An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Evaluation of Quality and Risk of Bias
2.6. Statistical Analysis
3. Results
3.1. Results of the Literature Search

3.2. Study Characteristics
| Summarized Characteristics of Reviewed Studies | |
|---|---|
| Total | 101 studies |
| Year of publication | 1929–2023 |
| Number of patients | |
| Total | 38,083 |
| Developed oral cancer | 606 |
| Sample size, range | 16–3568 patients |
| Diagnostic entity | |
| Oral lichen planus (OLP) | 97 studies (36,889 patients) |
| Oral lichenoid lesions (OLLs) | 8 studies (856 patients) |
| Lichenoid reactions (LRs) | 4 studies (164 patients) |
| OLP with dysplasia | 5 studies (174 patients) |
| Study design | |
| Retrospective longitudinal | 92 studies |
| Prospective longitudinal | 8 studies |
| Ambispective longitudinal | 1 study |
| Geographical region | |
| Europe | 51 studies (18 countries) |
| Asia | 26 studies (11 countries) |
| North America | 15 studies (2 countries) |
| South America | 4 studies (2 countries) |
| Oceania | 3 studies (2 countries) |
| Africa | 2 studies (1 country) |
| Total | 6 continents, 36 countries |
3.3. Qualitative Evaluation
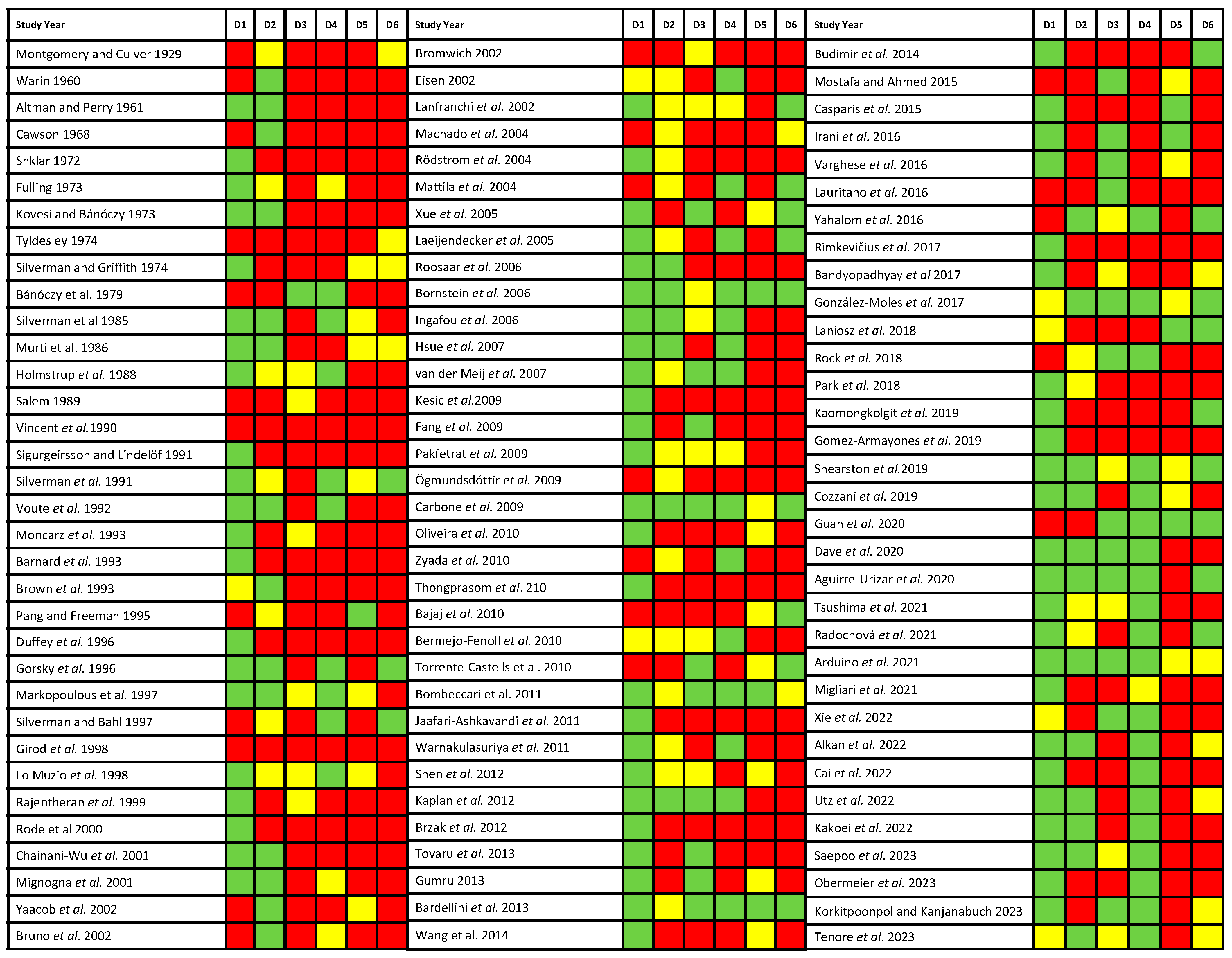
3.4. Quantitative Evaluation (Meta-Analysis)
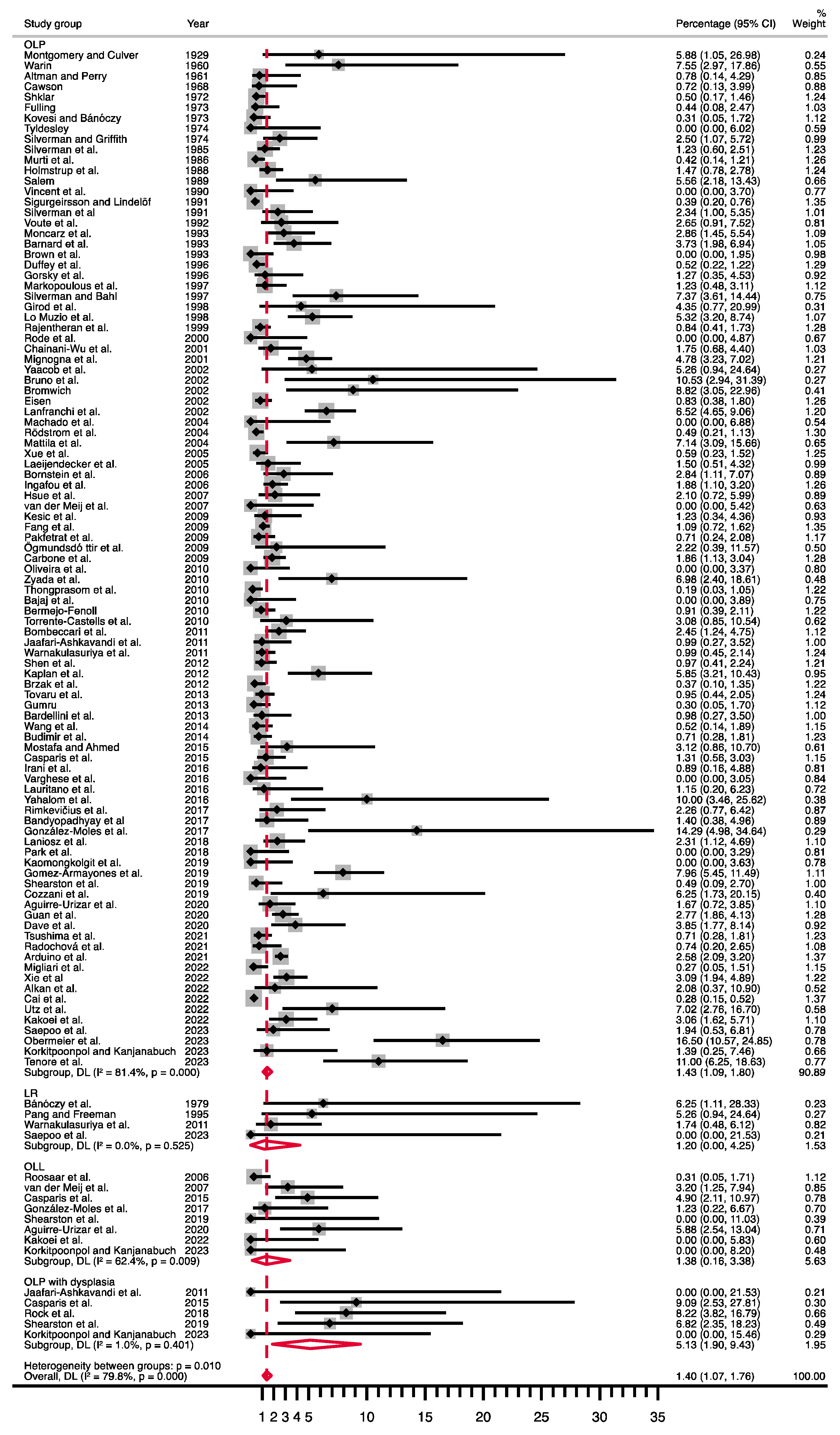
| Pooled Data | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|
| Analysis | No. of Studies | No. of Patients | Stat. Model | ES (95% CI) | p-Value | Q | Phet | I2 (%) |
| Diagnosis a | 0.001 b | |||||||
| OLP | 97 | 36,889 | R, d-l | PP = 1.43% (1.09–1.80) | 515.65 | <0.001 | 81.4 | |
| OLP with dysplasia | 5 | 174 | R, d-l | PP = 5.13% (1.90–9.43) | 4.04 | 0.40 | 1.0 | |
| Diagnosis a | 0.853 b | |||||||
| OLP | 97 | 36,889 | R, d-l | PP = 1.43% (1.09–1.80) | 515.65 | <0.001 | 81.4 | |
| OLL | 8 | 856 | R, d-l | PP = 1.38% (0.16–3.38) | 18.62 | 0.009 | 62.4 | |
| Diagnosis a | 0.328 b | |||||||
| OLP | 97 | 36,889 | R, d-l | PP = 1.43% (1.09–1.80) | 515.65 | <0.001 | 81.4 | |
| LR | 4 | 164 | R, d-l | PP = 1.20% (0.00–4.25) | 2.24 | 0.53 | 0.0 | |
| Criteria a | <0.001 b | |||||||
| Clinical and histopathological | 70 | 27,975 | R, d-l | PP = 1.92% (1.48–2.41) | 378.36 | <0.001 | 81.8 | |
| Clinical or non-exhaustive | 31 | 10,108 | R, d-l | PP = 0.61% (0.25–1.07) | 110.66 | <0.001 | 72.9 | |
| Sex c | ||||||||
| Male vs. Female | 59 | 29,297 | F, m-h | RR = 1.13 (0.93–1.38) | 0.208 | 44.81 | 0.898 | 0.0 |
| Smoking c | ||||||||
| Smokers vs. non-smokers | 24 | 7122 | F, m-h | RR = 1.60 (1.07–2.41) | 0.022 | 20.04 | 0.581 | 0.0 |
| Alcohol c | ||||||||
| Drinkers vs. non-drinkers | 11 | 3275 | F, m-h | RR = 2.11 (1.13–3.97) | 0.020 | 10.73 | 0.379 | 6.8 |
| HCV c | ||||||||
| HCV-positive vs. negative | 8 | 5433 | R, d-l | RR = 3.67 (1.48–9.14) | 0.005 | 17.40 | 0.015 | 59.8 |
| Localization c | ||||||||
| Tongue vs. others | 22 | 15,284 | F, m-h | RR = 1.82 (1.25–2.63) | 0.002 | 11.68 | 0.948 | 0.0 |
| Clinical aspect c | ||||||||
| Red vs. white | 39 | 14,515 | F, m-h | RR = 2.38 (1.85–3.07) | <0.001 | 27.90 | 0.885 | 0.0 |
| Appraisal of highest quality studies d | 0.849 b | |||||||
| OLP | 11 | 6379 | R, d-l | PP = 2.25% (1.65–2.94) | 17.49 | 0.064 | 42.8 | |
| OLLs | 3 | 197 | R, d-l | PP = 2.11% (0.01–6.33) | 3.69 | 0.158 | 45.8 | |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Moles, M.Á.; Ramos-García, P. Oral lichen planus and related lesions. What should we accept based on the available evidence? Oral Dis. 2023, 29, 2624–2637. [Google Scholar] [CrossRef]
- Scully, C.; Carrozzo, M. Oral mucosal disease: Lichen planus. Br. J. Oral Maxillofac. Surg. 2008, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Zhao, Z.Z.; Zhou, X.J.; Khan, A.; Seymour, G.J.; Bigby, M. The pathogenesis of oral lichen planus. Crit. Rev. Oral Biol. Med. 2002, 13, 350–365. [Google Scholar] [CrossRef]
- Lodi, G.; Scully, C.; Carrozzo, M.; Griffiths, M.; Sugerman, P.B.; Thongprasom, K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2005, 100, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O. Oral lichen planus. 1. A clinical evaluation of 115 cases. Oral Surg. Oral Med. Oral Pathol. 1968, 25, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Scully, C.; Gil-Montoya, J.A. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Lodi, G. Oral potentially malignant disorders: Proceedings from an expert symposium. Oral Dis. 2021, 27, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Urizar, J.M.; Lafuente-Ibáñez de Mendoza, I.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and meta-analysis of the last 5 years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P.; Warnakulasuriya, S. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Dis. 2021, 27, 1908–1918. [Google Scholar] [CrossRef]
- Ramos-García, P.; Gonzalez-Moles, M.A.; Warnakulasuriya, S. Oral cancer development in lichen planus and related conditions -3.0 evidence level-: A systematic review of systematic reviews. Oral Dis. 2021, 27, 1919–1935. [Google Scholar] [CrossRef]
- Kujan, O.; Mello, F.W.; Warnakulasuriya, S. Malignant transformation of oral submucous fibrosis: A systematic review and meta-analysis. Oral Dis. 2021, 27, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Odell, E.; Kujan, O.; Warnakulasuriya, S.; Sloan, P. Oral epithelial dysplasia: Recognition, grading and clinical significance. Oral Dis. 2021, 27, 1947–1976. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Mello, F.W.; Warnakulasuriya, S. Tissue biomarkers for predicting the risk of oral cancer in patients diagnosed with oral leukoplakia: A systematic review. Oral Dis. 2021, 27, 1977–1992. [Google Scholar] [CrossRef]
- Odell, E.W. Aneuploidy and loss of heterozygosity as risk markers for malignant transformation in oral mucosa. Oral Dis. 2021, 27, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.R.; Lodi, G. Management of oral potentially malignant disorders. Oral Dis. 2021, 27, 2008–2025. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.Á.; Mello, F.W.; Bagan, J.V.; Warnakulasuriya, S. Malignant transformation of oral proliferative verrucous leukoplakia: A systematic review and meta-analysis. Oral Dis. 2021, 27, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Van der Meij, E.H.; Van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Gil-Montoya, J.A.; Ruiz-Avila, I.; Bravo, M. Is oral cancer incidence among patients with oral lichen planus/oral lichenoid lesions underestimated? J. Oral Pathol. Med. 2017, 46, 148–153. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. J. Am. Med. Assoc. 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Tuckey, J. Transformations Related to the Angular and the Square Root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Jin, Z.-C.; Zhou, X.-H.; He, J. Statistical methods for dealing with publication bias in meta-analysis. Stat. Med. 2015, 34, 343–360. [Google Scholar] [CrossRef]
- Saepoo, J.; Kerdpon, D.; Pangsomboon, K. Malignant Transformation in Oral Lichen Planus and Lichenoid Reactions in Southern Thai Population. Oral. Sci. Rep. 2023, 44, 27–34. [Google Scholar] [CrossRef]
- Migliari, D.; Sugaya, N.; Hirota, S. A Survey of Brazilian Patients with Oral Lichen Planus Showing No Evidence of Malignancy. Dermatol. Res. Pract. 2022, 2022, 5937540. [Google Scholar] [CrossRef]
- Arduino, P.G.; Magliano, A.; Gambino, A.; Macciotta, A.; Carbone, M.; Conrotto, D.; Karimi, D.; Carrozzo, M.; Broccoletti, R. Risk of Malignant Transformation in 3173 Subjects with Histopathologically Confirmed Oral Lichen Planus: A 33-Year Cohort Study in Northern Italy. Cancers 2021, 13, 5740. [Google Scholar] [CrossRef] [PubMed]
- Radochová, V.; Koberová Ivančaková, R.; Heneberk, O.; Slezák, R. The Characteristics of Patients with Oral Lichen Planus and Malignant Transformation-A Retrospective Study of 271 Patients. Int. J. Environ. Res. Public Health 2021, 18, 6525. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, F.; Sakurai, J.; Uesugi, A.; Oikawa, Y.; Ohsako, T.; Mochizuki, Y.; Hirai, H.; Kayamori, K.; Harada, H. Malignant transformation of oral lichen planus: A retrospective study of 565 Japanese patients. BMC Oral Health 2021, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Dave, A.; Shariff, J.; Philipone, E. Association between oral lichen planus and systemic conditions and medications: Case-control study. Oral Dis. 2021, 27, 515–524. [Google Scholar] [CrossRef]
- Guan, G.; Mei, L.; Polonowita, A.; Hussaini, H.; Seo, B.; Rich, A.M. Malignant transformation in oral lichen planus and lichenoid lesions: A 14-year longitudinal retrospective cohort study of 829 patients in New Zealand. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 411–418. [Google Scholar] [CrossRef]
- Aguirre-Urizar, J.-M.; Alberdi-Navarro, J.; Lafuente-Ibáñez de Mendoza, I.; Marichalar-Mendia, X.; Martínez-Revilla, B.; Parra-Pérez, C.; Juan-Galíndez, A.-D.; Echebarria-Goicouria, M.-Á. Clinicopathological and prognostic characterization of oral lichenoid disease and its main subtypes: A series of 384 cases. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e554–e562. [Google Scholar] [CrossRef]
- Cozzani, E.; Russo, R.; Mazzola, F.; Garofolo, S.; Camerino, M.; Burlando, M.; Peretti, G.; Parodi, A. Narrow-band imaging: A useful tool for early recognition of oral lichen planus malignant transformation? Eur. J. Dermatol. 2019, 29, 500–506. [Google Scholar] [CrossRef]
- Shearston, K.; Fateh, B.; Tai, S.; Hove, D.; Farah, C.S. Oral lichenoid dysplasia and not oral lichen planus undergoes malignant transformation at high rates. J. Oral Pathol. Med. 2019, 48, 538–545. [Google Scholar] [CrossRef]
- Gomez-Armayones, S.; Chimenos-Küstner, E.; Marí, A.; Tous, S.; Penin, R.; Clavero, O.; Quirós, B.; Pavon, M.A.; Taberna, M.; Alemany, L.; et al. Human papillomavirus in premalignant oral lesions: No evidence of association in a Spanish cohort. PLoS ONE 2019, 14, e0210070. [Google Scholar] [CrossRef]
- Tenore, G.; Mohsen, A.; Rocchetti, F.; Rossi, G.; Cassoni, A.; Battisti, A.; Della Monaca, M.; Di Gioia, C.R.T.; De Felice, F.; Botticelli, A.; et al. Risk of Oral Squamous Cell Carcinoma in One Hundred Patients with Oral Lichen Planus: A Follow-Up Study of Umberto I University Hospital of Rome. Cancers 2023, 15, 3004. [Google Scholar] [CrossRef]
- Kaomongkolgit, R.; Daroonpan, P.; Tantanapornkul, W.; Palasuk, J. Clinical profile of 102 patients with oral lichen planus in Thailand. J. Clin. Exp. Dent. 2019, 11, e625–e629. [Google Scholar] [CrossRef] [PubMed]
- Laniosz, V.; Torgerson, R.R.; Ramos-Rodriguez, A.J.; Ma, J.E.; Mara, K.C.; Weaver, A.L.; Bruce, A.J. Incidence of squamous cell carcinoma in oral lichen planus: A 25-year population-based study. Int. J. Dermatol. 2019, 58, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Rock, L.D.; Laronde, D.M.; Lin, I.; Rosin, M.P.; Chan, B.; Shariati, B.; Zhang, L. Dysplasia Should Not Be Ignored in Lichenoid Mucositis. J. Dent. Res. 2018, 97, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.J.; Kim, S.H.; Kim, S.B.; Choi, Y.H.; Kim, Y.K.; Yun, P.Y. Factors affecting treatment outcomes in patients with oral lichen planus lesions: A retrospective study of 113 cases. J. Periodontal Implant Sci. 2018, 48, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rimkevičius, A.; Aleksejūnienė, J.; Pūrienė, A.; Šeinin, D.; Rastenienė, R. Oral lichen planus: A 4-year clinical follow-up study. Turkish J. Med. Sci. 2017, 47, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Banyopadhyay, A.; Sundar Behura, S.; Nisht, R.; Dash, K.; Bhuyan, L.; Ramachandra, S. Clinicopathological profile and malignant transformation in oral lichen planus: A retrospective study. J Int Soc Prev Community Dent. 2017, 7, S1–S7. [Google Scholar] [CrossRef]
- Lauritano, D.; Arrica, M.; Lucchese, A.; Valente, M.; Pannone, G.; Lajolo, C.; Ninivaggi, R.; Petruzzi, M. Oral lichen planus clinical characteristics in Italian patients: A retrospective analysis. Head Face Med. 2016, 12, 18. [Google Scholar] [CrossRef]
- Irani, S.; Esfahani, A.; Ghorbani, A. Dysplastic change rate in cases of oral lichen planus: A retrospective study of 112 cases in an Iranian population. J. Oral Maxillofac. Pathol. 2016, 20, 395. [Google Scholar] [CrossRef]
- Yahalom, R.; Yarom, N.; Shani, T.; Amariglio, N.; Kaplan, I.; Trakhtenbrot, L.; Hirshberg, A. Oral lichen planus patients exhibit consistent chromosomal numerical aberrations: A follow-up analysis. Head Neck 2016, 38, E741–E746. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.S.; George, G.B.; Sarojini, S.B.; Vinod, S.; Mathew, P.; Mathew, D.G.; Sebastian, J.; George, A. Epidemiology of Oral Lichen Planus in a Cohort of South Indian Population: A Retrospective Study. J. Cancer Prev. 2016, 21, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Korkitpoonpol, N.; Kanjanabuch, P. Direct immunofluorescence cannot be used solely to differentiate among oral lichen planus, oral lichenoid lesion, and oral epithelial dysplasia. J. Dent. Sci. 2023, 18, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, B.; Ahmed, E. Prevalence of oral lichen planus among a sample of the Egyptian population. J. Clin. Exp. Dent. 2015, 7, e7–e12. [Google Scholar] [CrossRef] [PubMed]
- Casparis, S.; Borm, J.M.; Tektas, S.; Kamarachev, J.; Locher, M.C.; Damerau, G.; Grätz, K.W.; Stadlinger, B. Oral lichen planus (OLP), oral lichenoid lesions (OLL), oral dysplasia, and oral cancer: Retrospective analysis of clinicopathological data from 2002–2011. Oral Maxillofac. Surg. 2015, 19, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Tail, Y.H.; Wang, W.C.; Chen, C.Y.; Kao, Y.H.; Chen, Y.K.; Chen, C.H. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health 2014, 14, 99. [Google Scholar] [CrossRef]
- Budimir, V.; Richter, I.; Andabak-Rogulj, A.; Vučićević-Boras, V.; Budimir, J.; Brailo, V. Oral lichen planus—Retrospective study of 563 Croatian patients. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e255–e260. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Flocchini, P.; Bonadeo, S.; Majorana, A. Clinicopathological features and malignant transformation of oral lichen planus: A 12-years retrospective study. Acta Odontol. Scand. 2013, 71, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Tovaru, S.; Parlatescu, I.; Gheorghe, C.; Tovaru, M.; Costache, M.; Sardella, A. Oral lichen planus: A retrospective study of 633 patients from Bucharest, Romania. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e201–e206. [Google Scholar] [CrossRef] [PubMed]
- Gümrü, B. A retrospective study of 370 patients with oral lichen planus in Turkey. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e427–e432. [Google Scholar] [CrossRef]
- Yaacob, H.B.; Tan, P.L.; Ngeow, W.C. Malignancy in oral lichen planus: A review of a group from the Malaysian population. J. Oral Sci. 2012, 44, 65–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaplan, I.; Ventura-Sharabi, Y.; Gal, G.; Calderon, S.; Anavi, Y. The Dynamics of Oral Lichen Planus: A Retrospective Clinicopathological Study. Head Neck Pathol. 2012, 6, 178–183. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, W.; Zhu, L.K.; Feng, J.Q.; Tang, G.Y.; Zhou, Z.T. A retrospective clinicopathological study on oral lichen planus and malignant transformation: Analysis of 518 cases. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e943–e947. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, K.T.; Wuersching, S.N.; Liokatis, P.; Smolka, W.; Poxleitner, P.; Kleye, C.; Ehrenfeld, M.; Kollmuss, M.; Otto, S. Metastases of OSCC Based on Oral Lichen Ruber Planus. Cancers 2023, 15, 4092. [Google Scholar] [CrossRef]
- Brzak, B.L.; Mravak-Stipetić, M.; Canjuga, I.; Baričević, M.; Baličević, D.; Sikora, M.; Filipović-Zore, I. Učestalost i stopa zloćudne preobrazbe oralnog lihena planusa i leukoplakije-retrospektivna studija. Coll. Antropol. 2012, 36, 773–777. [Google Scholar] [PubMed]
- Warnakulasuriya, S.; Kovacevic, T.; Madden, P.; Coupland, V.H.; Sperandio, M.; Odell, E.; Møller, H. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J. Oral Pathol. Med. 2011, 40, 677–683. [Google Scholar] [CrossRef]
- Jaafari-Ashkavandi, Z.; Mardani, M.; Pardis, S.; Amanpour, S. Oral mucocutaneous diseases: Clinicopathologic analysis and malignant transformation. J. Craniofac. Surg. 2011, 22, 949–951. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Guzzi, G.; Tettamanti, M.; Giann, A.B.; Baj, A.; Pallotti, F.; Spadari, F. Oral lichen planus and malignant transformation: A longitudinal cohort study. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Castells, E.; Figueiredo, R.; Berini-Aytés, L.; Gay-Escoda, C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e685–e690. [Google Scholar] [CrossRef]
- Thongprasom, K.; Youngnak-Piboonratanakit, P.; Pongsiriwet, S.; Laothumthut, T.; Kanjanabud, P.; Rutchakitprakarn, L. A multicenter study of oral lichen planus in Thai patients. J. Investig. Clin. Dent. 2010, 1, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Fenoll, A.; Sánchez-Siles, M.; López-Jornet, P.; Camacho-Alonso, F.; Salazar-Sánchez, N. A retrospective clinicopathological study of 550 patients with oral lichen planus in south-eastern Spain. J. Oral Pathol. Med. 2010, 39, 491–496. [Google Scholar] [CrossRef]
- Zyada, M.M.; Fikry, H.E. Immunohistochemical study of syndecan-1 down-regulation and the expression of P35 protein in oral lichen planus: A clinicopathologic correlation with hepatitis C infection in the Egyptian population. Ann. Diagn. Pathol. 2010, 14, 153–161. [Google Scholar] [CrossRef]
- Bajaj, D.R.; Khoso, N.A.; Devrajani, B.R.; Matlani, B.L.; Lohana, P. Oral lichen planus: A clinical study. J. Coll. Physicians Surg. Pakistan 2010, 20, 154–157. [Google Scholar]
- Oliveira Alves, M.G.; Almeida, J.D.; Balducci, I.; Guimarães Cabral, L.A. Oral lichen planus: A retrospective study of 110 Brazilian patients. BMC Res. Notes 2010, 3, E315–E318. [Google Scholar] [CrossRef] [PubMed]
- Kakoei, S.; Torabi, M.; Rad, M.; Karbasi, N.; Mafi, S. Retrospective Study of Oral Lichen Planus and Oral Lichenoid Lesions: Clinical Profile and Malignant Transformation. J. Dent. (Shiraz, Iran) 2022, 23, 452–458. [Google Scholar] [CrossRef]
- Ögmundsdóttir, H.M.; Björnsson, J.; Holbrook, W.P. Role of TP53 in the progression of pre-malignant and malignant oral mucosal lesions. A follow-up study of 144 patients. J. Oral Pathol. Med. 2009, 38, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhang, W.; Chen, Y.; He, Z. Malignant transformation of oral lichen planus: A retrospective study of 23 cases. Quintessence Int. 2009, 40, 235–242. [Google Scholar]
- Carbone, M.; Arduino, P.G.; Carrozzo, M.; Gandolfo, S.; Argiolas, M.R.; Bertolusso, G.; Conrotto, D.; Pentenero, M.; Broccoletti, R. Course of oral lichen planus: A retrospective study of 808 northern Italian patients. Oral Dis. 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Kesić, L.; Obradović, R.; Mihailović, D.; Radičvić, G.; Stanković, S.; Todorović, K. Incidence and treatment outcome of oral lichen planus in southeast Serbia in a 10-year period (1997–2007). Vojnosanit. Pregl. 2009, 66, 435–439. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, E.H.; Mast, H.; van der Waal, I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective five-year follow-up study of 192 patients. Oral Oncol. 2007, 43, 742–748. [Google Scholar] [CrossRef]
- Hsue, S.S.; Wang, W.C.; Chen, C.H.; Lin, C.C.; Chen, Y.K.; Lin, L.M. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: A follow-up study based in a Taiwanese hospital. J. Oral Pathol. Med. 2007, 36, 25–29. [Google Scholar] [CrossRef]
- Ingafou, M.; Leao, J.C.; Porter, S.R.; Scully, C. Oral lichen planus: A retrospective study of 690 British patients. Oral Dis. 2006, 12, 463–468. [Google Scholar] [CrossRef]
- Roosaar, A.; Yin, L.; Sandborgh-Englund, G.; Nyrén, O.; Axéll, T. On the natural course of oral lichen lesions in a Swedish population-based sample. J. Oral Pathol. Med. 2006, 35, 257–261. [Google Scholar] [CrossRef]
- Bornstein, M.; Kalas, L.; Lemp, S.; Altermatt Jörg, H.; Rees, D.; Buser, D. Oral lichen planus and malignant transformation: A retrospective follow-up study of clinical and histopathologic data. Quintessence Int. 2006, 37, 261. [Google Scholar]
- Laeijendecker, R.; Van Joost, T.; Kuizinga, M.C.; Tank, B.; Neumann, H.A.M. Premalignant nature of oral lichen planus. Acta Derm. Venereol. 2005, 85, 516–520. [Google Scholar] [CrossRef]
- Utz, S.; Suter, V.G.A.; Cazzaniga, S.; Borradori, L.; Feldmeyer, L. Outcome and long-term treatment protocol for topical tacrolimus in oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2459–2465. [Google Scholar] [CrossRef]
- Xue, J.L.; Fan, M.W.; Wang, S.Z.; Chen, X.M.; Li, Y.; Wang, L. A clinical study of 674 patients with oral lichen planus in China. J. Oral Pathol. Med. 2005, 34, 467–472. [Google Scholar] [CrossRef]
- Rödström, P.O.; Jontell, M.; Mattsson, U.; Holmberg, E. Cancer and oral lichen planus in a Swedish population. Oral Oncol. 2004, 40, 131–138. [Google Scholar] [CrossRef]
- Mattila, R.; Alanen, K.; Syrjänen, S. DNA content as a prognostic marker of oral lichen planus with a risk of cancer development. Anal. Quant. Cytol. Histol. 2004, 26, 278–284. [Google Scholar]
- Lanfranch, H.E.; Aguas, S.C.; Sano, S.M. Malignant transformation of atypical oral lichen planus: A review of 32 cases. Med. Oral 2003, 8, 2–9. [Google Scholar]
- Machado, A.C.P.; Sugaya, N.N.; Migliari, D.A.; Matthews, R.W. Oral lichen planus: Clinical aspects and management in fifty-two Brazilian patients. West Indian Med. J. 2003, 52, 203–207. [Google Scholar] [PubMed]
- Bruno, E.; Alessandrini, M.; Russo, S.; D’Erme, G.; Nucci, R.; Calabretta, F. Malignant degeneration of oral lichen planus: Our clinical experience and review of the literature. An. Otorrinolaringol. Ibero. Am. 2002, 29, 349–357. [Google Scholar] [PubMed]
- Eisen D The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J. Am. Acad. Dermatol. 2002, 46, 207–214. [CrossRef] [PubMed]
- Bromwich, M. Retrospective study of the progression of oral premalignant lesions to squamous cell carcinoma: A South Wales experience. J. Otolaryngol. 2002, 31, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N.; Silverman, S.; Lozada-Nur, F.; Mayer, P.; Watson, J. Oral lichen planus: Patient profile, disease progression, and treatment responses. J. Am. Dent. Assoc. 2001, 132, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, M.D.; Lo Muzio, L.; Lo Russo, L.; Fedele, S.; Ruoppo, E.; Bucci, E. Clinical guidelines in early detection of oral squamous cell carcinoma arising in oral lichen planus: A 5-year experience. Oral Oncol. 2001, 37, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, J.; Zhang, H.; Li, T. Overestimated risk of transformation in oral lichen planus. Oral Oncol. 2022, 133, 106025. [Google Scholar] [CrossRef]
- Rode, M.; Kansky, A.A.; Kogoj-Rode, M. Reticular form of oral lichen planus. A 19-year observation period in 75 patients from Slovenia. Acta Dermatovenerol. Alp. Panon. Adriat. 2000, 9, 137–141. [Google Scholar]
- Rajentheran, R.; McLean, N.R.; Kelly, C.G.; Reed, M.F.; Nolan, A. Malignant transformation of oral lichen planus. Eur. J. Surg. Oncol. 1999, 25, 520–523. [Google Scholar] [CrossRef]
- Girod, S.C.; Pfeiffer, P.; Ries, J.; Pape, H.D. Proliferative activity and loss of function of tumour suppressor genes as “biomarkers” in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 1998, 36, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lo Muzio, L.; Mignogna, M.D.; Favia, G.; Procaccini, M.; Testa, N.F.; Bucci, E. The possible association between oral lichen planus and oral squamous cell carcinoma: A clinical evaluation on 14 cases and a review of the literature. Oral Oncol. 1998, 34, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, A.K.; Antoniades, D.; Papanayotou, P.; Trigonidis, G. Malignant potential of oral lichen planus: A follow-up study of 326 patients. Eur. J. Cancer Part B Oral Oncol. 1997, 33, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S., Jr.; Bahl, S. Oral lichen planus update: Clinical characteristics, treatment responses, and malignant transformation. Am. J. Dent. 1997, 10, 259–263. [Google Scholar] [PubMed]
- Gorsky, M.; Raviv, M.; Moskona, D.; Laufer, M.; Bodner, L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Duffey, D.C.; Eversole, L.R.; Abemayor, E. Oral lichen planus and its association with squamous cell carcinoma: An update on pathogenesis and treatmentimplications. Laryngoscope 1996, 106, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.K.; Freeman, S. Oral lichenoid lesions caused by allergy to mercury in amalgam fillings. Contact Dermat. 1995, 33, 423–427. [Google Scholar] [CrossRef]
- Moncarz, V.; Ulmansky, M.; Lustmann, J. Lichen planus: Exploring its malignant potential. J. Am. Dent. Assoc. 1993, 124, 102–108. [Google Scholar] [CrossRef]
- Alkan, U.; Bachar, G.; Nachalon, Y.; Zlotogorsky, A.; Levin, E.G.; Kaplan, I. Proliferative verrucous leukoplakia: A clinicopathological comparative study. Int. J. Oral Maxillofac. Surg. 2022, 51, 1027–1033. [Google Scholar] [CrossRef]
- Barnard, N.A.; Scully, C.; Eveson, J.W.; Cunningham, S.; Porter, S.R. Oral cancer development in patients with oral lichen planus. J. Oral Pathol. Med. 1993, 22, 421–424. [Google Scholar] [CrossRef]
- Brown, R.S.; Bottomley, W.K.; Puente, E.; Lavigne, G.J. A retrospective evaluation of 193 patients with oral lichen planus. J. Oral Pathol. Med. 1993, 22, 69–72. [Google Scholar] [CrossRef]
- Voûte, A.B.E.; de Jong, W.F.B.; Schulten, E.A.J.M.; Snow, G.B.; van der Waal, I. Possible premalignant character of oral lichen planus the Amsterdam experience. J. Oral Pathol. Med. 1992, 21, 326–329. [Google Scholar] [CrossRef]
- Sigurgeirsson, B.; Lindelöf, B. Lichen Planus and Malignancy: An Epidemiologic Study of 2071 Patients and a Review of the Literature. Arch. Dermatol. 1991, 127, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Gorsky, M.; Lozada-Nur, F.; Giannotti, K. A prospective study of findings and management in 214 patients with oral lichen planus. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 665–670. [Google Scholar] [CrossRef]
- Vincent, S.D.; Fotos, P.G.; Baker, K.A.; Williams, T.P. Oral lichen planus: The clinical, historical, and therapeutic features of 100 cases. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 165–171. [Google Scholar] [CrossRef]
- Salem, G. Oral lichen planus among 4277 patients from Gizan, Saudi Arabia. Community Dent. Oral Epidemiol. 1989, 17, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, P.; Thorn, J.J.; Rindum, J.; Pindborg, J.J. Malignant development of lichen planus-affected oral mucosa. J. Oral Pathol. Med. 1988, 17, 219–225. [Google Scholar] [CrossRef]
- Murti, P.R.; Daftary, D.K.; Bhonsle, R.B.; Gupta, P.C.; Mehta, F.S.; Pindborg, J.J. Malignant potential of oral lichen planus: Observations in 722 patients from India. J. Oral Pathol. Med. 1986, 15, 71–77. [Google Scholar] [CrossRef]
- Silverman, S.; Gorsky, M.; Lozada-Nur, F. A prospective follow-up study of 570 patients with oral lichen planus: Persistence, remission, and malignant association. Oral Surg. Oral Med. Oral Pathol. 1985, 60, 30–34. [Google Scholar] [CrossRef]
- Xie, F.; Gleue, C.A.; Deschaine, M.; Dasari, S.; Lau, J.S.; Sartori-Valinotti, J.C.; Meves, A.; Lehman, J.S. Whole-exome sequencing of transforming oral lichen planus reveals mutations in DNA damage repair and apoptosis pathway genes. J. Oral Pathol. Med. 2022, 51, 395–404. [Google Scholar] [CrossRef]
- Bánóczy, J.; Roed-Petersen, B.; Pindborg, J.J.; Inovay, J. Clinical and histologic studies on electrogalvanically induced oral white lesions. Oral Surg. Oral Med. Oral Pathol. 1979, 48, 319–323. [Google Scholar] [CrossRef]
- Silverman, S.; Griffith, M. Studies on oral lichen planus. II. Follow-up on 200 patients, clinical characteristics, and associated malignancy. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 705–710. [Google Scholar] [CrossRef]
- Tyldesley, W. Oral lichen planus. Br. J. Oral Surg. 1974, 11, 187–206. [Google Scholar] [CrossRef]
- Fulling, H.J. Cancer Development in Oral Lichen Planus: A Follow-Up Study of 327 Patients. Arch. Dermatol. 1973, 108, 667–669. [Google Scholar] [CrossRef]
- Kövesi, G.; Bánóczy, J. Follow-up studies in oral lichen planus. Int. J. Oral Surg. 1973, 2, 13–19. [Google Scholar] [CrossRef]
- Shklar, G. Lichen planus as an oral ulcerative disease. Oral Surg. Oral Med. Oral Pathol. 1972, 33, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Cawson, R.A. Treatment of Oral Lichen Planus with Betamethasone. Br. Med. J. 1968, 1, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Perry, H.O. The Variations and Course of Lichen Planus. Arch. Dermatol. 1961, 84, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Warin, R.P. Epithelioma Following Lichen Planus of the Mouth. Br. J. Dermatol. 1960, 72, 288–291. [Google Scholar] [CrossRef]
- Montgomery, D.; Culver, G. Lichen Planus of the Mouth Alone. Br. J. Dermatol. 1929, 41, 45–50. [Google Scholar] [CrossRef]
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2019, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2020, 50, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Lo Muzio, L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2018, 25, 693–709. [Google Scholar] [CrossRef]
- Cheng, Y.-S.L.; Gould, A.; Kurago, Z.; Fantasia, J.; Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 332–354. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.-Á.; Warnakulasuriya, S.; González-Ruiz, I.; Ayén, Á.; González-Ruiz, L.; Ruiz-Ávila, I.; Ramos-García, P. Dysplasia in oral lichen planus: Relevance, controversies and challenges. A position paper. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e541–e548. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Aguilar-Ruiz, M.; Ramos-García, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef]
- González-Ruiz, I.; Ramos-García, P.; Ruiz-Ávila, I.; González-Moles, M.Á. Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution. Cancers 2023, 15, 3270. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.; de Porras-Carrique, T.; Ramos-García, P. Association of oral lichen planus with hepatic disorders and hepatocellular carcinoma: Systematic review and meta-analysis. Med. Oral Patol. Oral y Cir. Bucal 2023, 28, e229–e237. [Google Scholar] [CrossRef] [PubMed]
- De Porras-Carrique, T.; Ramos-García, P.; Aguilar-Diosdado, M.; Warnakulasuriya, S.; González-Moles, M.Á. Autoimmune disorders in oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2023, 29, 1382–1394. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; López-Ansio, M.; Ramos-García, P. Hallmarks of Cancer Applied to Oral and Oropharyngeal Carcinogenesis: A Scoping Review of the Evidence Gaps Found in Published Systematic Reviews. Cancers 2022, 14, 3834. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Keim-Del Pino, C.; Ramos-García, P. Hallmarks of Cancer Expression in Oral Lichen Planus: A Scoping Review of Systematic Reviews and Meta-Analyses. Int. J. Mol. Sci. 2022, 23, 13099. [Google Scholar] [CrossRef]
- Clark, O.A.C.; Castro, A.A. Searching the Literatura Latino Americana e do Caribe em Ciências da Saúde (LILACS) database improves systematic reviews. Int. J. Epidemiol. 2002, 31, 112–114. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Wang, J.; Spijker, R.; Bossuyt, P.M. Should we search Chinese biomedical databases when performing systematic reviews? Syst. Rev. 2015, 4, 23. [Google Scholar] [CrossRef]
- Dickersin, K. The Existence of Publication Bias and Risk Factors for Its Occurrence. JAMA J. Am. Med. Assoc. 1990, 263, 1385–1389. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Moles, M.Á.; Ramos-García, P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 608. https://doi.org/10.3390/cancers16030608
González-Moles MÁ, Ramos-García P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(3):608. https://doi.org/10.3390/cancers16030608
Chicago/Turabian StyleGonzález-Moles, Miguel Ángel, and Pablo Ramos-García. 2024. "An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis" Cancers 16, no. 3: 608. https://doi.org/10.3390/cancers16030608
APA StyleGonzález-Moles, M. Á., & Ramos-García, P. (2024). An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers, 16(3), 608. https://doi.org/10.3390/cancers16030608







