Insights, Advantages, and Barriers of Teledermatology vs. Face-to-Face Dermatology for the Diagnosis and Follow-Up of Non-Melanoma Skin Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
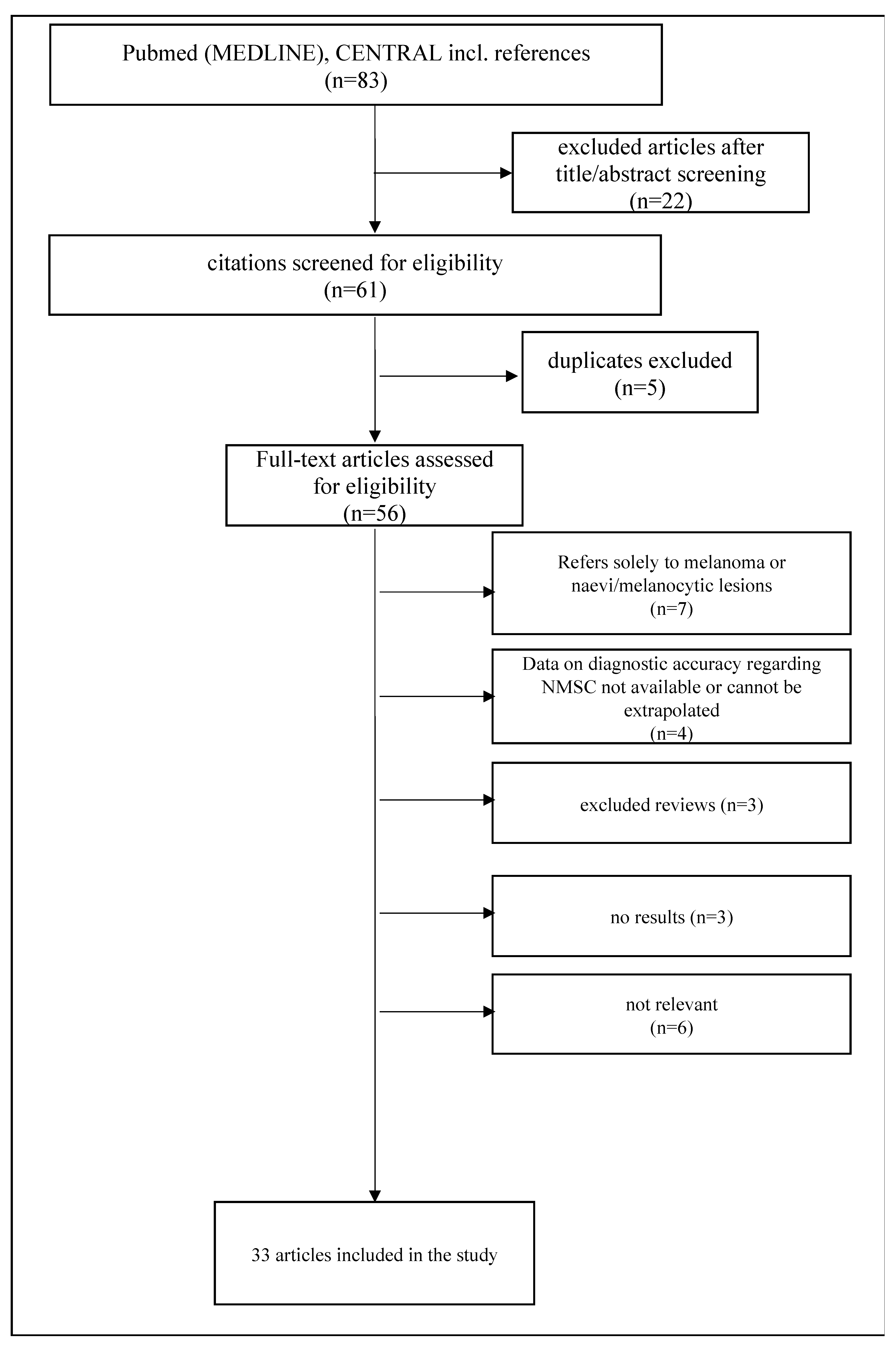
2. Materials and Methods
3. Results
3.1. Teledermatology for Non-Melanoma Skin Cancer: Triage in Primary Care
3.2. Diagnostic Concordance of TD vs. FTF: Not Such a Simple Matter
3.3. Some Help along the Way: Introducing Teledermoscopy (TDS) to Facilitate NMSC TD Diagnosis
3.4. Teledermatology and Occupational Dermatology: Screening and Follow-Up of High-Risk Populations
3.5. Image Quality and Digital Health Innovations as a Tool for TD Improvement for Diagnosis of NMSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elsner, P.; Bauer, A.; Diepgen, T.L.; Drexler, H.; Fartasch, M.; John, S.M.; Schliemann, S.; Wehrmann, W.; Tittelbach, J. Position Paper: Telemedicine in Occupational Dermatology–Current Status and Perspectives. JDDG J. Dtsch. Dermatol. Ges. 2018, 16, 969–974. [Google Scholar] [CrossRef]
- Elsner, P. Teledermatology in the Times of COVID-19–A Systematic Review. JDDG J. Dtsch. Dermatol. Ges. 2020, 18, 841–845. [Google Scholar] [CrossRef]
- Düker, I.; Elsner, P. Dermatology in telemedicine. Possibilities and limits. Hautarzt 2002, 53, 11–17. [Google Scholar] [CrossRef]
- Cumsky, H.J.L.; Maly, C.J.; Costello, C.M.; Buras, M.R.; Ranieri, L.A.M.; Grover, M.L.; Comfere, N.I.; Nelson, S.A.; Pittelkow, M.R.; Mangold, A.R. Impact of Standardized Templates and Skin Cancer Learning Modules for Teledermatology Consultations. Int. J. Dermatol. 2019, 58, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Finnane, A.; Dallest, K.; Janda, M.; Soyer, H.P. Teledermatology for the Diagnosis and Management of Skin Cancer: A Systematic Review. JAMA Dermatol. 2017, 153, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Jemec, G.B.E. Diagnosis of Nonmelanoma Skin Cancer/Keratinocyte Carcinoma: A Review of Diagnostic Accuracy of Nonmelanoma Skin Cancer Diagnostic Tests and Technologies. JDDG J. Dtsch. Dermatol. Ges. 2007, 33, 1158–1174. [Google Scholar] [CrossRef]
- Livingstone, J.; Solomon, J. An Assessment of the Cost-Effectiveness, Safety of Referral and Patient Satisfaction of a General Practice Teledermatology Service. Lond. J. Prim. Care 2015, 7, 31–35. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Santos, A.; Cordioli, E. Benefits of Teledermatology for Geriatric Patients: Population-Based Cross-Sectional Study. J. Med. Internet Res. 2020, 22, e16700. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Downie, F.; Auld, S.; Smith, B.; van der Pol, M.; Baughan, P.; Wells, J.; Wootton, R. Community Photo-Triage for Skin Cancer Referrals: An Aid to Service Delivery. Clin. Exp. Dermatol. 2011, 36, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.L.; Oh, D.H. The Impact of Store-and-Forward Teledermatology on Skin Cancer Diagnosis and Treatment. J. Am. Acad. Dermatol. 2008, 59, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Massone, C.; Maak, D.; Hofmann-Wellenhof, R.; Soyer, H.P.; Frühauf, J. Teledermatology for Skin Cancer Prevention: An Experience on 690 Austrian Patients. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Naka, F.; Lu, J.; Porto, A.; Villagra, J.; Wu, Z.H.; Anderson, D. Impact of Dermatology EConsults on Access to Care and Skin Cancer Screening in Underserved Populations: A Model for Teledermatology Services in Community Health Centers. J. Am. Acad. Dermatol. 2018, 78, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tandjung, R.; Badertscher, N.; Kleiner, N.; Wensing, M.; Rosemann, T.; Braun, R.P.; Senn, O. Feasibility and Diagnostic Accuracy of Teledermatology in Swiss Primary Care: Process Analysis of a Randomized Controlled Trial. J. Eval. Clin. Pract. 2015, 21, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, S.; Frühauf, J.; Campbell, T.M.; Massone, C.; Schwantzer, G.; Soyer, H.P.; Hofmann-Wellenhof, R. Mobile Teledermatology for Skin Tumour Screening: Diagnostic Accuracy of Clinical and Dermoscopic Image Tele-Evaluation Using Cellular Phones. Br. J. Dermatol. 2011, 164, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Börve, A.; Dahlén Gyllencreutz, J.; Terstappen, K.; Johansson Backman, E.; Alden-Bratt, A.; Danielsson, M.; Gillstedt, M.; Sandberg, C.; Paoli, J. Smartphone Teledermoscopy Referrals: A Novel Process for Improved Triage of Skin Cancer Patients. Acta Derm. Venereol. 2015, 95, 186–190. [Google Scholar] [CrossRef]
- Jobbágy, A.; Kiss, N.; Meznerics, F.A.; Farkas, K.; Plázár, D.; Bozsányi, S.; Fésűs, L.; Bartha, Á.; Szabó, E.; Lőrincz, K.; et al. Emergency Use and Efficacy of an Asynchronous Teledermatology System as a Novel Tool for Early Diagnosis of Skin Cancer during the First Wave of COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 2699. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Gravely, A.A.; Nelson, D.B. Reliability of Store and Forward Teledermatology for Skin Neoplasms. J. Am. Acad. Dermatol. 2015, 72, 426–435. [Google Scholar] [CrossRef]
- Lamel, S.A.; Haldeman, K.M.; Ely, H.; Kovarik, C.L.; Pak, H.; Armstrong, A.W. Application of Mobile Teledermatology for Skin Cancer Screening. J. Am. Acad. Dermatol. 2012, 67, 576–581. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; Azevedo, M.F.D.; Sousa, R.M.; Cordioli, E. Part II: Accuracy of Teledermatology in Skin Neoplasms. Front. Med. 2020, 7, 598903. [Google Scholar] [CrossRef]
- Zink, A.; Kolbinger, A.; Leibl, M.; Léon Suarez, I.; Gloning, J.; Merkel, C.; Winkler, J.; Biedermann, T.; Ring, J.; Eberlein, B. Teledermatoskopie Mittels Smartphone: Zuverlässige Hilfe Bei Der Diagnostik von Hautläsionen? Hautarzt 2017, 68, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.; Tarantino, V.; Zavattaro, E.; Biacchi, F.; Airoldi, C.; Salvi, M.; Seoni, S.; Branciforti, F.; Meiburger, K.M.; Savoia, P. Teledermoscopy in the Diagnosis of Melanocytic and Non-Melanocytic Skin Lesions: NurugoTM Derma Smartphone Microscope as a Possible New Tool in Daily Clinical Practice. Diagnostics 2022, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Paget, S.; Zaman, S.; Patel, N.P. Intradisciplinary Team Meeting for Teledermatology: An Aid to Improving Clinician Confidence. Clin. Exp. Dermatol. 2022, 47, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, S.S.; Fevrier, H.; Alexeeff, S.; Crowley, E.; Haiman, M.; Pham, N.; Tuerk, M.J.; Wukda, D.; Hartmann, M.; Herrinton, L.J. Comparative Effectiveness Study of Face-to-Face and Teledermatology Workflows for Diagnosing Skin Cancer. J. Am. Acad. Dermatol. 2019, 81, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- IBowns, I.; Collins, K.; Walters, S.J.; McDonagh, A.J. Telemedicine in Dermatology: A Randomised Controlled Trial. Health Technol. Assess. 2006, 10, 3–39. [Google Scholar]
- Ferrándiz, L.; Ojeda-Vila, T.; Corrales, A.; Martín-Gutiérrez, F.J.; Ruíz-de-Casas, A.; Galdeano, R.; Álvarez-Torralba, I.; Sánchez-Ibáñez, F.; Domínguez-Toro, J.M.; Encina, F.; et al. Internet-Based Skin Cancer Screening Using Clinical Images Alone or in Conjunction with Dermoscopic Images: A Randomized Teledermoscopy Trial. J. Am. Acad. Dermatol. 2017, 76, 676–682. [Google Scholar] [CrossRef]
- Senel, E.; Sabancılar, E.; Mansuroğlu, C.; Demir, E. A Preliminary Study of the Contribution of Telemicroscopy to the Diagnosis and Management of Skin Tumours in Teledermatology. J. Telemed. Telecare 2014, 20, 178–183. [Google Scholar] [CrossRef]
- Cheung, C.M.M.; Muttardi, K.; Chinthapalli, S.; Ismail, F. Pilot Teledermatology Service for Assessing Solitary Skin Lesions in a Tertiary London Dermatology Center. J. Healthc. Qual. 2019, 41, E1–E6. [Google Scholar] [CrossRef]
- Tan, E.; Yung, A.; Jameson, M.; Oakley, A.; Rademaker, M. Successful Triage of Patients Referred to a Skin Lesion Clinic Using Teledermoscopy (IMAGE IT Trial). Br. J. Dermatol. 2010, 162, 803–811. [Google Scholar] [CrossRef]
- Tan, E.; Oakley, A.; Soyer, H.P.; Haskett, M.; Marghoob, A.; Jameson, M.; Rademaker, M. Interobserver Variability of Teledermoscopy: An International Study. Br. J. Dermatol. 2010, 163, 1276–1281. [Google Scholar] [CrossRef]
- May, C.; Giles, L.; Gupta, G. Prospective Observational Comparative Study Assessing the Role of Store and Forward Teledermatology Triage in Skin Cancer. Clin. Exp. Dermatol. 2008, 33, 736–739. [Google Scholar] [CrossRef]
- Sola-Ortigosa, J.; Muñoz-Santos, C.; Masat-Ticó, T.; Isidro-Ortega, J.; Guilabert, A. The Role of Teledermatology and Teledermoscopy in the Diagnosis of Actinic Keratosis and Field Cancerization. J. Investig. Dermatol. 2020, 140, 1976–1984.e4. [Google Scholar] [CrossRef]
- Saranath, R.; Fernandez, B.; Gomez, J.; Miller, M.; Novack, D.; Parvathala, N.; Shah, E.; Wong-Michalak, S.; Rodman, J.; Fong, M.W.; et al. A Retrospective Analysis of Rates of Dermatology Follow-up and New Skin Cancer Diagnosis among Solid Organ Transplant Recipients during the COVID-19 Pandemic. JAAD Int. 2023, 10, 53–54. [Google Scholar] [CrossRef]
- van der Heijden, J.P.; Thijssing, L.; Witkamp, L.; Spuls, P.I.; de Keizer, N.F. Accuracy and Reliability of Teledermatoscopy with Images Taken by General Practitioners during Everyday Practice. J. Telemed. Telecare 2013, 19, 320–325. [Google Scholar] [CrossRef]
- Mahendran, R.; Goodfield, M.J.D.; Sheehan-Dare, R.A. An Evaluation of the Role of a Store-and-Forward Teledermatology System in Skin Cancer Diagnosis and Management. Clin. Exp. Dermatol. 2005, 30, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mehrtens, S.H.; Shall, L.; Halpern, S.M. A 14-Year Review of a UK Teledermatology Service: Experience of over 40 000 Teleconsultations. Clin. Exp. Dermatol. 2019, 44, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Hames, S.C.; Sinnya, S.; Tan, J.-M.; Morze, C.; Sahebian, A.; Soyer, H.P.; Prow, T.W. Automated Detection of Actinic Keratoses in Clinical Photographs. PLoS ONE 2015, 10, e0112447. [Google Scholar] [CrossRef]
- Silveira, C.E.G.; Silva, T.B.; Fregnani, J.H.G.T.; da Costa Vieira, R.A.; Haikel, R.L.J.; Syrjänen, K.; Carvalho, A.L.; Mauad, E.C. Digital Photography in Skin Cancer Screening by Mobile Units in Remote Areas of Brazil. BMC Dermatol. 2014, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Escalé-Besa, A.; Yélamos, O.; Vidal-Alaball, J.; Fuster-Casanovas, A.; Miró Catalina, Q.; Börve, A.; Ander-Egg Aguilar, R.; Fustà-Novell, X.; Cubiró, X.; Rafat, M.E.; et al. Exploring the Potential of Artificial Intelligence in Improving Skin Lesion Diagnosis in Primary Care. Sci. Rep. 2023, 13, 4293. [Google Scholar] [CrossRef]
- Chuchu, N.; Dinnes, J.; Takwoingi, Y.; Matin, R.N.; Bayliss, S.E.; Davenport, C.; Moreau, J.F.; Bassett, O.; Godfrey, K.; O’sullivan, C.; et al. Teledermatology for Diagnosing Skin Cancer in Adults. Cochrane Database Syst. Rev. 2018, 2018, 1–179. [Google Scholar] [CrossRef]
- Reinders, P.; Augustin, M.; Kirsten, N.; Fleyder, A.; Otten, M. Digital Health Interventions in Dermatology—Mapping Technology and Study Parameters of Systematically Identified Publications. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Oakley, A.M.M.; Rademaker, M. Better, Sooner, More Convenient: A Successful Teledermoscopy Service. Australas. J. Dermatol. 2012, 53, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Duniphin, D.D. Limited Access to Dermatology Specialty Care: Barriers and Teledermatology. Dermatol. Pract. Concept. 2023, 13, e2023031. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Country | Study Design | Study Population (n) | Intervention/Study Arms | Assessment TD Method | Reference Standard | Outcome | Potential Limitations/Bias |
|---|---|---|---|---|---|---|---|---|
| Livingstone et al. [8] | United Kingdom | retrospective monocentric comparative study | 248 patients referred after initial GP assessment | Skin cancer diagnosis including NMSC: 102 direct referrals/FTF vs. 146 via TD | SAF: clinical photos | unknown—diagnosis from secondary care provider | TD cost-effective, timely assessment, patient satisfaction | specificity—follow-up for “benign” cases was performed exclusively by GP |
| Bianchi et al. [9] | Brazil | retrospective multicenter cohort study | 12,770 lesions/6633 individuals | TD assessment and referral for biopsy OR FTF assessment OR return to GP for treatment | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | 2/3 of cases were returned to GP, AK between the most common diagnoses, comfortable for the elderly | specificity—follow-up for “benign” cases was performed exclusively by GP |
| Morton et al. [10] | United Kingdom | prospective monocentric observational study | 642 lesions suspicious for skin cancer | Conventional GP referrals vs. TD consultations prior to FTF assessment/treatment | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | TD use as triage tool, improved waiting times, reduction of the burden of FTF dermatology, photo-triage increased the sensitivity for NMSC | (-) |
| Hsiao et al. [11] | United States | retrospective monocentric chart review | 169 patients | patients treated for skin cancer after FTF or TD assessment | SAF: not specified | histopathology | diagnostic accuracy between FTF and TD was comparable for NMSC, wait time to skin cancer surgery for TD was shorter | very specific population characteristics might not mirror the general population |
| Massone et al. [12] | Austria | observational multicenter study | 955 lesions | TD evaluation of suspected skin cancer according to pre-trained GPs followed by referral for excision or FTF evaluation or follow-up | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | diagnostic accuracy was 94% and the sensitivity 100%, only 1% of the TD group were referred for an FTF evaluation | (-) |
| Naka et al. [13] | United States | descriptive retrospective cohort study | 2385 referrals | TD evaluation (44%) of suspected skin cancer from underserved US populations followed by referral for FTF evaluation or vs. direct FTF evaluation | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | TD reduced wait times, increased primary care satisfaction, no direct head-to head comparison of diagnostic accuracy | very specific population characteristics might not mirror the general population, no data on follow-up of “benign” lesions |
| Author(s) | Country | Study Design | Study Population (n) | Intervention/Study Arms | Assessment TD Method | Reference Standard | Outcome | Potential Limitations/Bias |
|---|---|---|---|---|---|---|---|---|
| Tandjung et al. [14] | Switzerland | randomized control trial | 979 skin lesions | TD evaluation of images made in primary care and categorization into “no further investigation”, “clinical observation”, “biopsy” and “other”. | SAF: clinical photos | FTF diagnosis or histopathology if performed | small number of avoided visits through TD, safety concerns concerning specificity of TD diagnosis (2 NMSC cases missed) | long-term data missing |
| Kroemer et al. [15] | Switzerland | comparative prospective study | 113 skin tumors from 88 patients | clinical TD evaluation vs. dermoscopic TD evaluation of GP- and self-referrals for skin tumors and subsequent categorization to benign melanocytic, benign nonmelanocytic, malignant melanocytic and malignant nonmelanocytic lesions | SAF: clinical photos and dermoscopy images with a mobile phone camera | histopathology (malignant tumors) and FTF diagnosis (non-malignant tumors) | high concordance in differentiating benign from malignant (90%), and similar specificity of FTF in comparison to TD for NMSC, no advantages of teledermoscopy over macroscopic TD evaluation | (-) |
| Börve et al. [16] | Sweden | open, controlled, multicenter, prospective observational study | 1562 patients | TDS evaluation via smartphone app and compatible digital microscope vs. FTF diagnosis | SAF: clinical photos and dermoscopy images | inter-rater agreement of dermatologists after FTF evaluation or histopathology | reduced waiting time from NMSC requiring surgery, increased reliability for triage through TDS, 40% of the patients could have avoided FTF | only 62% of the deemed malignant cases had a histopathologic evaluation |
| Jobbàgy et al. [17] | Hungary | retrospective monocentric study | 749 patients with 779 lesions | TD evaluation of skin cancer lesions during the COVID-19 pandemic and categorization in 11 diagnostic groups (among them scc, BCC, AK) and triage groups followed by FTF | SAF: clinical photos | histopathology (malignant lesions) and FTF diagnosis (non-malignant lesions) | diagnostic concordance was substantial for primary (85:3%) and aggregated diagnoses (87:9%), kappa coefficient was moderate for SCC and higher for BCC | precancerous lesions (AK) were included in malignant lesions |
| Warshaw et al. [18] | United States | cross-sectional repeated measures equivalence study | 2152 patients with 3021 lesions | TD evaluation of suspected skin cancer referrals and categorization to 1 of 17 diagnoses with up to 2 differential diagnoses, choice between 4 management plans and level of diagnostic confidence followed by FTF | SAF: clinical photos | FTF diagnosis or histopathology if performed | Diagnostic agreement had a moderate/substantial kappa of 0:32–0:86 for non-pigmented lesions incl. NMSC | male Caucasian population, teledermatologists were aware of the study, no evaluation of intra-rater reliability |
| Lamel et al. [19] | United States | prospective monocentric single-blind observational study | 86 patients with 137 lesions | TD evaluation of lesions during a scan screening event and FTF diagnosis from another dermatologist, blinded to the TD evaluation | instant evaluation of clinical images | FTF diagnosis or histopathology if performed | substantial diagnostic agreement on primary diagnosis and management with with AK and BCC being the third and fourth most common diagnoses. | technical difficulties, no TDS |
| Giavina-Bianchi et al. [20] | Brazil | retrospective cohort study | 30,976 patients with 55,012 lesions | TD evaluation on skin cancer (10 most frequent skin neoplasms) vs. FTF evaluation or histopathology reports with focus on diagnostic accuracy | SAF: clinical photos | FTF diagnosis or histopathology if performed | low to moderate diagnostic concordance (kappa −0:146 to 0:326) for NMSC and AK | no assessment of false negative cases |
| Author(s) | Country | Study Design | Study Population (n) | Intervention/Study Arms | Assessment TD Method | Reference Standard | Outcome | Potential Limitations/Bias |
|---|---|---|---|---|---|---|---|---|
| Zink et al. [21] | Germany | prospective pilot study | 26 patients | TD/TDS evaluation using a mobile phone camera and a vs. FTF diagnosis with dermoscopy | SAF: clinical photos | FTF diagnosis or histopathology if performed | diagnostic concordance in 92:3% of the cases | histology available only in 23% of the cases, FTF was performed by residents, while TD evaluation by a senior consultant |
| Veronese et al. [22] | Italy | retrospective observational study | 144 images of suspected skin cancer lesions | FTF diagnosis using a dermatoscope vs. TDS using a dermatoscope vs. TDS using a novel smart-phone image capture device vs. TDS using the former with the interposition of a slide | SAF: clinical photos and dermoscopy images | histopathology (malignant lesions) and FTF diagnosis incl. Conventional dermoscopy with follow-up (non-malignant lesions) | TDS using conventional dermatoscopy had substantial diagnostic concordance, higher than the other two methods | retrospective character of the study |
| Paget et al. [23] | United Kingdom | retrospective cohort study | 400 cases | TD/TDS evaluation before and aftera weekly teledermatology intradisciplinary team meeting | SAF: clinical photos and dermoscopy images | not mentioned | increase of direct discharge rate and decrease of biopsy rate after implementation of the meeting, no change in requested FTF rate | retrospective character of the study |
| Marwaha et al. [24] | United States | retrospective cohort study | 59,729 patients | several workflows of TD/TDS evaluation of skin cancer lesions vs. direct FTF referral | SAF: clinical photos and dermoscopy images | histopathology (malignant lesions) and FTF diagnosis (non-malignant lesions) | workflow of high-resolution images with TDS had 9% higher probability of cancer detection in comparison to FTF, reduction of FTF rate by 40%, reduced wait times | potential selections bias |
| Bowns et al. [25] | United Kingdom | multicenter randomized control trial | 208 patients | TD/TDS evaluation of GP referrals vs. FTF diagnosis | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | modest diagnostic concordance (68%) between the two arms, sensitive but not specific | higher loss of control cases in comparison to intervention cases |
| Ferrándiz et al. [26] | Spain | single-center double-blind randomized control trial | 454 patients | TD evaluation alone vs. TD plus TDS evaluation for suspected skin cancer, using images captured with a professional dermatoscope | SAF: clinical photos and dermoscopy images | FTF diagnosis after consulation | Diagnostic concordance with FTF increased by using TDS, increased confidence level to avoid FTF in benign lesions and was cost-effective | no data on histopathology of the lesions, regarding the reference standard |
| Senel et al. [27] | Turkey | retrospective monocentric cohort study | 120 skin tumor lesions | TD evaluation of random benign and malignant tumors vs. TS/TDS evaluation 2 months later, conducted from the same two dermatologists vs. histopathology | SAF: clinical photos and dermoscopy images | histopathology | reliability was substantial with TD and almost perfect after TD/TDS, TDS increased diagnostic accuracy especially for SCC, BCC, actinic keratosis | TDS only with 30-fold magnification |
| Cheung et al. [28] | Unitred Kingdom | pilot monocentric study | 76 primary care referrals of suspected skin cancer | TD evaluation vs. FTF diagnosis for single lesions suspected for skin cancer in non-hair bearing or genital sites | SAF: clinical photos | FTF diagnosis or histopathology if performed | 68% of TD evaluation confident benign diagnoses were made, no FTF-assessment needed | limited size, high-rate of non-attendance for FTF diagnosis (unavailable follow-up data) |
| Tan et al. [29] | New Zealand | retrospective monocentric cohort study | 200 patients with 491 lesions | TD/TDS evaluation of referrals in a dermatology clinic vs. FTF diagnosis | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | TD/TDS showed approximate 100% sensitivity and 90% specificity for NMSC, 74% of TD/TDS evaluations did not need FTF evaluation | recall bias due to using the same dermatologist for TDS and FTF evaluation |
| Tan et al. [30] | New Zealand | retrospective study | 206 patients with 979 lesions | TD/TDS evaluation for different cancerous and precancerous lesions from 5 experienced dermatologists | SAF: clinical photos and dermoscopy images | agreed TD diagnosis by all dermatologists | interrater agreement for AK/SCC in situ was moderate, moderate to very good for BCC and poor for SCC | no comparison with histopathology, selected population of elderly Fitzpatrick II patients |
| May et al. [31] | United Kingdom | prospective monocentric observational comparative study | 43 patients with SCC out of 451 new patients | TD evaluation of melanoma and SCC vs. conventional FTF diagnosis after referral via post/fax | SAF: clinical photos | (-) | 10-day decrease of waiting waiting time for SCC | sample size |
| Sola Ortigosa et al. [32] | Spain | prospective single-center comparative study | 636 patients with 1000 keratotic skin lesions | TD±TDS evaluation vs. FTF evaluation of keratotic lesions after initial primary care assessment | SAF: clinical photos and dermoscopy images | Consensus of FTF diagnosis or histopathology (in case of disagreement) | TD: High diagnostic concordance for AK and field cancerization, further increased by TDS incl. diagnotic concordance on AK subtypes | Biopsies only for 22:5% of cases |
| Saranath et al. [33] | United States | retrospective medical chart review | 1569 solid organ transplant recipients | TD evaluation vs. FTF evaluation of NMSC for this population during the pandemic | SAF: clinical photos | not mentioned | superior diagnostic accuracy of FTF approach than TD | gold standard not mentioned, results refer to a special population |
| Van der Heijden [34] | The Netherlands | prospective comparative study | 76 patients | TD/TDS evaluation of lesions using images taken from GPs vs. FTF diagnosis | SAF: clinical photos and dermoscopy images | FTF diagnosis or histopathology if performed | The inter-observer reliability on diagnosis was 0:65 (substantial), the diagnostic concordance of TD/TDS with histopathology was 0:41–0:63 (moderate) and 0:90 for FTF-diagnosis | Over 1/3 of the images were reported to have bad quality |
| Mahendran et al. [35] | United Kingdom | prospective monocentric cohort study | 163 patients | TD evaluation of suspected skin cancer GP referrals from a consultant or experienced resident vs. FTF-diagnosis from a consultant | SAF: clinical photos | FTF diagnosis or histopathology if performed | 48% of the consultant’s diagnoses were identical with FTF diagnosis, less for the trainee | no statistical analysis of diagnostic agreement, recall bias possible |
| Mehrtens et al. [36] | United Kingdom | retrospective chart review | 40,201 teleconsultations | TD evaluation of skin lesions vs. diagnosis as obtained from patient notes and histology records | SAF: clinical images and option for dermoscopic images | FTF diagnosis or histopathology if performed | 10% of TD did not provide any diagnosis, diagnostic concordance with biopsied samples was 68%, BCC, AK and SCC were third to fifth most common diagnosis | retrospective character of the study |
| Hames et al. [37] | Australia | retrospective chart review | 20 volunteers | Automatic analysis of pictures frompatients with and without actinic keratosis based on color based transforms and erythema vs. FTF approach | analysis of clinical images | TD evaluation | Correlation between of automated analysis and TD evaluation was moderate | (-) |
| Silveira et al. [38] | Brazil | monocentric retrospective study | 416 lesions | TD evaluation of suspected skin cancer lesions by two oncologistsand classification as malignant, benign, unknown and low quality image vs. FTF approach | SAF: clinical photos | histopathology | High diagnostic accuracy (>85%) in comparison to FTF, BCC and SCC were the most common tumors | no dermnoscopic images, medical history to accompany TD missing |
| Escalé-Besa et al. [39] | Spain | prospective multicenter observational feasiblity study | 100 patients/44 patients with a skin disease | GP evaluation of skin lesions vs. TD evaluation of GP-acquired images via smartphone camera vs. evaluation through a machine learning model | SAF: clinical photos | histopathology or concentual FTF diagnosis | diagnostic accuracy was lower for the ML model concerning the primary diagnosis and higher for the TD evl | AK was considered a benign tumor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolakis, G.; Vaiopoulos, A.G.; Georgopoulos, I.; Papakonstantinou, E.; Gaitanis, G.; Zouboulis, C.C. Insights, Advantages, and Barriers of Teledermatology vs. Face-to-Face Dermatology for the Diagnosis and Follow-Up of Non-Melanoma Skin Cancer: A Systematic Review. Cancers 2024, 16, 578. https://doi.org/10.3390/cancers16030578
Nikolakis G, Vaiopoulos AG, Georgopoulos I, Papakonstantinou E, Gaitanis G, Zouboulis CC. Insights, Advantages, and Barriers of Teledermatology vs. Face-to-Face Dermatology for the Diagnosis and Follow-Up of Non-Melanoma Skin Cancer: A Systematic Review. Cancers. 2024; 16(3):578. https://doi.org/10.3390/cancers16030578
Chicago/Turabian StyleNikolakis, Georgios, Aristeidis G. Vaiopoulos, Ioannis Georgopoulos, Eleni Papakonstantinou, George Gaitanis, and Christos C. Zouboulis. 2024. "Insights, Advantages, and Barriers of Teledermatology vs. Face-to-Face Dermatology for the Diagnosis and Follow-Up of Non-Melanoma Skin Cancer: A Systematic Review" Cancers 16, no. 3: 578. https://doi.org/10.3390/cancers16030578
APA StyleNikolakis, G., Vaiopoulos, A. G., Georgopoulos, I., Papakonstantinou, E., Gaitanis, G., & Zouboulis, C. C. (2024). Insights, Advantages, and Barriers of Teledermatology vs. Face-to-Face Dermatology for the Diagnosis and Follow-Up of Non-Melanoma Skin Cancer: A Systematic Review. Cancers, 16(3), 578. https://doi.org/10.3390/cancers16030578







