Emerging Therapies in Management of Cholangiocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
Current Management of CCA
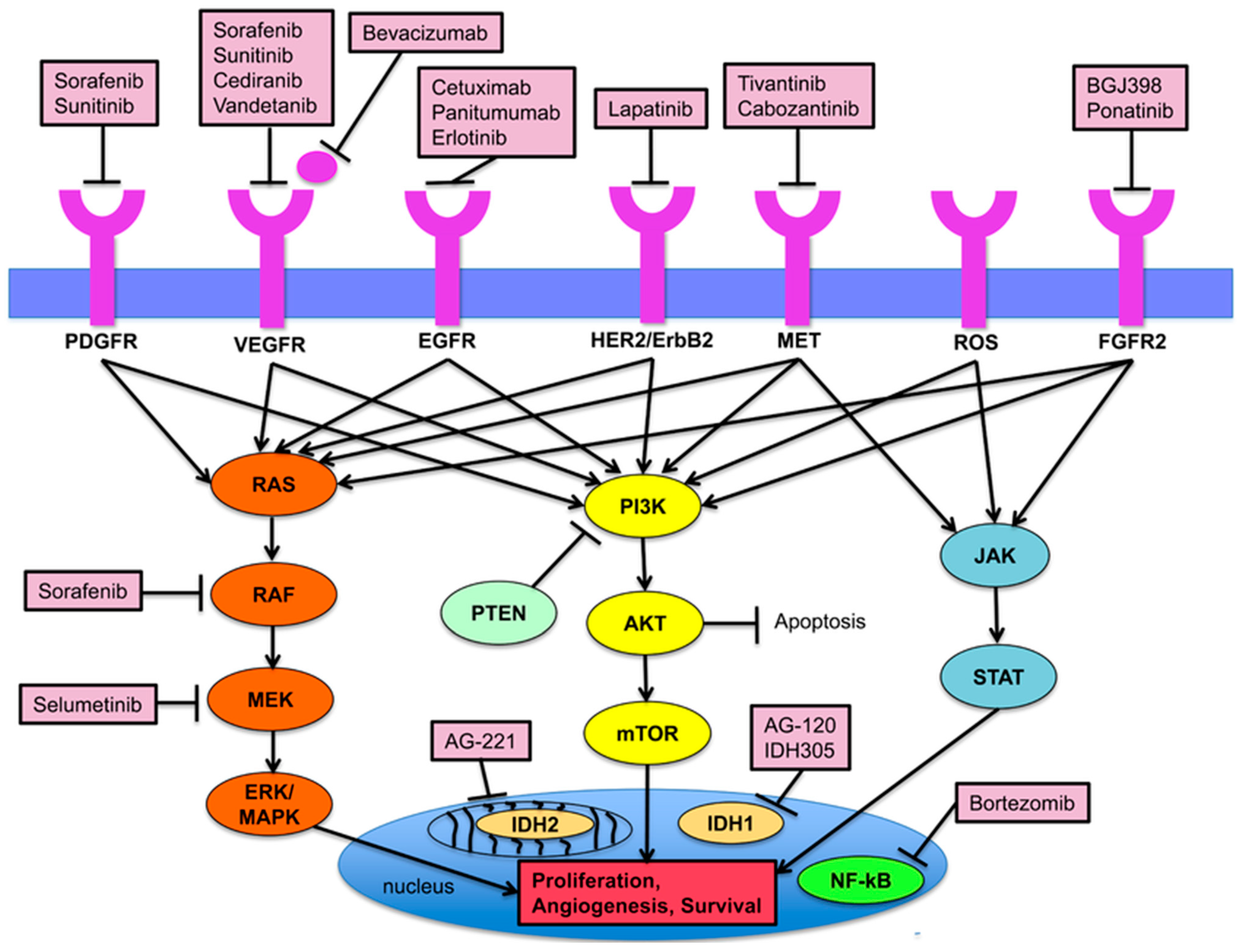
2. Emerging Therapies
2.1. FGFR Inhibitors
2.2. IDH Inhibitors
2.3. VEGF Inhibitors
2.4. EGFR Inhibitors
2.5. HGF/MET Inhibitors
2.6. ROS1/ALK Inhibitors
2.7. PI3K/PTEN/AKT/mTOR Signaling Pathway Inhibitors
2.8. RAS/RAF/MEK/ERK Signaling Pathway Inhibitors
2.9. Other
2.10. Immunotherapy: PD-1/PDL-1 Inhibitors
| Treatment | Target | Population | Phase | ClinicalTrials.gov Identifier | Status |
|---|---|---|---|---|---|
| Ivosidenib | IDH1 | advanced solid tumors including CCA | I | NCT02073994 [79] | active |
| TRK-950 in combination with selected anti-cancer treatment regimens | CAPRIN-1 + multiple drug targets | advanced solid tumors including CCA | I | NCT03872947 [80] | active |
| Pemigatinib OR Ivosidenib + gemcitabine/cisplatin | FGFR2 + IDH | advanced CCA | I | NCT04088188 [81] | active |
| LY3410738 | IDH1/2 | advanced solid tumors including CCA | I | NCT04521686 [82] | active |
| CAR-macrophages (CT-0508) | CAR macrophages | HER2-overexpressing solid tumors including CCA | I | NCT04660929 [83] | active |
| MIV 818 + Lenvatinib OR Pembrolizumab | liver-targeting prodrug of TRX-MP | HCC and iCCA | I/II | NCT03781934 [84] | active |
| Durvalumab + Tremelimumab + gemcitabine/cisplatin | PDL-1 + CTLA4 | iCCA | I/II | NCT04989218 [85] | active |
| Futibatinib | FGFR2 | advanced solid tumors including CCA | I/II | NCT05727176 [86] | active |
| IMM2902 | HER2 + CD47 | HER2-expressing advanced solid tumors including CCA | I/II | NCT05805956 [87] | active |
| Entrectinib | TrkA/B/C + ROS1 + ALK | solid tumors with TRKA/B/C, ROS1, or ALK gene rearrangements including CCA | II | NCT02568267 [88] | active |
| Erdafitinib | FGFR | non-small cell lung cancer, urothelial cancer, esophageal cancer, and CCA | II | NCT02699606 [89] | active |
| Olaparib | IDH1/2 | glioma, CCA, and other solid tumors | II | NCT03212274 [90] | active |
| Olaparib + Ceralasertib | IDH1/2 + ATR kinase | advanced solid tumors including CCA | II | NCT03878095 [91] | suspended, pending data analysis |
| Olaparib + Durvalumab | IDH + PD-L1 | glioma and CCA | II | NCT03991832 [92] | active |
| DKN-01 + Nivolumab | DKK1 | advanced BTC | II | NCT04057365 [93] | active |
| Pemigatinib after SBRT | FGFR2 | iCCA | II | NCT04088188 [81] | active |
| Toripalimab + Lenvatinib | PD-1 + multikinase inhibitor | advanced BTC | II | NCT04211168 [94] | active |
| Infigratinib | FGFR | advanced solid tumors including CCA | II | NCT04233567 [95] | active |
| Pemigatinib | FGFR2 | advanced CCA | II | NCT04256980 [96] | active |
| AZD6738 + Durvalumab | ATR + PDL-1 | BTC | II | NCT04298008 [97] | active |
| Pembrolizumab + Olaparib | PD-1 + PARP | advanced CCA | II | NCT04306367 [98] | active |
| Camrelizumab + Apatinib | PD-1 and VEGFR2 | advanced iCCA | II | NCT04454905 [99] | active |
| Zanidatamab | HER2 | advanced BTC | II | NCT04466891 [100] | active |
| Atezolizumab + Derazantinib | FGFR2 | advanced iCCA | II | NCT05174650 [101] | active |
| Durvalumab + GemCis | PDL-1 | resectable iCCA | II | NCT05672537 [102] | active |
| Futibatinib | FGFR2 | advanced CCA | II | NCT05727176 [86] | active |
| SMT-NK + Pembrolizumab OR Pembrolizumab monotherapy | allogeneic natural killer cells + PD-1 inhibitor | advanced BTC | II/III | NCT05429697 [103] | active |
| Pemigatinib + gemcitabine/cisplatin | FGFR2 | advanced CCA | III | NCT03656536 [104] | active |
| Pembrolizumab + gemcitabine/cisplatin | PD-1 | advanced BTC | III | NCT04003636 [105] | active |
| Futibatinib | FGFR2 | advanced iCCA | III | NCT04093362 [106] | active |
| Toripalimab + Lenvatinib + GEMOX | PD-1 + multikinase inhibitor | unresectable iCCA | III | NCT05342194 [107] | not yet recruiting |
| Ivosidenib | FGFR2 | previously treated CCA | IIIb | NCT05876754 [108] | active |
3. Conclusions
4. Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 66. [CrossRef]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global Trends in Mortality from Intrahepatic and Extrahepatic Cholangiocarcinoma. J. Hepatol. 2019, 71, 104–111. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Chong, D.Q.; Zhu, A.X. The Landscape of Targeted Therapies for Cholangiocarcinoma: Current Status and Emerging Targets. Oncotarget 2016, 7, 46750–46767. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhou, Z.; Chen, Z.; Xu, G.; Chen, Y. Fibroblast Growth Factor Receptors (FGFRs): Structures and Small Molecule Inhibitors. Cells 2019, 8, 614. [Google Scholar] [CrossRef]
- Vogel, A.; Segatto, O.; Stenzinger, A.; Saborowski, A. FGFR2 Inhibition in Cholangiocarcinoma. Annu. Rev. Med. 2023, 74, 293–306. [Google Scholar] [CrossRef]
- Patel, T.H.; Marcus, L.; Horiba, M.N.; Donoghue, M.; Chatterjee, S.; Mishra-Kalyani, P.S.; Schuck, R.N.; Li, Y.; Zhang, X.; Fourie Zirkelbach, J.; et al. FDA Approval Summary: Pemigatinib for Previously Treated, Unresectable Locally Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusion or Other Rearrangement. Clin. Cancer Res. 2023, 29, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Futibatinib: First Approval. Drugs 2022, 82, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Infigratinib: First Approval. Drugs 2021, 81, 1355–1360. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Bibeau, K.; Féliz, L.; Lihou, C.F.; Ren, H.; Abou-Alfa, G.K. Progression-Free Survival in Patients With Cholangiocarcinoma With or Without FGF/FGFR Alterations: A FIGHT-202 Post Hoc Analysis of Prior Systemic Therapy Response. JCO Precis. Oncol. 2022, 6, e2100414. [Google Scholar] [CrossRef]
- Shi, G.-M.; Huang, X.-Y.; Wen, T.-F.; Song, T.-Q.; Kuang, M.; Mou, H.-B.; Bao, L.-Q.; Zhao, H.-T.; Zhao, H.; Feng, X.-L.; et al. Pemigatinib in Previously Treated Chinese Patients with Locally Advanced or Metastatic Cholangiocarcinoma Carrying FGFR2 Fusions or Rearrangements: A Phase II Study. Cancer Med. 2023, 12, 4137–4146. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-Line Pemigatinib vs Gemcitabine plus Cisplatin for Advanced Cholangiocarcinoma with FGFR2 Rearrangements. Future Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Rockich, K.; Epstein, N.; Overholt, H.; Wang, P.; Chen, X.; Punwani, N.; Yeleswaram, S. Evaluation of the Pharmacokinetics of Pemigatinib in Patients with Impaired Hepatic or Renal Function. Br. J. Clin. Pharmacol. 2022, 88, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in Previously Treated Patients with Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusions or Rearrangements: Mature Results from a Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib Is a Novel Irreversible FGFR 1-4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res. 2020, 80, 4986–4997. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in Advanced or Inoperable FGFR2 Gene Fusion-Positive Intrahepatic Cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Ahn, D.H.; Uson Junior, P.L.S.; Masci, P.; Kosiorek, H.; Halfdanarson, T.R.; Mody, K.; Babiker, H.; DeLeon, T.; Sonbol, M.B.; Gores, G.; et al. A Pilot Study of Pan-FGFR Inhibitor Ponatinib in Patients with FGFR-Altered Advanced Cholangiocarcinoma. Investig. New Drugs 2022, 40, 134–141. [Google Scholar] [CrossRef]
- Casak, S.J.; Pradhan, S.; Fashoyin-Aje, L.A.; Ren, Y.; Shen, Y.-L.; Xu, Y.; Chow, E.C.Y.; Xiong, Y.; Zirklelbach, J.F.; Liu, J.; et al. FDA Approval Summary: Ivosidenib for the Treatment of Patients with Advanced Unresectable or Metastatic, Chemotherapy Refractory Cholangiocarcinoma with an IDH1 Mutation. Clin. Cancer Res. 2022, 28, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Burris, H.A.; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and Activity of Ivosidenib in Patients with IDH1-Mutant Advanced Cholangiocarcinoma: A Phase 1 Study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Cleary, J.M.; Rouaisnel, B.; Daina, A.; Raghavan, S.; Roller, L.A.; Huffman, B.M.; Singh, H.; Wen, P.Y.; Bardeesy, N.; Zoete, V.; et al. Secondary IDH1 Resistance Mutations and Oncogenic IDH2 Mutations Cause Acquired Resistance to Ivosidenib in Cholangiocarcinoma. Npj Precis. Oncol. 2022, 6, 61. [Google Scholar] [CrossRef]
- Eder, J.P.; Doroshow, D.B.; Do, K.T.; Keedy, V.L.; Sklar, J.S.; Glazer, P.; Bindra, R.; Shapiro, G.I. Clinical Efficacy of Olaparib in IDH1/IDH2-Mutant Mesenchymal Sarcomas. JCO Precis. Oncol. 2021, 5, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Foà, R.; Vitale, A.; Vignetti, M.; Meloni, G.; Guarini, A.; De Propris, M.S.; Elia, L.; Paoloni, F.; Fazi, P.; Cimino, G.; et al. Dasatinib as First-Line Treatment for Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Blood 2011, 118, 6521–6528. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Balasubramanian, B.; Pongchaikul, P.; Tohtong, R.; Chutipongtanate, S. Molecularly Guided Drug Repurposing for Cholangiocarcinoma: An Integrative Bioinformatic Approach. Genes 2022, 13, 271. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, B.; Cloyd, J.M.; Alaimo, L.; Xu, G.; Du, S.; Mao, Y.; Pawlik, T.M. Novel Drug Candidate Prediction for Intrahepatic Cholangiocarcinoma via Hub Gene Network Analysis and Connectivity Mapping. Cancers 2022, 14, 3284. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and Prognostic Significance of EGFR, VEGF, and HER2 Expression in Cholangiocarcinoma. Br. J. Cancer 2008, 98, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yang, X.; Lin, J.; Yang, X.; Wang, D.; Zhang, L.; Bai, Y.; Bian, J.; Long, J.; Xie, F.; et al. Apatinib as Non-First-Line Treatment in Patients with Intrahepatic Cholangiocarcinoma. J. Cancer 2021, 12, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gong, S.; Pang, L.; Hou, L.; He, W. Efficacy and Safety of Apatinib Treatment for Advanced Cholangiocarcinoma After Failed Gemcitabine-Based Chemotherapy: An Open-Label Phase II Prospective Study. Front. Oncol. 2021, 11, 659217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shroff, R.T.; Makawita, S.; Xiao, L.; Danner De Armas, A.; Bhosale, P.; Reddy, K.; Shalaby, A.; Raghav, K.; Pant, S.; et al. Phase II Study of Ramucirumab in Advanced Biliary Tract Cancer Previously Treated By Gemcitabine-Based Chemotherapy. Clin. Cancer Res. 2022, 28, 2229–2236. [Google Scholar] [CrossRef]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.-Y.; Orlova, R.; Van Cutsem, E.; Adeva, J.; Chen, L.-T.; Obermannova, R.; et al. Addition of Ramucirumab or Merestinib to Standard First-Line Chemotherapy for Locally Advanced or Metastatic Biliary Tract Cancer: A Randomised, Double-Blind, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 1468–1482. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A Single-Arm, Multicenter, Open-Label Phase 2 Trial of Surufatinib in Patients with Unresectable or Metastatic Biliary Tract Cancer. Cancer 2021, 127, 3975–3984. [Google Scholar] [CrossRef]
- Murahashi, M.; Tsuruta, T.; Yamada, K.; Hijikata, Y.; Ogata, H.; Kishimoto, J.; Yoshimura, S.; Hikichi, T.; Nakanishi, Y.; Tani, K. Clinical Trial of a Cancer Vaccine Targeting VEGF and KIF20A in Advanced Biliary Tract Cancer. Anticancer Res. 2021, 41, 1485–1496. [Google Scholar] [CrossRef]
- Javle, M.M.; Oh, D.-Y.; Ikeda, M.; Yong, W.-P.; Hsu, K.; Lindmark, B.; McIntyre, N.; Firth, C. Varlitinib plus Capecitabine in Second-Line Advanced Biliary Tract Cancer: A Randomized, Phase II Study (TreeTopp). ESMO Open 2022, 7, 100314. [Google Scholar] [CrossRef]
- Amin, N.E.L.; Hansen, T.F.; Fernebro, E.; Ploen, J.; Eberhard, J.; Lindebjerg, J.; Jensen, L.H. Randomized Phase II Trial of Combination Chemotherapy with Panitumumab or Bevacizumab for Patients with Inoperable Biliary Tract Cancer without KRAS Exon 2 Mutations. Int. J. Cancer 2021, 149, 119–126. [Google Scholar] [CrossRef]
- Fu, J.; Su, X.; Li, Z.; Deng, L.; Liu, X.; Feng, X.; Peng, J. HGF/c-MET Pathway in Cancer: From Molecular Characterization to Clinical Evidence. Oncogene 2021, 40, 4625–4651. [Google Scholar] [CrossRef]
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision Medicine in Cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40. [Google Scholar] [CrossRef]
- Pant, S.; Saleh, M.; Bendell, J.; Infante, J.R.; Jones, S.; Kurkjian, C.D.; Moore, K.M.; Kazakin, J.; Abbadessa, G.; Wang, Y.; et al. A Phase I Dose Escalation Study of Oral C-MET Inhibitor Tivantinib (ARQ 197) in Combination with Gemcitabine in Patients with Solid Tumors. Ann. Oncol. 2014, 25, 1416–1421. [Google Scholar] [CrossRef]
- He, A.R.; Cohen, R.B.; Denlinger, C.S.; Sama, A.; Birnbaum, A.; Hwang, J.; Sato, T.; Lewis, N.; Mynderse, M.; Niland, M.; et al. First-in-Human Phase I Study of Merestinib, an Oral Multikinase Inhibitor, in Patients with Advanced Cancer. Oncologist 2019, 24, e930–e942. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, L.N.; Larkins, E.; Akinboro, O.; Roy, P.; Amatya, A.K.; Fiero, M.H.; Mishra-Kalyani, P.S.; Helms, W.S.; Myers, C.E.; Skinner, A.M.; et al. FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations. Clin. Cancer Res. 2022, 28, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Lefler, D.S.; Tierno, M.B.; Bashir, B. Partial Treatment Response to Capmatinib in MET-Amplified Metastatic Intrahepatic Cholangiocarcinoma: Case Report & Review of Literature. Cancer Biol. Ther. 2022, 23, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Turpin, A.; Descarpentries, C.; Grégoire, V.; Farchi, O.; Cortot, A.B.; Jamme, P. Response to Capmatinib in a MET Fusion-Positive Cholangiocarcinoma. Oncologist 2023, 28, 80–83. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Q.; Li, W.; Qu, Y.; Zhang, Y.; Liu, T. Identification of a Novel EHBP1-MET Fusion in an Intrahepatic Cholangiocarcinoma Responding to Crizotinib. Oncologist 2020, 25, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, C.; Adjei, A.; Opyrchal, M.; Evans, R.; Ghasemi, M.; Attwood, K.; Groman, A.; Bshara, W.; Goey, A.; Wilton, J.; et al. A Phase I Study of the Anaplastic Lymphoma Kinase Inhibitor Ceritinib in Combination with Gemcitabine-Based Chemotherapy in Patients with Advanced Solid Tumors. Int. J. Cancer 2021, 149, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A Phase 2 and Biomarker Study of Cabozantinib in Patients with Advanced Cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef]
- Gu, T.-L.; Deng, X.; Huang, F.; Tucker, M.; Crosby, K.; Rimkunas, V.; Wang, Y.; Deng, G.; Zhu, L.; Tan, Z.; et al. Survey of Tyrosine Kinase Signaling Reveals ROS Kinase Fusions in Human Cholangiocarcinoma. PLoS ONE 2011, 6, e15640. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, K.B.; Kim, T.Y.; Han, S.W.; Oh, D.Y.; Im, S.A.; Kim, T.Y.; Yi, N.J.; Lee, K.W.; Suh, K.S.; et al. Clinical and Pathological Significance of ROS1 Expression in Intrahepatic Cholangiocarcinoma. BMC Cancer 2015, 15, 721. [Google Scholar] [CrossRef]
- Yothaisong, S.; Dokduang, H.; Techasen, A.; Namwat, N.; Yongvanit, P.; Bhudhisawasdi, V.; Puapairoj, A.; Riggins, G.J.; Loilome, W. Increased Activation of PI3K/AKT Signaling Pathway Is Associated with Cholangiocarcinoma Metastasis and PI3K/mTOR Inhibition Presents a Possible Therapeutic Strategy. Tumour Biol. 2013, 34, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Ren, Z.; Fan, J.; Gao, Q. Genetic Profiling of Intrahepatic Cholangiocarcinoma and its Clinical Implication in Targeted Therapy. Am. J. Cancer Res. 2016, 6, 577–586. [Google Scholar] [PubMed]
- Varnier, R.; Puszkiel, A.; Tod, M.; Calattini, S.; Payen, L.; Lopez, J.; Guitton, J.; Schwiertz, V.; Fontaine, J.; Peron, J.; et al. Clinical Results of the EVESOR Trial, a Multiparameter Phase I Trial of Everolimus and Sorafenib Combination in Solid Tumors. Cancer Chemother. Pharmacol. 2023, 91, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.S.; Cao, B.; Kim, J.; Al-Toubah, T.E.; Mehta, R.; Centeno, B.A.; Kim, R.D. Phase 2 Study of Copanlisib in Combination with Gemcitabine and Cisplatin in Advanced Biliary Tract Cancers. Cancer 2021, 127, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Rovida, E.; Tusa, I. Targeting MAPK in Cancer 2.0. Int. J. Mol. Sci. 2022, 23, 5702. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Zhang, D.; Yang, M.; Wang, L.; Yuan, Y. Vemurafenib Effectively Controlled Chemotherapy-Refractory Intrahepatic Cholangiocarcinoma with BRAF V600E Mutation: A Case Report and Literature Review. Z. Gastroenterol. 2022, 60, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Eigentler, T.K. Vemurafenib. In Small Molecules in Oncology; Recent Results in Cancer Research; Springer: Cham, Switzerland, 2018; Volume 211, pp. 77–89. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600E-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Wabitsch, S.; Tandon, M.; Ruf, B.; Zhang, Q.; McCallen, J.D.; McVey, J.C.; Ma, C.; Green, B.L.; Diggs, L.P.; Heinrich, B.; et al. Anti-PD-1 in Combination with Trametinib Suppresses Tumor Growth and Improves Survival of Intrahepatic Cholangiocarcinoma in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1166–1178. [Google Scholar] [CrossRef]
- Ewald, F.; Nörz, D.; Grottke, A.; Hofmann, B.T.; Nashan, B.; Jücker, M. Dual Inhibition of PI3K-AKT-mTOR- and RAF-MEK-ERK-Signaling Is Synergistic in Cholangiocarcinoma and Reverses Acquired Resistance to MEK-Inhibitors. Investig. New Drugs 2014, 32, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Anichini, G.; Carrassa, L.; Stecca, B.; Marra, F.; Raggi, C. The Role of the Hedgehog Pathway in Cholangiocarcinoma. Cancers 2021, 13, 4774. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Molina, L.; Tao, J.; Liu, S.; Hassan, M.; Singh, S.; Poddar, M.; Bell, A.; Sia, D.; Oertel, M.; et al. NOTCH-YAP1/TEAD-DNMT1 Axis Drives Hepatocyte Reprogramming Into Intrahepatic Cholangiocarcinoma. Gastroenterology 2022, 163, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, S.; Serino, G.; Dituri, F.; Cigliano, A.; Ribback, S.; Wang, J.; Chen, X.; Calvisi, D.F.; Giannelli, G. Crenigacestat, a Selective NOTCH1 Inhibitor, Reduces Intrahepatic Cholangiocarcinoma Progression by Blocking VEGFA/DLL4/MMP13 Axis. Cell Death Differ. 2020, 27, 2330–2343. [Google Scholar] [CrossRef]
- Mancarella, S.; Gigante, I.; Serino, G.; Pizzuto, E.; Dituri, F.; Valentini, M.F.; Wang, J.; Chen, X.; Armentano, R.; Calvisi, D.F.; et al. Crenigacestat Blocking Notch Pathway Reduces Liver Fibrosis in the Surrounding Ecosystem of Intrahepatic CCA viaTGF-β Inhibition. J. Exp. Clin. Cancer Res. 2022, 41, 331. [Google Scholar] [CrossRef] [PubMed]
- Massard, C.; Cassier, P.A.; Azaro, A.; Anderson, B.; Yuen, E.; Yu, D.; Oakley, G.; Benhadji, K.A.; Pant, S. A Phase 1b Study of Crenigacestat (LY3039478) in Combination with Gemcitabine and Cisplatin or Gemcitabine and Carboplatin in Patients with Advanced or Metastatic Solid Tumors. Cancer Chemother. Pharmacol. 2022, 90, 335–344. [Google Scholar] [CrossRef]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167. [Google Scholar] [CrossRef]
- Di Maira, G.; Gentilini, A.; Pastore, M.; Caligiuri, A.; Piombanti, B.; Raggi, C.; Rovida, E.; Lewinska, M.; Andersen, J.B.; Borgo, C.; et al. The Protein Kinase CK2 Contributes to the Malignant Phenotype of Cholangiocarcinoma Cells. Oncogenesis 2019, 8, 61. [Google Scholar] [CrossRef]
- Borad, M.J.; Bai, L.-Y.; Richards, D.; Mody, K.; Hubbard, J.; Rha, S.Y.; Soong, J.; McCormick, D.; Tse, E.; O’Brien, D.; et al. Silmitasertib plus Gemcitabine and Cisplatin First-Line Therapy in Locally Advanced/Metastatic Cholangiocarcinoma: A Phase 1b/2 Study. Hepatology 2023, 77, 760–773. [Google Scholar] [CrossRef]
- Yao, W.Y.; Gong, W. Immunotherapy in Cholangiocarcinoma: From Concept to Clinical Trials. Surg. Pract. Sci. 2021, 5, 100028. [Google Scholar] [CrossRef]
- Fontugne, J.; Augustin, J.; Pujals, A.; Compagnon, P.; Rousseau, B.; Luciani, A.; Tournigand, C.; Cherqui, D.; Azoulay, D.; Pawlotsky, J.-M.; et al. PD-L1 Expression in Perihilar and Intrahepatic Cholangiocarcinoma. Oncotarget 2017, 8, 24644–24651. [Google Scholar] [CrossRef]
- Ma, K.; Wei, X.; Dong, D.; Wu, Y.; Geng, Q.; Li, E. PD-L1 and PD-1 Expression Correlate with Prognosis in Extrahepatic Cholangiocarcinoma. Oncol. Lett. 2017, 14, 250–256. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Shi, G.-M.; Huang, X.-Y.; Wu, D.; Sun, H.-C.; Liang, F.; Ji, Y.; Chen, Y.; Yang, G.-H.; Lu, J.-C.; Meng, X.-L.; et al. Toripalimab Combined with Lenvatinib and GEMOX Is a Promising Regimen as First-Line Treatment for Advanced Intrahepatic Cholangiocarcinoma: A Single-Center, Single-Arm, Phase 2 Study. Signal Transduct. Target. Ther. 2023, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A Randomized Phase 2 Trial of Nivolumab, Gemcitabine, and Cisplatin or Nivolumab and Ipilimumab in Previously Untreated Advanced Biliary Cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, S.; Gu, S.; Ren, Z.; Chen, Z.; Xiong, J.; Liu, Y.; Meng, Z.; Zhang, X.; Wang, L.; et al. Camrelizumab plus Oxaliplatin-Based Chemotherapy as First-Line Therapy for Advanced Biliary Tract Cancer: A Multicenter, Phase 2 Trial. Int. J. Cancer 2021, 149, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Javle, M.M.; Verdaguer Mata, H.; de Braud, F.; Trojan, J.; Raoul, J.-L.; Kim, J.W.; Ueno, M.; Lee, C.-K.; Hijioka, S.; et al. Phase 2 Trial of Bintrafusp Alfa as Second-Line Therapy for Patients with Locally Advanced/Metastatic Biliary Tract Cancers. Hepatology 2023, 78, 758–770. [Google Scholar] [CrossRef]
- Study of Orally Administered AG-120 in Subjects with Advanced Solid Tumors, Including Glioma, with and IDH1 Mutation. ClinicalTrials.gov Identifier: NCT02073994. Updated 5 December 2023. Available online: https://clinicaltrials.gov/study/NCT02073994 (accessed on 10 January 2024).
- Bauernschub, Vicki. A Study of TRK-950 in Combinations with Anti-Cancer Treatment Regimens in Patients with Advanced Solid Tumors. ClinicalTrials.gov Identifier: NCT03872947. Updated 18 November 2023. Available online: https://clinicaltrials.gov/study/NCT03872947 (accessed on 10 January 2024).
- Gemcitabine and Cisplatin With Ivosidenib or Pemigatinib for the Treatment of Unresectable or Metastatic Cholangiocarcinoma. ClinicalTrials.gov Identifier: NCT04088188. Updated 5 July 2023. Available online: https://clinicaltrials.gov/study/NCT04088188 (accessed on 10 January 2024).
- Study of LY3410738 Administered to Patients with Advanced Solid Tumors with IDH1 or IDH2 Mutations. ClinicalTrials.gov Identifier: NCT04521686. Updated 1 September 2023. Available online: https://clinicaltrials.gov/study/NCT04521686 (accessed on 10 January 2024).
- Swaby, Ramona. CAR-Macrophages for the Treatment of HER2 Overexpressing Solid Tumors. ClinicalTrials.gov Identifier: NCT04660929. Updated 19 December 2023. Available online: https://clinicaltrials.gov/study/NCT04660929 (accessed on 10 January 2024).
- A Study to Evaluate MIV-818 in Patients with Liver Cancer Manifestations. ClinicalTrials.gov Identifier: NCT03781934. Updated 11 September 2023. Available online: https://clinicaltrials.gov/study/NCT03781934 (accessed on 10 January 2024).
- Isaac, Shiney. Durvalumab and Tremelimumab with Platinum-based Chemotherapy in Intrahepatic Cholangiocarcinoma. ClinicalTrials.gov Identifier: NCT04989218. Updated 3 January 2024. Available online: https://clinicaltrials.gov/study/NCT04989218 (accessed on 10 January 2024).
- Study of Futibatinib in Patients with Advanced Cholangiocarcinoma with FGFR2 Fusion or Rearrangement (FOENIX-CCA4). ClinicalTrials.gov Identifier: NCT05727176. Updated 21 December 2023. Available online: https://clinicaltrials.gov/study/NCT05727176 (accessed on 10 January 2024).
- Chen, D. IMM2902 in Patients with Advanced Solid Tumors Expressing HER2. ClinicalTrials.gov Identifier: NCT05805956. Updated 10 April 2023. Available online: https://clinicaltrials.gov/study/NCT05805956 (accessed on 10 January 2024).
- Basket Study of Entrectinib (RXDX-101) for the Treatment of Patients with Solid Tumors Harboring NTRK 1/2/3 (Trk A/B/C), ROS1, or ALK Gene Rearrangements (Fusions) (STARTRK-2). ClinicalTrials.gov Identifier: NCT02568267. 3 January 2024. Available online: https://clinicaltrials.gov/study/NCT02568267 (accessed on 10 January 2024).
- A Study to Evaluate the Clinical Efficacy of JNJ-42756493 (Erdafitinib), A Pan-Fibroblast Growth Factor Receptor (FGFR) Tyrosine Kinase Inhibitor, In Asian Participants with Advanced Non-Small-Cell Lung Cancer, Urothelial Cancer, Esophageal Cancer Or Cholangiocarcinoma. ClinicalTrials.gov Identifier: NCT02699606. Updated 3 January 2024. Available online: https://clinicaltrials.gov/study/NCT02699606 (accessed on 10 January 2024).
- Olaparib in Treating Patients with Advanced Glioma, Cholangiocarcinoma, or Solid Tumors with IDH1 or IDH2 Mutations. ClinicalTrials.gov Identifier: NCT03212274. Updated 12 December 2023. Available online: https://clinicaltrials.gov/study/NCT03212274 (accessed on 10 January 2024).
- Testing Olaparib and AZD6738 in IDH1 and IDH2 Mutant Tumors. ClinicalTrials.gov Identifier: NCT03878095. Updated 6 December 2023. Available online: https://clinicaltrials.gov/study/NCT03878095 (accessed on 10 January 2024).
- Chen, E. Study of Olaparib and Durvalumab in IDH-Mutated Solid Tumors (SOLID). ClinicalTrials.gov Identifier: NCT03991832. Updated 10 January 2023. Available online: https://clinicaltrials.gov/study/NCT03991832 (accessed on 10 January 2024).
- Goyal, L. Study of the Combination of DKN-01 and Nivolumab in Previously Treated Patients with Advanced Biliary Tract Cancer (BTC). ClinicalTrials.gov Identifier: NCT04057365. Updated 26 January 2021. Available online: https://clinicaltrials.gov/study/NCT04057365 (accessed on 10 January 2024).
- Yang, X. Toripalimab Plus Lenvatinib as Second-line Treatment in Advanced Biliary Tract Cancers. ClinicalTrials.gov Identifier: NCT04211168. Updated 29 March 2023. Available online: https://clinicaltrials.gov/study/NCT04211168 (accessed on 10 January 2024).
- Roychowdhury, S. Infigratinib for the Treatment of Advanced or Metastatic Solid Tumors in Patients With FGFR Gene Mutations. ClinicalTrials.gov Identifier: NCT04233567. Updated 18 September 2023. Available online: https://clinicaltrials.gov/study/NCT04233567 (accessed on 10 January 2024).
- Pemigatinib in Treating Patients with Advanced/Metastatic or Surgically Unresectable Cholangiocarcinoma Including FGFR2 Rearrangement. ClinicalTrials.gov Identifier: NCT04256980. Updated 4 October 2022. Available online: https://clinicaltrials.gov/study/NCT04256980 (accessed on 10 January 2024).
- Oh, D.-Y. AZD6738 Plus Durvalumab in Biliary Tract Cancer. ClinicalTrials.gov Identifier: NCT04298008. Updated 12 April 2023. Available online: https://clinicaltrials.gov/study/NCT04298008 (accessed on 10 January 2024).
- Study of Pembrolizumab and Olaparib in Bile Duct Cancer. ClinicalTrials.gov Identifier: NCT04306367. Updated 5 April 2023. Available online: https://clinicaltrials.gov/study/NCT04306367 (accessed on 10 January 2024).
- Xu, L. Camrelizumab in Combination with Apatinib in Advanced ICC: A Single-arm Phase II Study. ClinicalTrials.gov Identifier: NCT04454905. Updated 19 April 2023. Available online: https://clinicaltrials.gov/study/NCT04454905 (accessed on 10 January 2024).
- A Study of ZW25 (Zanidatamab) in Subjects with Advanced or Metastatic HER2-Amplified Biliary Tract Cancers (HERIZON-BTC-01). ClinicalTrials.gov Identifier: NCT04466891. Updated 7 December 2023. Available online: https://clinicaltrials.gov/study/NCT04466891 (accessed on 10 January 2024).
- Vogel, A. Treatment of Atezolizumab and Derazantinib in Patients with Advanced iCCA With FGFR2 Fusions/Rearrangements. ClinicalTrials.gov Identifier: NCT05174650. Updated 23 May 2023. Available online: https://clinicaltrials.gov/study/NCT05174650 (accessed on 10 January 2024).
- Durvalumab Combined with GemCis Neoadjuvant Therapy of Resectable Intrahepatic Cholangiocarcinoma with High Recurrence Risk. ClinicalTrials.gov Identifier: NCT05672537. Updated 5 January 2023. Available online: https://clinicaltrials.gov/study/NCT05672537 (accessed on 10 January 2024).
- Im, J. Study of SMT-NK Inj. Plus Pembrolizumab vs Pembrolizumab Monotherapy in Patients with Advanced Biliary Tract Cancer. ClinicalTrials.gov Identifier: NCT05429697. Updated 27 September 2022. Available online: https://clinicaltrials.gov/study/NCT05429697 (accessed on 10 January 2024).
- A Study to Evaluate the Efficacy and Safety of Pemigatinib Versus Chemotherapy in Unresectable or Metastatic Cholangiocarcinoma (FIGHT-302). ClinicalTrials.gov Identifier: NCT03656536. Updated 5 January 2024. Available online: https://clinicaltrials.gov/study/NCT03656536 (accessed on 10 January 2024).
- Pembrolizumab (MK-3475) Plus Gemcitabine/Cisplatin Versus Placebo Plus Gemcitabine/Cisplatin for First-Line Advanced and/or Unresectable Biliary Tract Carcinoma (BTC) (MK-3475-966/KEYNOTE-966) (KEYNOTE-966). ClinicalTrials.gov Identifier: NCT04003636. Updated 12 December 2022. Available online: https://clinicaltrials.gov/study/NCT04003636 (accessed on 10 January 2024).
- Futibatinib Versus Gemcitabine-Cisplatin Chemotherapy as First-Line Treatment of Patients with Advanced Cholangiocarcinoma Harboring FGFR2 Gene Rearrangements (FOENIX-CCA3). ClinicalTrials.gov Identifier: NCT04093362. Updated 9 January 2024. Available online: https://clinicaltrials.gov/study/NCT04093362 (accessed on 10 January 2024).
- Yan, J. Toripalimab Plus Lenvatinib and Gemcitabine-based Chemotherapy in 1L Treatment of Advanced ICC: A Phase III Study. ClinicalTrials.gov Identifier: NCT05342194. Updated 15 August 2022. Available online: https://clinicaltrials.gov/study/NCT05342194 (accessed on 10 January 2024).
- An Early Access Study of Ivosidenib in Patients with a Pretreated Locally Advanced or Metastatic Cholangiocarcinoma (ProvIDHe). ClinicalTrials.gov Identifier: NCT05876754. Updated 7 December 2023. Available online: https://clinicaltrials.gov/study/NCT05876754 (accessed on 10 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speckart, J.; Rasmusen, V.; Talib, Z.; GnanaDev, D.A.; Rahnemai-Azar, A.A. Emerging Therapies in Management of Cholangiocarcinoma. Cancers 2024, 16, 613. https://doi.org/10.3390/cancers16030613
Speckart J, Rasmusen V, Talib Z, GnanaDev DA, Rahnemai-Azar AA. Emerging Therapies in Management of Cholangiocarcinoma. Cancers. 2024; 16(3):613. https://doi.org/10.3390/cancers16030613
Chicago/Turabian StyleSpeckart, Jessica, Veronica Rasmusen, Zohray Talib, Dev A. GnanaDev, and Amir A. Rahnemai-Azar. 2024. "Emerging Therapies in Management of Cholangiocarcinoma" Cancers 16, no. 3: 613. https://doi.org/10.3390/cancers16030613
APA StyleSpeckart, J., Rasmusen, V., Talib, Z., GnanaDev, D. A., & Rahnemai-Azar, A. A. (2024). Emerging Therapies in Management of Cholangiocarcinoma. Cancers, 16(3), 613. https://doi.org/10.3390/cancers16030613





