Severe Acute Kidney Injury in Hospitalized Cancer Patients: Epidemiology and Predictive Model of Renal Replacement Therapy and In-Hospital Mortality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Identification and Classification of Severe AKI
2.3. AKI Etiology
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Incidence and Characterization of Severe AKI
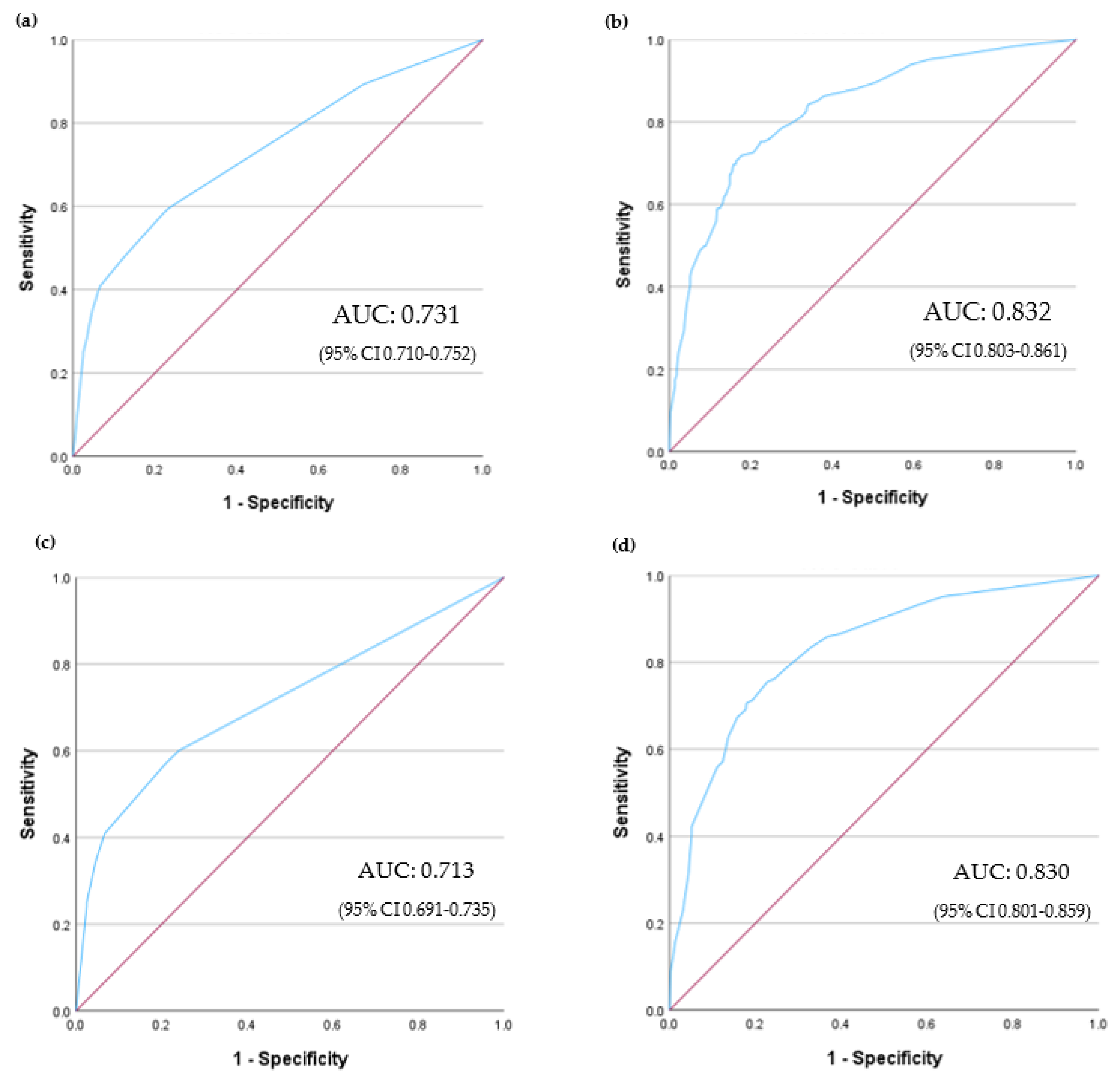
3.3. Need for RRT
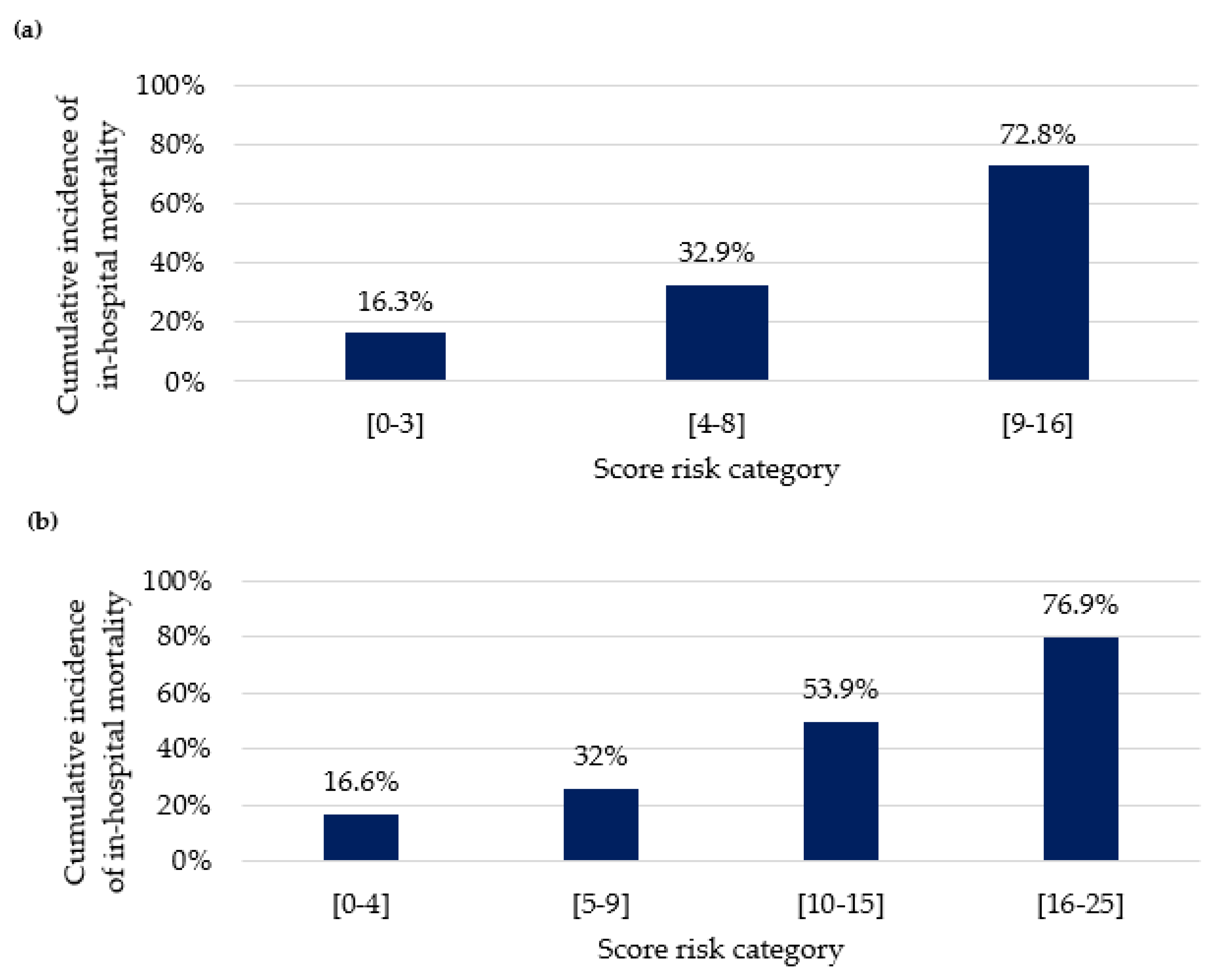
3.4. In-Hospital Mortality and Risk Score Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meraz-Munoz, A.; Langote, A.; Jhaveri, K.D.; Izzedine, H.; Gudsoorkar, P. Acute Kidney Injury in the Patient with Cancer. Diagnostics 2021, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112, S92–S107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Nie, S.; Li, L.; Li, Y.; Liu, D.; Xiong, M.; Wang, L.; Ge, S.; Xu, G.; on behalf of the EACH Study Investigators. Epidemiology and outcomes of acute kidney injury in hospitalized cancer patients in China. Int. J. Cancer 2019, 144, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; McArthur, E.; Amir, E.; Booth, C.M.; Sutradhar, R.; Majeed, H.; Nash, D.M.; A Silver, S.; Garg, A.X.; Chan, C.T.; et al. Acute Kidney Injury in Patients Receiving Systemic Treatment for Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2019, 111, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.F.; Johansen, M.B.; Langeberg, W.J.; Fryzek, J.P.; Sørensen, H.T. Incidence of acute kidney injury in cancer patients: A Danish population-based cohort study. Eur. J. Intern. Med. 2011, 22, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Darmon, M.; Ostermann, M. Onco-nephrology: What the intensivist needs to know. Intensive Care Med. 2022, 48, 1234–1236. [Google Scholar] [CrossRef]
- Lam, A.Q.; Humphreys, B.D. Onco-nephrology: AKI in the cancer patient. Clin. J. Am. Soc. Nephrol. 2012, 7, 1692–1700. [Google Scholar] [CrossRef]
- Perazella, M.A.; Moeckel, G.W. Nephrotoxicity from Chemotherapeutic Agents: Clinical Manifestations, Pathobiology, and Prevention/Therapy. Semin. Nephrol. 2010, 30, 570–581. [Google Scholar] [CrossRef]
- Sprangers, B.; Sandhu, G.; Rosner, M.H.; Tesarova, P.; Stadler, W.M.; Malyszko, J. Drug dosing in cancer patients with decreased kidney function: A practical approach. Cancer Treat. Rev. 2021, 93, 102139. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Wang, J.-N.; Diao, Z.-L.; Guan, Y.-M.; Liu, W.-H. Acute Kidney Injury in Oncology Patients. J. Cancer 2020, 11, 4700–4708. [Google Scholar] [CrossRef]
- Givens, M.L.; Wethern, J. Renal complications in oncologic patients. Emerg. Med. Clin. N. Am. 2009, 27, 283–291. [Google Scholar] [CrossRef]
- van der Veen, A.; De Vusser, K.; De Moor, B.; Wildiers, H.; Cosmai, L.; Sprangers, B. How to use dialysis wisely in cancer patients? J. Onco-Nephrol. 2021, 5, 79–86. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Uchino, S.; Takinami, M.; Bellomo, R. Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.K.; Doshi, S.M.; Pawar, T.; Nowshad, G.; Lahoti, A.; Shah, P. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin. J. Am. Soc. Nephrol. 2013, 8, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-M.; Cleeve, L.K.; Milner, A.D.; Pitman, A.G. Malignant Ureteral Obstruction: Outcomes After Intervention. Have Things Changed? J. Urol. 2007, 178, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Reis, M.; Ferreira, H.; Coelho, I.; Chuva, T.; Paiva, A.; Costa, J.M. Post-renal acute kidney injury in patients with cancer: Clinical presentation and kidney and patient outcomes. J. Onco-Nephrol. 2023, 7, 120–129. [Google Scholar] [CrossRef]
- O’reilly, M.; Mellotte, G.; Ryan, B.; O’connor, A. Gastrointestinal side effects of cancer treatments. Ther. Adv. Chronic Dis. 2020, 11, 2040622320970354. [Google Scholar] [CrossRef]
- Gupta, S.; Gudsoorkar, P.; Jhaveri, K.D. Acute Kidney Injury in Critically Ill Patients with Cancer. Clin. J. Am. Soc. Nephrol. 2022, 17, 1385–1398. [Google Scholar] [CrossRef]
- Chen, C.; Xie, D.; Gewirtz, D.A.; Li, N. Nephrotoxicity in cancer treatment: An update. Adv. Cancer Res. 2022, 155, 77–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, W.; Yang, L.; Li, J.; Yi, C.; Pu, Y.; Yin, T.; Na, F.; Zhang, L.; Fu, P.; et al. Incidence and risk factors of acute kidney injury in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1173952. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Muñoz, A.; Amir, E.; Ng, P.; Avila-Casado, C.; Ragobar, C.; Chan, C.; Kim, J.; Wald, R.; Kitchlu, A. Acute kidney injury associated with immune checkpoint inhibitor therapy: Incidence, risk factors and outcomes. J. Immunother. Cancer 2020, 8, e000467. [Google Scholar] [CrossRef] [PubMed]
- Pierson-Marchandise, M.; Gras, V.; Moragny, J.; Micallef, J.; Gaboriau, L.; Picard, S.; Choukroun, G.; Masmoudi, K.; Liabeuf, S. The drugs that mostly frequently induce acute kidney injury: A case-noncase study of a pharmacovigilance database. Br. J. Clin. Pharmacol. 2017, 83, 1341–1349. [Google Scholar] [CrossRef]
- Heeg, M.; Mertens, A.; Ellenberger, D.; A Müller, G.; Patschan, D. Prognosis of AKI in malignant diseases with and without sepsis. BMC Anesthesiol. 2013, 13, 36. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.J.; Kim, W.Y. Non-recovery of renal function was correlated with increased mortality in the cancer cohort with septic shock. Cancer Commun. (Lond.) 2021, 41, 1420–1422. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, J.; Chen, X.; Chen, R.; Wang, H. Incidence, risk factors and clinical outcomes of septic acute renal injury in cancer patients with sepsis admitted to the ICU: A retrospective study. Front. Med. (Lausanne) 2022, 9, 1015735. [Google Scholar] [CrossRef]
- Benoit, D.D.; Hoste, E.A. Acute kidney injury in critically ill patients with cancer. Crit. Care Clin. 2010, 26, 151–179. [Google Scholar] [CrossRef]
- Parikh, C.R.; Yarlagadda, S.G.; Storer, B.; Sorror, M.; Storb, R.; Sandmaier, B. Impact of acute kidney injury on long-term mortality after nonmyeloablative hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2008, 14, 309–315. [Google Scholar] [CrossRef]
- Hsu, C.; Ordoñez, J.; Chertow, G.; Fan, D.; McCulloch, C.; Go, A. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | Period A (1995–2010) | Period B (2011–2023) | p-Value |
|---|---|---|---|---|
| Patients | 3201 | 1588 | 1613 | |
| Male | 1836 (57.4) | 852 (53.7) | 984 (61.0) | <0.001 |
| Age, mean ± SD | 62.5 ± 17.2 | 59.2 ± 19.2 | 65.7 ± 14.6 | <0.001 |
| ≥40 years | 2880 (90.0) | 1360 (85.5) | 1523 (94.2) | <0.001 |
| <40 years | 321 (10.0) | 228 (14.4) | 93 (5.8) | |
| Multiple primary tumors | 96 (3.0) | 32 (2.0) | 64 (4.0) | 0.001 |
| Cancer | ||||
| Hematological | 779 (24.3) | 466 (29.3) | 313 (19.4) | <0.001 |
| HSCT | 284 (36.4) | 172 (36.9) | 112 (35.8) | <0.001 |
| Allo | 133 (17.1) | 77 (4.8) | 54 (3.3) | |
| Auto | 148 (19.0) | 95 (6.0) | 58 (3.6) | |
| Solid | 2422 (75.7) | 1122 (70.7) | 1300 (80.6) | <0.001 |
| Gastrointestinal | 749 (30.9) | 319 (28.4) | 430 (33.1) | |
| Gynecological | 571 (23.5) | 224 (20.0) | 347 (26.7) | |
| Urological | 438 (18.1) | 287 (25.6) | 151 (11.6) | |
| Head and neck | 172 (7.1) | 87 (7.8) | 85 (6.5) | |
| Breast | 128 (5.3) | 79 (7.0) | 49 (3.8) | |
| Lung | 123 (5.1) | 31 (2.8) | 92 (7.1) | |
| Sarcoma | 115 (4.7) | 49 (4.4) | 66 (5.1) | |
| AKI etiology | ||||
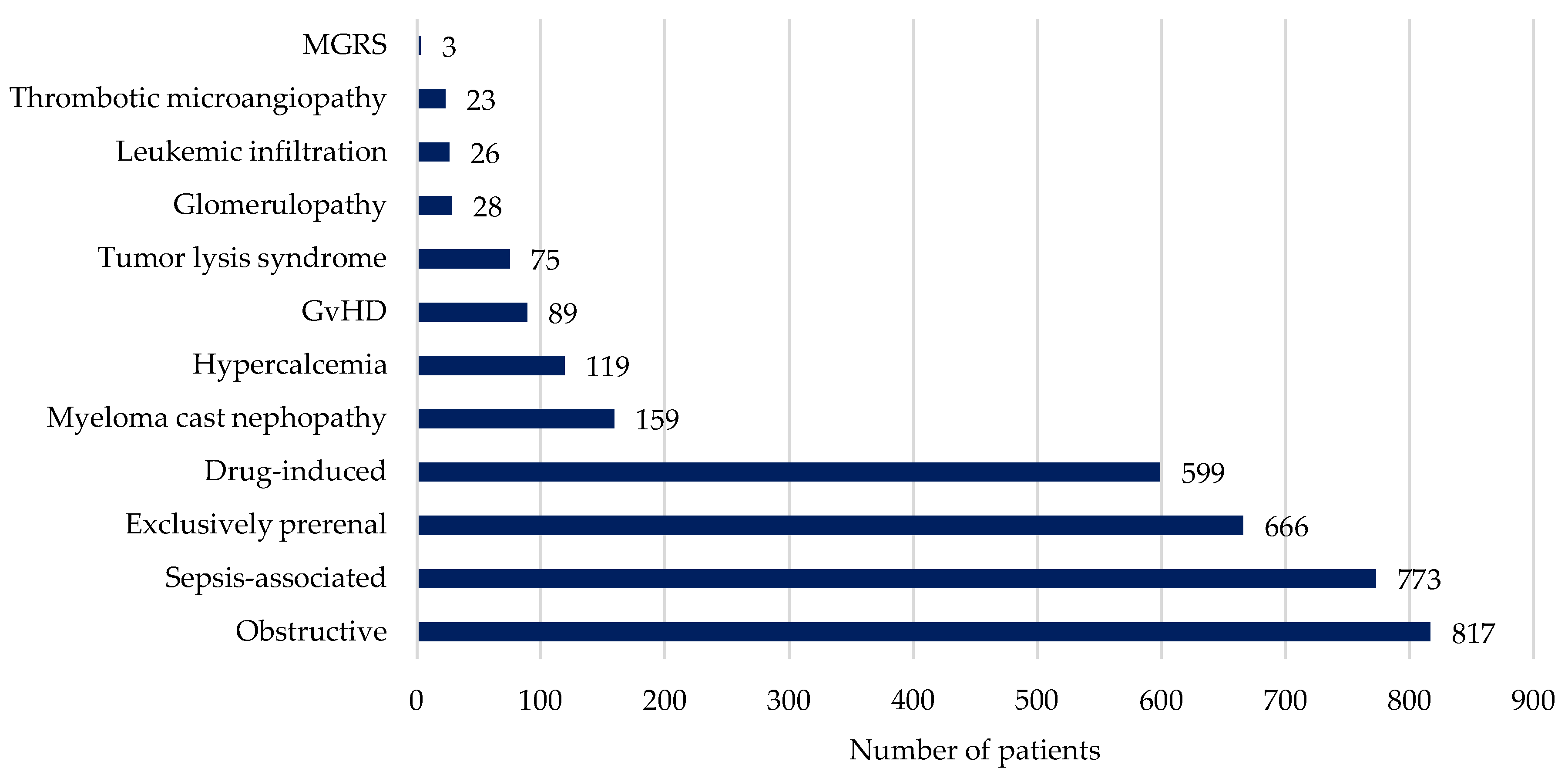
| Exclusively prerenal | 666 (20.8) | 277 (17.6) | 389 (24.1) | <0.001 |
| Sepsis-associated | 773 (24.1) | 308 (19.4) | 465 (28.8) | <0.001 |
| Drug-induced | 599 (18.7) | 265 (16.8) | 334 (20.7) | 0.006 |
| Immunotherapy | 52 (1.6) | 0 | 52 (3.2) | <0.001 |
| Obstructive | 889 (27.8) | 503 (32.0) | 314 (19.5) | <0.001 |
| Pos-operative AKI | 321 (10) | 187 (11.8) | 134 (8.3) | 0.001 |
| Outcomes | ||||
| ICU admission | 637 (19.9) | 326 (20.5) | 311 (19.3) | 0.377 |
| IMV | 423 (13.2) | 233 (14.7) | 190 (11.8) | 0.016 |
| RRT | 539 (16.8) | 279 (17.6) | 260 (22.3) | 0.002 |
| CRRT | 364 (11.4) | 165 (10.4) | 199 (12.3) | |
| IHD | 310 (9.7) | 131 (8.2) | 179 (11.1) | |
| Mortality | 846 (26.4) | 464 (29.2) | 382 (23.7) | <0.001 |
| Characteristics | Obstructive n = 817 (24.2%) | Sepsis-Associated n = 773 (22.9%) | Prerenal n = 666 (19.7%) | Drug-Induced n = 599 (17.7%) | |
|---|---|---|---|---|---|
| Male sex | 403 (49.3) | 475 (61.4) | 385 (57.8) | 344, (57.4) | |
| Age, mean ± SD | 65.4 ± 14.6 | 59.5 ± 18.1 | 68.0 ± 13.2 | 59.1 ± 19.4 | |
| Most common neoplasms | Urological 269 (32.9) | Hematological 336 (43.5) | Gastrointestinal 235 (35.3) | Hematological 242 (40.4) | |
| Gynecological 260 (31.8) | Gastrointestinal 152 (19.7) | Urological 121 (18.2) | Gastrointestinal 99 (16.5) | ||
| Gastrointestinal 202 (24.7) | Urological 109 (14.1) | Gynecological 72 (10.8) | Head and neck 60 (10.0) | ||
| ICU admission | 18 (2.2) | 454 (58.7) | 54 (8.1) | 164 (27.4) | |
| Outcomes | IMV | 6 (0.7) | 365 (47.2) | 45 (6.8) | 129 (21.5) |
| RRT | 49 (6.0) | 355 (45.9) | 69 (10.4) | 170 (28.4) | |
| Mortality | 110 (13.5) | 406 (52.5) | 131 (19.7) | 329 (32.9) | |
| Factors | Multivariate Analysis | ||
|---|---|---|---|
| B Coefficient | OR (95% CI) | p-Value | |
| IMV | |||
| No | - | Ref. | |
| Yes | 2.008 | 7.450 (5.455–10.174) | <0.001 |
| Type of cancer | |||
| Solid | - | Ref. | |
| Hematological | 0.844 | 2.325 (1.849–2.925) | <0.001 |
| Sepsis-associated AKI | |||
| No | - | Ref. | |
| Yes | 0.246 | 1.279 (0.980–1.670) | 0.071 |
| Prerenal AKI | |||
| No | - | Ref. | |
| Yes | −3.968 | 0.019 (0.006–0.060) | <0.001 |
| Obstructive AKI | |||
| No | - | Ref. | |
| Yes | −1.083 | 0.339 (0.251–0.457) | <0.001 |
| Factors | Multivariate Analysis | ||
|---|---|---|---|
| B Coefficient | OR (95% CI) | p-Value | |
| Type of cancer | |||
| Solid | - | Ref. | |
| Hematological | 1.267 | 3.552 (2.886–4.372) | <0.001 |
| Sepsis-associated AKI | |||
| No | - | Ref. | |
| Yes | 1.111 | 3.037 (2.462–3.746) | <0.001 |
| Prerenal AKI | |||
| No | - | Ref. | |
| Yes | −3.939 | 0.019 (0.006–0.061) | <0.001 |
| Obstructive AKI | |||
| No | - | Ref. | |
| Yes | −1.110 | 0.330 (0.246–0.442) | <0.001 |
| Factors | Multivariate Analysis | ||
|---|---|---|---|
| B Coefficient | OR (95% CI) | p-Value | |
| IMV | |||
| No | - | Ref. | |
| Yes | 2.445 | 11.533 (7.954–16.723) | <0.001 |
| Age | |||
| ≥40 years | - | Ref. | |
| <40 years | 0.406 | 1.501 (1.001–2.250) | 0.049 |
| Prerenal AKI | |||
| No | - | Ref. | |
| Yes | −2.886 | 0.056 (0.013–0.247) | <0.001 |
| Obstructive AKI | |||
| No | - | Ref. | |
| Yes | −0.782 | 0.457 (0.245–0.854) | 0.014 |
| Characteristics | Univariate Analysis | ||||
|---|---|---|---|---|---|
| Overall n = 3201 | Survivors n = 2355 | Non-Survivors n = 846 | OR (95% CI) | p-Value | |
| Sex | |||||
| Female | 1365 (42.6) | 1030 (43.7) | 335 (39.6) | Ref. | |
| Male | 1836 (57.4) | 1325 (56.3) | 511 (60.4) | 1.186 (1.010–1.392) | 0.037 |
| Age | |||||
| ≥40 years | 2880 (90.0) | 2150 (91.3) | 730 (86.3) | Ref. | |
| <40 years | 321 (10.0) | 205 (8.7) | 116 (13.7) | 1.667 (1.308–2.124) | <0.001 |
| Multiple primary tumors | |||||
| No | 3105 (97.0) | 2285 (97.0) | 820 (96.9) | Ref. | |
| Yes | 96 (3.0) | 70 (3.0) | 26 (3.1) | 1.035 (0.655–1.635) | 0.883 |
| Type of cancer | |||||
| Solid | 2422 (75.7) | 1903 (80.8) | 519 (61.3) | Ref. | |
| Hematological | 779 (24.3) | 452 (19.2) | 327 (38.7) | 2.653 (2.233–3.151) | <0.001 |
| ICU admission | |||||
| No | 2564 (80.1) | 2113 (89.7) | 451 (53.3) | Ref. | |
| Yes | 637 (19.9) | 242 (10.3) | 395 (46.7) | 7.647 (6.327–9.243) | <0.001 |
| IMV | |||||
| No | 2778 (86.8) | 2235 (94.9) | 543 (64.2) | Ref. | |
| Yes | 423 (13.2) | 120 (5.1) | 303 (35.8) | 10.393 (8.247–13.097) | <0.001 |
| AKI etiology | |||||
| Exclusively prerenal | |||||
| No | 2535 (79.2) | 1820 (77.3) | 715 (84.5) | Ref. | |
| Yes | 666 (20.8) | 535 (22.7) | 131 (15.5) | 0.623 (0.505–0.769) | <0.001 |
| Sepsis-associated | |||||
| No | 2428 (75.9) | 1988 (84.4) | 440 (52.0) | Ref. | |
| Yes | 773 (24.1) | 367 (15.6) | 406 (48.0) | 4.998 (4.196–5.954) | <0.001 |
| Drug-induced | |||||
| No | 2602 (81.3) | 1953 (82.9) | 649 (76.7) | Ref. | |
| Yes | 599 (18.7) | 402 (17.1) | 197 (23.3) | 1.475 (1.217–1.787) | <0.001 |
| Obstructive | |||||
| No | 2312 (72.2) | 1593 (67.6) | 719 (85.0) | Ref. | |
| Yes | 889 (27.8) | 762 (32.4) | 127 (15.0) | 0.369 (0.300–0.454) | <0.001 |
| Postoperative AKI | |||||
| No | 2877 (89.9) | 2125 (90.2) | 752 (88.9) | Ref. | |
| Yes | 324 (10.1) | 230 (9.8) | 94 (11.1) | 1.144 (0.888–1.475) | 0.298 |
| Need for RRT | |||||
| No | 2562 (80.0) | 2066 (87.7) | 496 (58.6) | Ref. | |
| Yes | 639 (20.0) | 289 (12.3) | 350 (41.4) | 5.045 (4.197–6.064) | <0.001 |
| Hematological malignancy | |||||
| HSCT | |||||
| No | 498 (63.9) | 336 (74.3) | 162 (49.5) | Ref. | |
| Auto | 148 (19.0) | 65 (14.4) | 83 (25.4) | 2.648 (1.820–3.853) | <0.001 |
| Allo | 133 (17.1) | 51 (11.3) | 82 (25.1) | 3.335 (2.243–4.958) | |
| GvHD | |||||
| No | 694 (89.1) | 415 (91.8) | 279 (85.3) | Ref. | |
| Yes | 85 (10.9) | 37 (8.2) | 48 (14.7) | 1.930 (1.224–3.041) | <0.001 |
| Factors | Multivariate Analysis | |||
|---|---|---|---|---|
| B Coefficient | OR (95% CI) | p-Value | Score Points | |
| IMV | ||||
| No | - | Ref. | ||
| Yes | 1.336 | 3.804 (2.844–5.089) | <0.001 | 8 |
| Sepsis-associated AKI | ||||
| No | - | Ref. | ||
| Yes | 0.772 | 2.164 (1.731–2.706) | <0.001 | 4 |
| Need for RRT | ||||
| No | - | Ref. | ||
| Yes | 0.716 | 2.046 (1.640–2.552) | <0.001 | 4 |
| Obstructive AKI | ||||
| No | - | Ref. | ||
| Yes | −1.537 | 0.647 (0.518–0.809) | <0.001 | −1 |
| Factors | Multivariate Analysis | |||
|---|---|---|---|---|
| B Coefficient | OR (95% CI) | p-Value | Score Points | |
| IMV | ||||
| No | - | Ref. | ||
| Yes | 1.511 | 4.533 (2.817–7.294) | <0.001 | 9 |
| Sepsis-associated AKI | ||||
| No | - | Ref. | ||
| Yes | 1.059 | 2.883 (1.903–4.368) | <0.001 | 6 |
| HSCT | ||||
| No | - | Ref. | ||
| Auto | 0.751 | 2.118 (1.289–3.482) | 0.003 | 4 |
| Allo | 0.754 | 2.125 (1.268–3.559) | 0.004 | 4 |
| Need for RRT | ||||
| No | - | Ref. | ||
| Yes | 0.372 | 1.451 (0.975–2.159) | 0.067 | 3 |
| Drug-induced AKI | ||||
| No | - | Ref. | ||
| Yes | 0.546 | 1.727 (1.179–2.529) | 0.005 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calças Marques, R.; Reis, M.; Pimenta, G.; Sala, I.; Chuva, T.; Coelho, I.; Ferreira, H.; Paiva, A.; Costa, J.M. Severe Acute Kidney Injury in Hospitalized Cancer Patients: Epidemiology and Predictive Model of Renal Replacement Therapy and In-Hospital Mortality. Cancers 2024, 16, 561. https://doi.org/10.3390/cancers16030561
Calças Marques R, Reis M, Pimenta G, Sala I, Chuva T, Coelho I, Ferreira H, Paiva A, Costa JM. Severe Acute Kidney Injury in Hospitalized Cancer Patients: Epidemiology and Predictive Model of Renal Replacement Therapy and In-Hospital Mortality. Cancers. 2024; 16(3):561. https://doi.org/10.3390/cancers16030561
Chicago/Turabian StyleCalças Marques, Roberto, Marina Reis, Gonçalo Pimenta, Inês Sala, Teresa Chuva, Inês Coelho, Hugo Ferreira, Ana Paiva, and José Maximino Costa. 2024. "Severe Acute Kidney Injury in Hospitalized Cancer Patients: Epidemiology and Predictive Model of Renal Replacement Therapy and In-Hospital Mortality" Cancers 16, no. 3: 561. https://doi.org/10.3390/cancers16030561
APA StyleCalças Marques, R., Reis, M., Pimenta, G., Sala, I., Chuva, T., Coelho, I., Ferreira, H., Paiva, A., & Costa, J. M. (2024). Severe Acute Kidney Injury in Hospitalized Cancer Patients: Epidemiology and Predictive Model of Renal Replacement Therapy and In-Hospital Mortality. Cancers, 16(3), 561. https://doi.org/10.3390/cancers16030561






