–8675. [Google Scholar] [CrossRef]
- Albertini, F.; Bolsi, A.; Lomax, A.J.; Rutz, H.P.; Timmerman, B.; Goitein, G. Sensitivity of intensity modulated proton therapy plans to changes in patient weight. Radiother. Oncol. 2008, 86, 187–194. [Google Scholar] [CrossRef]
- Berger, T.; Petersen, J.B.B.; Lindegaard, J.C.; Fokdal, L.U.; Tanderup, K. Impact of bowel gas and body outline variations on total accumulated dose with intensity-modulated proton therapy in locally advanced cervical cancer patients. Acta Oncol. 2017, 56, 1472–1478. [Google Scholar] [CrossRef]
- Uh, J.; Krasin, M.J.; Hua, C.H. Technical Note: Feasibility of MRI-based estimation of water-equivalent path length to detect changes in proton range during treatment courses. Med. Phys. 2018, 45, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Kato, T.; Takemasa, K.; Sato, H.; Ikeda, T.; Harada, T.; Oyama, S.; Murakami, M. Dosimetric impact of simulated changes in large bowel content during proton therapy with simultaneous integrated boost for locally advanced pancreatic cancer. J. Appl. Clin. Med. Phys. 2021, 22, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.S.; Rompokos, V.; Bizzocchi, N.; Gillies, C.; Gosling, A.; Royle, G.; Chang, Y.C.; Gaze, M.N.; Gains, J.E. Pencil Beam Scanning Proton Therapy Case Selection for Paediatric Abdominal Neuroblastoma: Effects of Tumour Location and Bowel Gas. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, e132–e142. [Google Scholar] [CrossRef] [PubMed]
- Gravgaard Andersen, A.; Casares-Magaz, O.; Petersen, J.; Toftegaard, J.; Bentzen, L.; Thörnqvist, S.; Muren, L.P. Beam angle evaluation to improve inter-fraction motion robustness in pelvic lymph node irradiation with proton therapy. Acta Oncol. 2017, 56, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Newly Diagnosed Patient’s with Wilm’s Tumor Requiring Abdominal Radiation Delivered with Proton Beam Irradiation. Available online: https://clinicaltrials.gov/study/NCT04968990 (accessed on 20 July 2021).
- Schneider, U.; Pedroni, E.; Lomax, A. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys. Med. Biol. 1996, 41, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.K.; Sharp, G.; Busse, P.; Winey, B. Water equivalent path length calculations using scatter-corrected head and neck CBCT images to evaluate patients for adaptive proton therapy. Phys. Med. Biol. 2017, 62, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Veiga, C.; Janssens, G.; Teng, C.L.; Baudier, T.; Hotoiu, L.; McClelland, J.R.; Royle, G.; Lin, L.; Yin, L.; Metz, J.; et al. First Clinical Investigation of Cone Beam Computed Tomography and Deformable Registration for Adaptive Proton Therapy for Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 549–559. [Google Scholar] [CrossRef]
- Gorgisyan, J.; Perrin, R.; Lomax, A.J.; Persson, G.F.; Josipovic, M.; Engelholm, S.A.; Weber, D.C.; Munck Af Rosenschold, P. Impact of beam angle choice on pencil beam scanning breath-hold proton therapy for lung lesions. Acta Oncol. 2017, 56, 853–859. [Google Scholar] [CrossRef]
- Casares-Magaz, O.; Toftegaard, J.; Muren, L.P.; Kallehauge, J.F.; Bassler, N.; Poulsen, P.R.; Petersen, J.B. A method for selection of beam angles robust to intra-fractional motion in proton therapy of lung cancer. Acta Oncol. 2014, 53, 1058–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Ho, M.W.; Li, Z. A Beam-Angle-Selection Method to Improve Inter-Fraction Motion Robustness for Lung Tumor Irradiation with Passive Proton Scattering. Technol. Cancer Res. Treat. 2020, 19, 1533033820948052. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.K.; Sharp, G.; Busse, P.; Winey, B. Beam angle optimization using angular dependency of range variation assessed via water equivalent path length (WEPL) calculation for head and neck proton therapy. Phys. Med. 2020, 69, 19–27. [Google Scholar] [CrossRef]
- Andersen, A.G.; Casares-Magaz, O.; Muren, L.P.; Toftegaard, J.; Bentzen, L.; Thörnqvist, S.; Petersen, J.B. A method for evaluation of proton plan robustness towards inter-fractional motion applied to pelvic lymph node irradiation. Acta Oncol. 2015, 54, 1643–1650. [Google Scholar] [CrossRef]




| Patient Number | Patient Age (Years) | Sex | Disease Site | Beam Arrangements | Target Volume | Prescription Dose (GyRBE) | Setup (mm)/Range (%) Uncertainty in Original Plan |
|---|---|---|---|---|---|---|---|
| 1 | 2 | M | R Kidney | RAO, LPO, PA | CTV_BWT | 10.8 | 3/3 |
| 2 | 1 | F | R Abdomen | RPO, PA | CTV_P | 10.8 | 3/3 |
| 3 | 5 | M | R Abdomen | RL, PA | CTV_P | 10.5 | 3/3 |
| 4 | 3 | M | L Abdomen | PA, LAO | CTV_P + CTV_LN | 10.5 | 5/5 |
| 5 | 4 | F | Partial Liver | PA, RL | CTV_P | 10.5 | 5/5 |
| 6 | 13 | M | L/R Abdomen | AP, RPO, LAO | ITV_Liver + GTV_P | 10.8 | 5/5 |
| 7 | 9 | F | L Abdomen | PA, LAO | ITV_P + ITV_LN | 10.8 | 3/3 |
| 8 | 4 | F | L Abdomen | LPO, LAO | ITV_P | 10.8 | 3/3 |
| 9 | 5 | F | L Abdomen | LPO, LL | ITV_P | 10.8 | 3/3 |
| 10 | 5 | M | L/R Partial Kidney | PA, RAO, LAO | CTV_BWT | 10.5 | 3/3 |
| 11 | 6 | F | Liver | PA, RAO, LAO | ITV_Liver | 10.5 | 3/3 |
| 12 | 4 | M | Partial Liver | PA, RAO | ITV_P | 10.8 | 5/5 |
| 13 | 4 | F | L Kidney | PA, LAO | ITV_BWT | 10.8 | 3/3 |
| Patient Number | Target D100% in Original Plan (%) | Target D100% in Daily Accumulation (%) | Contralateral Kidney D50% in Original Plan (GyRBE) | Contralateral Kidney D50% in Daily Accumulation (GyRBE) | Liver D50% in Original Plan (GyRBE) | Liver D50% in Daily Accumulation (GyRBE) | Target ΔD100% (%) | ΔWEPL Mean ± SD (mm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 98.9 | 98.4 | N/A* | N/A* | 0.1 | 0.1 | 0.5 | 1.0 ± 0.8 |
| 2 | 99.6 | 99.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 ± 0.9 |
| 3 | 97.0 | 97.0 | 0.0 | 0.0 | 0.7 | 0.7 | 0.0 | 1.9 ± 2.2 |
| 4 | 97.9 | 92.8 | 0.1 | 0.1 | 0.6 | 0.7 | 5.1 | 5.7 ± 6.5 |
| 5 | 99.6 | 99.5 | 0.0 | 0.0 | 8.2 | 8.2 | 0.1 | 3.9 ± 2.3 |
| 6 | 99.5 | 91.5 | 0.0 | 0.0 | 0.0 | 0.0 | 8.0 | 11.3 ± 9.6 |
| 7 | 97.0 | 88.8 | 0.2 | 0.3 | 0.0 | 0.0 | 8.2 | 3.4 ± 3.2 |
| 8 | 99.0 | 92.1 | 0.0 | 0.0 | 0.0 | 0.0 | 6.9 | 3.4 ± 3.0 |
| 9 | 97.6 | 96.1 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 1.3 ± 1.4 |
| 10 | 98.6 | 93.1 | 7.4 | 7.4 | 0.0 | 0.0 | 5.5 | 3.8 ± 2.5 |
| 11 | 85.4 | 85.0 | 0.1 | 0.1 | N/A** | N/A** | 0.4 | 2.0 ± 0.8 |
| 12 | 87.4 | 87.3 | 0.0 | 0.0 | 8.5 | 8.6 | 0.1 | 1.7 ± 1.7 |
| 13 | 99.0 | 98.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 2.3 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ates, O.; Pirlepesov, F.; Uh, J.; Hua, C.-h.; Merchant, T.E.; Boria, A.; Davidoff, A.M.; Graetz, D.E.; Krasin, M.J. Evaluating the Impact of Bowel Gas Variations for Wilms’ Tumor in Pediatric Proton Therapy. Cancers 2024, 16, 642. https://doi.org/10.3390/cancers16030642
Ates O, Pirlepesov F, Uh J, Hua C-h, Merchant TE, Boria A, Davidoff AM, Graetz DE, Krasin MJ. Evaluating the Impact of Bowel Gas Variations for Wilms’ Tumor in Pediatric Proton Therapy. Cancers. 2024; 16(3):642. https://doi.org/10.3390/cancers16030642
Chicago/Turabian StyleAtes, Ozgur, Fakhriddin Pirlepesov, Jinsoo Uh, Chia-ho Hua, Thomas E. Merchant, Andrew Boria, Andrew M. Davidoff, Dylan E. Graetz, and Matthew J. Krasin. 2024. "Evaluating the Impact of Bowel Gas Variations for Wilms’ Tumor in Pediatric Proton Therapy" Cancers 16, no. 3: 642. https://doi.org/10.3390/cancers16030642
APA StyleAtes, O., Pirlepesov, F., Uh, J., Hua, C.-h., Merchant, T. E., Boria, A., Davidoff, A. M., Graetz, D. E., & Krasin, M. J. (2024). Evaluating the Impact of Bowel Gas Variations for Wilms’ Tumor in Pediatric Proton Therapy. Cancers, 16(3), 642. https://doi.org/10.3390/cancers16030642






