The Importance of Th2 Immune Responses in Mediating the Progression of Gastritis-Associated Metaplasia to Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Inflammation in the Stomach and Gastric Metaplasia
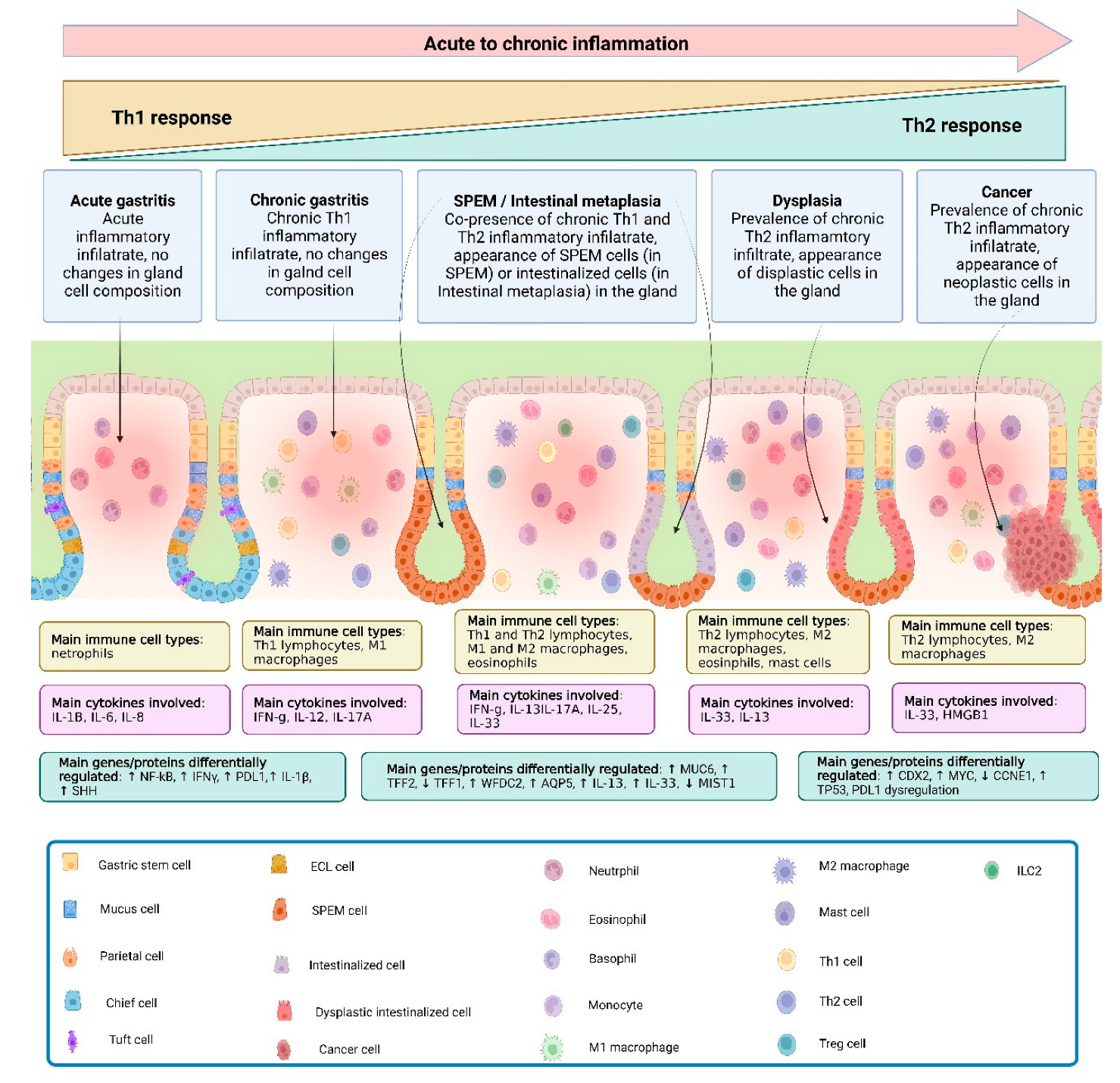
2.1. Gastric Metaplasia Occurs in Different Forms, Possibly Representing Separate Stages of Carcinogenesis
2.2. Different Murine Models Help to Understand Different Aspects of Metaplasia Progression to Overt Cancer
2.3. Chronic Inflammation and Metaplasia Are Key Processes for Advancement to Gastric Carcinogenesis
3. Th2 Pathways in Gastric Metaplasia
3.1. Th2 Immune Responses, Specifically via IL-13, Is Central to the Pathogenesis of Gastric Cancer
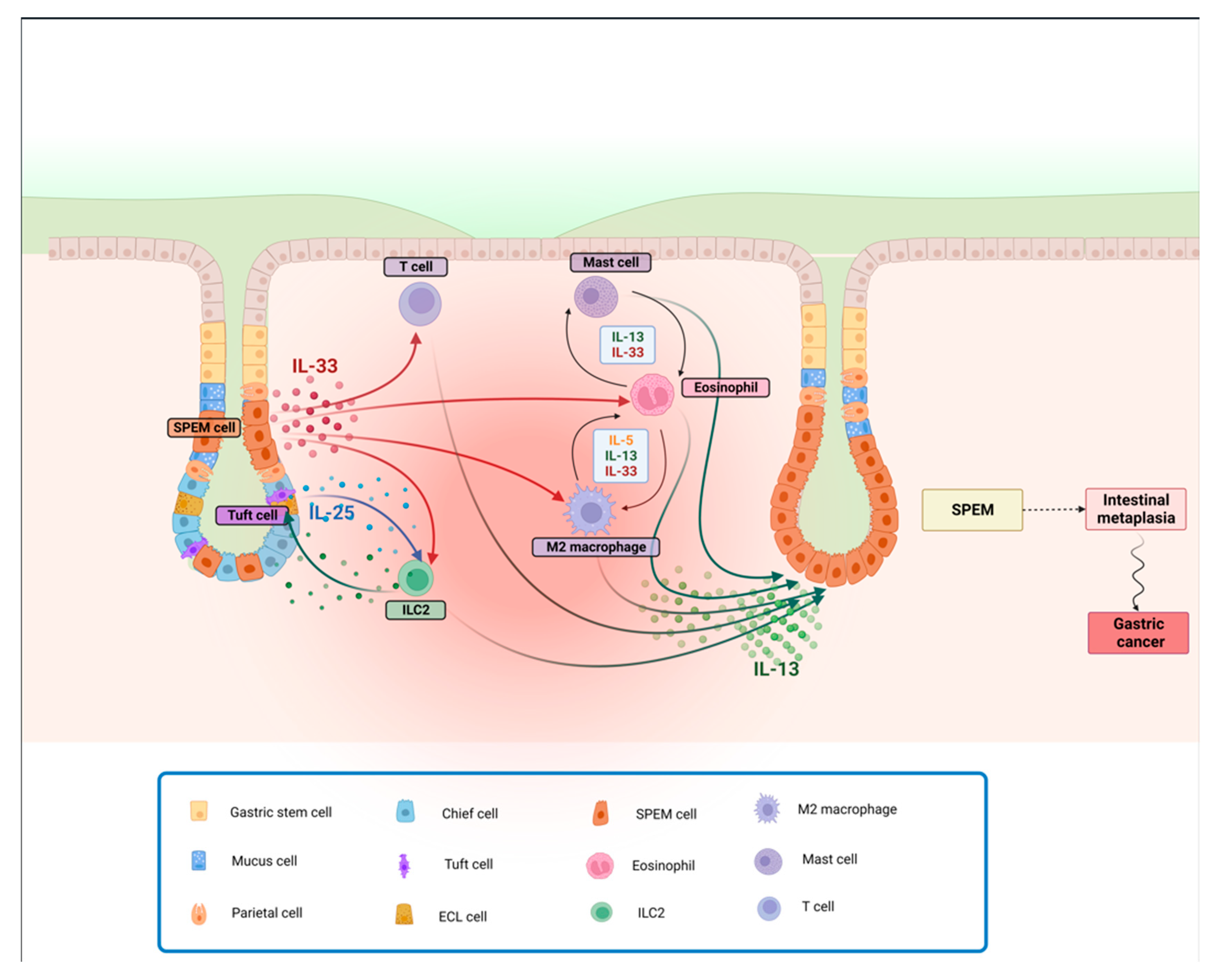
3.2. IL-33 Is a Crucial Inducer of Th2 Responses Primarily, but Not Exclusively, by Inducing IL-13
3.3. Cell Types Associated with the Cascade of Events Leading to Gastritis, and Subsequently to Gastric Metaplasia
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estimated Age-Standardized Mortality Rates (World) in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-multi-bars?v=2020&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&type_multiple=%257B%2522inc%2522%253Afalse%252C%2522mort%2522%253Atrue%252C%2522prev%2522%253Afalse%257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522bottom%2522%253Afalse%257D (accessed on 12 October 2021).
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Liu, X.; Chu, K.M. E-cadherin and gastric cancer: Cause, consequence, and applications. Biomed. Res. Int. 2014, 2014, 637308. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef]
- Uemura, N.; Oomoto, Y.; Mukai, T.; Okamoto, S.; Yamaguchi, S.; Mashiba, H.; Taniyama, K.; Sasaki, N.; Sumii, K.; Haruma, K.; et al. Gastric corpus IL-8 concentration and neutrophil infiltration in duodenal ulcer patients. Aliment. Pharmacol. Ther. 1997, 11, 793–800. [Google Scholar] [CrossRef]
- Nakajima, N.; Kuwayama, H.; Ito, Y.; Iwasaki, A.; Arakawa, Y. Helicobacter pylori, neutrophils, interleukins, and gastric epithelial proliferation. J. Clin. Gastroenterol. 1997, 25 (Suppl. S1), S198–S202. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic. Res. 2000, 33, 785–794. [Google Scholar] [CrossRef]
- Drumm, B.; Perez-Perez, G.I.; Blaser, M.J.; Sherman, P.M. Intrafamilial clustering of Helicobacter pylori infection. N. Engl. J. Med. 1990, 322, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Shallcross, T.M.; Wyatt, J.I.; Taylor, J.D.; Heatley, R.V.; Rathbone, B.J.; Losowsky, M.S. Mucosal humoral immune response to Helicobacter pylori in patients with duodenitis. Dig. Dis. Sci. 1991, 36, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, R.A.; Karttunen, T.J.; Yousfi, M.M.; el-Zimaity, H.M.; Graham, D.Y.; el-Zaatari, F.A. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand. J. Gastroenterol. 1997, 32, 22–27. [Google Scholar] [CrossRef] [PubMed]
- D’Elios, M.M.; Manghetti, M.; Almerigogna, F.; Amedei, A.; Costa, F.; Burroni, D.; Baldari, C.T.; Romagnani, S.; Telford, J.L.; Del Prete, G. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur. J. Immunol. 1997, 27, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- D’Elios, M.M.; Manghetti, M.; De Carli, M.; Costa, F.; Baldari, C.T.; Burroni, D.; Telford, J.L.; Romagnani, S.; Del Prete, G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 1997, 158, 962–967. [Google Scholar] [CrossRef]
- Bamford, K.B.; Fan, X.; Crowe, S.E.; Leary, J.F.; Gourley, W.K.; Luthra, G.K.; Brooks, E.G.; Graham, D.Y.; Reyes, V.E.; Ernst, P.B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenteroly 1998, 114, 482–492. [Google Scholar] [CrossRef]
- Sommer, F.; Faller, G.; Konturek, P.; Kirchner, T.; Hahn, E.G.; Zeus, J.; Rollinghoff, M.; Lohoff, M. Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 1998, 66, 5543–5546. [Google Scholar] [CrossRef]
- Lindholm, C.; Quiding-Jarbrink, M.; Lonroth, H.; Hamlet, A.; Svennerholm, A.M. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 1998, 66, 5964–5971. [Google Scholar] [CrossRef]
- Berstad, A.E.; Hogasen, K.; Bukholm, G.; Moran, A.P.; Brandtzaeg, P. Complement activation directly induced by Helicobacter pylori. Gastroenterology 2001, 120, 1108–1116. [Google Scholar] [CrossRef]
- Edwards, P.D.; Carrick, J.; Turner, J.; Lee, A.; Mitchell, H.; Cooper, D.A. Helicobacter pylori-associated gastritis is rare in AIDS: Antibiotic effect or a consequence of immunodeficiency? Am. J. Gastroenterol. 1991, 86, 1761–1764. [Google Scholar] [PubMed]
- Varsky, C.G.; Correa, M.C.; Sarmiento, N.; Bonfanti, M.; Peluffo, G.; Dutack, A.; Maciel, O.; Capece, P.; Valentinuzzi, G.; Weinstock, D. Prevalence and etiology of gastroduodenal ulcer in HIV-positive patients: A comparative study of 497 symptomatic subjects evaluated by endoscopy. Am. J. Gastroenterol. 1998, 93, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Lichterfeld, M.; Lorenz, C.; Nischalke, H.D.; Scheurlen, C.; Sauerbruch, T.; Rockstroh, J.K. Decreased prevalence of Helicobacter pylori infection in HIV patients with AIDS defining diseases. Z. Gastroenterol. 2002, 40, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Callaghan, J.M.; Gleeson, P.A.; Mori, Y.; Masuda, T.; Toh, B.H. The parietal cell autoantigens recognized in neonatal thymectomy-induced murine gastritis are the alpha and beta subunits of the gastric proton pump. Gastroenterology 1991, 101, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bettington, M.; Brown, I. Autoimmune gastritis: Novel clues to histological diagnosis. Pathology 2013, 45, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Primers 2020, 6, 56. [Google Scholar] [CrossRef]
- Suri-Payer, E.; Amar, A.Z.; McHugh, R.; Natarajan, K.; Margulies, D.H.; Shevach, E.M. Post-thymectomy autoimmune gastritis: Fine specificity and pathogenicity of anti-H/K ATPase-reactive T cells. Eur. J. Immunol. 1999, 29, 669–677. [Google Scholar] [CrossRef]
- Harakal, J.; Rival, C.; Qiao, H.; Tung, K.S. Regulatory T Cells Control Th2-Dominant Murine Autoimmune Gastritis. J. Immunol. 2016, 197, 27–41. [Google Scholar] [CrossRef]
- De Salvo, C.; Pastorelli, L.; Petersen, C.P.; Butto, L.F.; Buela, K.A.; Omenetti, S.; Locovei, S.A.; Ray, S.; Friedman, H.R.; Duijser, J.; et al. Interleukin 33 Triggers Early Eosinophil-Dependent Events Leading to Metaplasia in a Chronic Model of Gastritis-Prone Mice. Gastroenterology 2021, 160, 302–316.e7. [Google Scholar] [CrossRef]
- Chu, D.K.; Jimenez-Saiz, R.; Verschoor, C.P.; Walker, T.D.; Goncharova, S.; Llop-Guevara, A.; Shen, P.; Gordon, M.E.; Barra, N.G.; Bassett, J.D.; et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J. Exp. Med. 2014, 211, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.A.; Zellner, K.R.; Colbert, D.; Lee, N.A.; Lee, J.J. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J. Immunol. 2011, 187, 6059–6068. [Google Scholar] [CrossRef]
- Tian, L.; Altin, J.A.; Makaroff, L.E.; Franckaert, D.; Cook, M.C.; Goodnow, C.C.; Dooley, J.; Liston, A. Foxp3(+) regulatory T cells exert asymmetric control over murine helper responses by inducing Th2 cell apoptosis. Blood 2011, 118, 1845–1853. [Google Scholar] [CrossRef]
- Ren, Z.; Pang, G.; Clancy, R.; Li, L.C.; Lee, C.S.; Batey, R.; Borody, T.; Dunkley, M. Shift of the gastric T-cell response in gastric carcinoma. J. Gastroenterol. Hepatol. 2001, 16, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B.; Wilson, K.T. Pathology of gastric intestinal metaplasia: Clinical implications. Am. J. Gastroenterol. 2010, 105, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, Y.S.; Heo, N.J.; Lim, J.H.; Yang, S.Y.; Chung, G.E.; Kim, J.S. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal Metaplasia. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Wyatt, J.I.; Trejdosiewicz, L.K.; Peichl, P.; Nichols, P.H.; Ramsay, N.; Primrose, J.N.; Lindley, I.J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J. Clin. Pathol. 1994, 47, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Stack, A.; Katzowitsch, E.; Aizawa, S.I.; Suerbaum, S.; Josenhans, C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 2003, 5, 1345–1356. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, A.; Sebkova, L.; Monteleone, G.; Guarnieri, G.; Imeneo, M.; Pallone, F.; Luzza, F. Interleukin-12 drives the Th1 signaling pathway in Helicobacter pylori-infected human gastric mucosa. Infect. Immun. 2007, 75, 1738–1744. [Google Scholar] [CrossRef]
- Arachchi, P.S.; Fernando, N.; Weerasekera, M.M.; Senevirathna, B.; Weerasekera, D.D.; Gunasekara, C.P. Proinflammatory Cytokine IL-17 Shows a Significant Association with Helicobacter pylori Infection and Disease Severity. Gastroenterol. Res. Pract. 2017, 2017, 6265150. [Google Scholar] [CrossRef] [PubMed]
- Rokkas, T.; Rokka, A.; Portincasa, P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann. Gastroenterol. 2017, 30, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Marotti, B.; Rocco, A.; De Colibus, P.; Compare, D.; de Nucci, G.; Staibano, S.; Tatangelo, F.; Romano, M.; Nardone, G. Interleukin-13 mucosal production in Helicobacter pylori-related gastric diseases. Dig. Liver Dis. 2008, 40, 240–247. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Larussa, T.; Nemati, M.; Jalapour, S. T cell subsets play an important role in the determination of the clinical outcome of Helicobacter pylori infection. Microb. Pathog. 2018, 116, 227–236. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Battista, S.; Ambrosio, M.R.; Limarzi, F.; Gallo, G.; Saragoni, L. Molecular Alterations in Gastric Preneoplastic Lesions and Early Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6652. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libanio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef]
- Xia, H.H.; Yang, Y.; Lam, S.K.; Wong, W.M.; Leung, S.Y.; Yuen, S.T.; Elia, G.; Wright, N.A.; Wong, B.C. Aberrant epithelial expression of trefoil family factor 2 and mucin 6 in Helicobacter pylori infected gastric antrum, incisura, and body and its association with antralisation. J. Clin. Pathol. 2004, 57, 861–866. [Google Scholar] [CrossRef]
- Jencks, D.S.; Adam, J.D.; Borum, M.L.; Koh, J.M.; Stephen, S.; Doman, D.B. Overview of Current Concepts in Gastric Intestinal Metaplasia and Gastric Cancer. Gastroenterol. Hepatol. 2018, 14, 92–101. [Google Scholar]
- Babu, S.D.; Jayanthi, V.; Devaraj, N.; Reis, C.A.; Devaraj, H. Expression profile of mucins (MUC2, MUC5AC and MUC6) in Helicobacter pylori infected pre-neoplastic and neoplastic human gastric epithelium. Mol. Cancer 2006, 5, 10. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Sanz-Anquela, J.M.; Gisbert, J.P.; Correa, P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int. J. Cancer 2013, 133, 1023–1032. [Google Scholar] [CrossRef]
- Schmidt, P.H.; Lee, J.R.; Joshi, V.; Playford, R.J.; Poulsom, R.; Wright, N.A.; Goldenring, J.R. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Investig. 1999, 79, 639–646. [Google Scholar] [PubMed]
- Keeley, T.M.; Samuelson, L.C. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1241–G1251. [Google Scholar] [CrossRef]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Actman, L.; McNally, S.R.; Silberg, D.G.; Furth, E.E.; Kaestner, K.H. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology 2005, 128, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Oshima, M.; Inaba, K.; Taketo, M.M. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004, 23, 1669–1678. [Google Scholar] [CrossRef]
- Oshima, H.; Matsunaga, A.; Fujimura, T.; Tsukamoto, T.; Taketo, M.M.; Oshima, M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 2006, 131, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Chuang, L.S.; Ito, T.; Chang, T.L.; Fukamachi, H.; Salto-Tellez, M.; Ito, Y. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology 2011, 140, 1536–1546.e8. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Takenaka, Y.; Yamaguchi, H.; Tetsuya, T.; Tanaka, H.; Tatematsu, M.; Nomura, S.; Goldenring, J.R.; Kaminishi, M. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab. Investig. 2007, 87, 1265–1276. [Google Scholar] [CrossRef]
- Nam, K.T.; Lee, H.J.; Mok, H.; Romero-Gallo, J.; Crowe, J.E., Jr.; Peek, R.M., Jr.; Goldenring, J.R. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology 2009, 136, 1288–1296. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N.A. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010, 138, 2207–2210.e1. [Google Scholar] [CrossRef] [PubMed]
- Leushacke, M.; Tan, S.H.; Wong, A.; Swathi, Y.; Hajamohideen, A.; Tan, L.T.; Goh, J.; Wong, E.; Denil, S.; Murakami, K.; et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat. Cell Biol. 2017, 19, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Ariyama, H.; Stancikova, J.; Sakitani, K.; Asfaha, S.; Renz, B.W.; Dubeykovskaya, Z.A.; Shibata, W.; Wang, H.; Westphalen, C.B.; et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015, 28, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, S.; Min, M.; Ni, Y.; Lu, Z.; Sun, X.; Wu, J.; Liu, B.; Ying, X.; Liu, Y. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut 2021, 70, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Willet, S.G.; Lewis, M.A.; Miao, Z.F.; Liu, D.; Radyk, M.D.; Cunningham, R.L.; Burclaff, J.; Sibbel, G.; Lo, H.G.; Blanc, V.; et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J. 2018, 37, e98311. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more (Review). Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef]
- Meyer, A.R.; Engevik, A.C.; Willet, S.G.; Williams, J.A.; Zou, Y.; Massion, P.P.; Mills, J.C.; Choi, E.; Goldenring, J.R. Cystine/Glutamate Antiporter (xCT) Is Required for Chief Cell Plasticity after Gastric Injury. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 379–405. [Google Scholar] [CrossRef]
- Miao, Z.F.; Sun, J.X.; Adkins-Threats, M.; Pang, M.J.; Zhao, J.H.; Wang, X.; Tang, K.W.; Wang, Z.N.; Mills, J.C. DDIT4 Licenses Only Healthy Cells to Proliferate During Injury-induced Metaplasia. Gastroenterology 2021, 160, 260–271.e10. [Google Scholar] [CrossRef]
- Choi, E.; Hendley, A.M.; Bailey, J.M.; Leach, S.D.; Goldenring, J.R. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology 2016, 150, 918–930.e13. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, B.; Min, J.; Contreras-Panta, E.W.; Presentation, K.S.; Delgado, A.G.; Piazuelo, M.B.; Choi, E.; Goldenring, J.R. Up-regulation of Aquaporin 5 Defines Spasmolytic Polypeptide-Expressing Metaplasia and Progression to Incomplete Intestinal Metaplasia. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 199–217. [Google Scholar] [CrossRef]
- Burclaff, J.; Osaki, L.H.; Liu, D.; Goldenring, J.R.; Mills, J.C. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 2017, 152, 762–766.e7. [Google Scholar] [CrossRef]
- Nam, K.T.; Lee, H.J.; Sousa, J.F.; Weis, V.G.; O’Neal, R.L.; Finke, P.E.; Romero-Gallo, J.; Shi, G.; Mills, J.C.; Peek, R.M., Jr.; et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010, 139, 2028–2037. [Google Scholar] [CrossRef]
- Huh, W.J.; Khurana, S.S.; Geahlen, J.H.; Kohli, K.; Waller, R.A.; Mills, J.C. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012, 142, 21–24.e7. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.P.; Weis, V.G.; Nam, K.T.; Sousa, J.F.; Fingleton, B.; Goldenring, J.R. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology 2014, 146, 1727–1738.e8. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.P.; Meyer, A.R.; De Salvo, C.; Choi, E.; Schlegel, C.; Petersen, A.; Engevik, A.C.; Prasad, N.; Levy, S.E.; Peebles, R.S.; et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 2018, 67, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Weis, V.G.; Petersen, C.P.; Weis, J.A.; Meyer, A.R.; Choi, E.; Mills, J.C.; Goldenring, J.R. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G67–G76. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Goldenring, J.R.; Dangler, C.; Ito, S.; Mueller, A.; Jeon, W.K.; Koh, T.J.; Fox, J.G. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 1998, 114, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Dangler, C.A.; Chen, D.; Goldenring, J.R.; Koh, T.; Raychowdhury, R.; Coffey, R.J.; Ito, S.; Varro, A.; Dockray, G.J.; et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 2000, 118, 36–47. [Google Scholar] [CrossRef]
- Roth, K.A.; Kapadia, S.B.; Martin, S.M.; Lorenz, R.G. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 1999, 163, 1490–1497. [Google Scholar] [CrossRef]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Lee, J.Y.; Amieva, M.R.; Roers, A.; Flavell, R.A.; Sparwasser, T.; Muller, A. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 2011, 140, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Hirata, Y.; Hayakawa, Y.; Suzuki, N.; Sakitani, K.; Hikiba, Y.; Ihara, S.; Kinoshita, H.; Nakagawa, H.; Tateishi, K.; et al. Gastric Metaplasia Induced by Helicobacter pylori Is Associated with Enhanced SOX9 Expression via Interleukin-1 Signaling. Infect. Immun. 2016, 84, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Sayi, A.; Kohler, E.; Toller, I.M.; Flavell, R.A.; Muller, W.; Roers, A.; Muller, A. TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J. Immunol. 2011, 186, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Thamphiwatana, S.; Carmona, E.M.; Rickman, B.; Doran, K.S.; Obonyo, M. Deficiency of the myeloid differentiation primary response molecule MyD88 leads to an early and rapid development of Helicobacter-induced gastric malignancy. Infect. Immun. 2014, 82, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, R.E.; Rose, S.; Westphalen, C.B.; Shibata, W.; Muthupalani, S.; Tailor, Y.; Friedman, R.A.; Han, W.; Fox, J.G.; Ferrante, A.W., Jr.; et al. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut 2014, 63, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, J.L.; Whary, M.T.; Ge, Z.; Muthupalani, S.; Taylor, N.S.; Mobley, M.; Potter, A.; Varro, A.; Eibach, D.; Suerbaum, S.; et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 2011, 140, 210–220. [Google Scholar] [CrossRef]
- Jain, R.N.; Al-Menhali, A.A.; Keeley, T.M.; Ren, J.; El-Zaatari, M.; Chen, X.; Merchant, J.L.; Ross, T.S.; Chew, C.S.; Samuelson, L.C. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J. Clin. Investig. 2008, 118, 2459–2470. [Google Scholar] [CrossRef][Green Version]
- Reuter, B.K.; Pastorelli, L.; Brogi, M.; Garg, R.R.; McBride, J.A.; Rowlett, R.M.; Arrieta, M.C.; Wang, X.M.; Keller, E.J.; Feldman, S.H.; et al. Spontaneous, immune-mediated gastric inflammation in SAMP1/YitFc mice, a model of Crohn’s-like gastritis. Gastroenterology 2011, 141, 1709–1719. [Google Scholar] [CrossRef]
- De Salvo, C.; Pizarro, T.T. Reply. Gastroenterology 2021, 160, 2630–2631. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Mills, J.C. Cellular Plasticity, Reprogramming, and Regeneration: Metaplasia in the Stomach and Beyond. Gastroenterology 2022, 162, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Macarthur, M.; Hold, G.L.; El-Omar, E.M. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G515–G520. [Google Scholar] [CrossRef]
- Weis, V.G.; Sousa, J.F.; LaFleur, B.J.; Nam, K.T.; Weis, J.A.; Finke, P.E.; Ameen, N.A.; Fox, J.G.; Goldenring, J.R. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut 2013, 62, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Radyk, M.D.; Burclaff, J.; Willet, S.G.; Mills, J.C. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology 2018, 154, 839–843.e2. [Google Scholar] [CrossRef] [PubMed]
- Maywald, R.L.; Doerner, S.K.; Pastorelli, L.; De Salvo, C.; Benton, S.M.; Dawson, E.P.; Lanza, D.G.; Berger, N.A.; Markowitz, S.D.; Lenz, H.J.; et al. IL-33 activates tumor stroma to promote intestinal polyposis. Proc. Natl. Acad. Sci. USA 2015, 112, E2487–E2496. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, R.; Karttunen, T.; Ekre, H.P.; MacDonald, T.T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 1995, 36, 341–345. [Google Scholar] [CrossRef]
- Bontems, P.; Robert, F.; Van Gossum, A.; Cadranel, S.; Mascart, F. Helicobacter pylori modulation of gastric and duodenal mucosal T cell cytokine secretions in children compared with adults. Helicobacter 2003, 8, 216–226. [Google Scholar] [CrossRef]
- Bergman, M.; Del Prete, G.; van Kooyk, Y.; Appelmelk, B. Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nat. Rev. Microbiol. 2006, 4, 151–159. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper T lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef]
- Tu, S.; Bhagat, G.; Cui, G.; Takaishi, S.; Kurt-Jones, E.A.; Rickman, B.; Betz, K.S.; Penz-Oesterreicher, M.; Bjorkdahl, O.; Fox, J.G.; et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 2008, 14, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Syu, L.J.; El-Zaatari, M.; Eaton, K.A.; Liu, Z.; Tetarbe, M.; Keeley, T.M.; Pero, J.; Ferris, J.; Wilbert, D.; Kaatz, A.; et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am. J. Pathol. 2012, 181, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Shibata, W.; Ariyama, H.; Westphalen, C.B.; Worthley, D.L.; Muthupalani, S.; Asfaha, S.; Dubeykovskaya, Z.; Quante, M.; Fox, J.G.; Wang, T.C. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut 2013, 62, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Asfaha, S.; Dubeykovskiy, A.N.; Tomita, H.; Yang, X.; Stokes, S.; Shibata, W.; Friedman, R.A.; Ariyama, H.; Dubeykovskaya, Z.A.; Muthupalani, S.; et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013, 144, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Noto, C.N.; Hoft, S.G.; Bockerstett, K.A.; Jackson, N.M.; Ford, E.L.; Vest, L.S.; Di Paolo, R.J. IL13 Acts Directly on Gastric Epithelial Cells to Promote Metaplasia Development During Chronic Gastritis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Miska, J.; Lui, J.B.; Toomer, K.H.; Devarajan, P.; Cai, X.; Houghton, J.; Lopez, D.M.; Abreu, M.T.; Wang, G.; Chen, Z. Initiation of inflammatory tumorigenesis by CTLA4 insufficiency due to type 2 cytokines. J. Exp. Med. 2018, 215, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Cao, G.; Zhang, B.; Wei, L.; Zhang, X.; Jin, M.; He, B.; Zhang, B.; He, Z.; Bie, Q. TNF+ regulatory T cells regulate the stemness of gastric cancer cells through the IL13/STAT3 pathway. Front. Oncol. 2023, 13, 1162938. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Pastorelli, L.; Garg, R.R.; Hoang, S.B.; Spina, L.; Mattioli, B.; Scarpa, M.; Fiocchi, C.; Vecchi, M.; Pizarro, T.T. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc. Natl. Acad. Sci. USA 2010, 107, 8017–8022. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef]
- Joshi, A.D.; Oak, S.R.; Hartigan, A.J.; Finn, W.G.; Kunkel, S.L.; Duffy, K.E.; Das, A.; Hogaboam, C.M. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010, 11, 52. [Google Scholar] [CrossRef]
- Stolarski, B.; Kurowska-Stolarska, M.; Kewin, P.; Xu, D.; Liew, F.Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 2010, 185, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.Y.; Lewkowich, I.P.; Dawson, L.A.; Downey, J.; Yang, Y.; Smith, D.E.; Herbert, D.R. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc. Natl. Acad. Sci. USA 2013, 110, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ben, Q.; Tu, S.; Dong, W.; Qi, X.; Wu, Y. Serum interleukin-33 levels in patients with gastric cancer. Dig. Dis. Sci. 2011, 56, 3596–3601. [Google Scholar] [CrossRef]
- Ye, X.L.; Zhao, Y.R.; Weng, G.B.; Chen, Y.C.; Wei, X.N.; Shao, J.P.; Ji, H. IL-33-induced JNK pathway activation confers gastric cancer chemotherapy resistance. Oncol. Rep. 2015, 33, 2746–2752. [Google Scholar] [CrossRef]
- Yu, X.X.; Hu, Z.; Shen, X.; Dong, L.Y.; Zhou, W.Z.; Hu, W.H. IL-33 Promotes Gastric Cancer Cell Invasion and Migration Via ST2-ERK1/2 Pathway. Dig. Dis. Sci. 2015, 60, 1265–1272. [Google Scholar] [CrossRef]
- Grunig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef]
- Engevik, A.C.; Feng, R.; Choi, E.; White, S.; Bertaux-Skeirik, N.; Li, J.; Mahe, M.M.; Aihara, E.; Yang, L.; DiPasquale, B.; et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 605–624. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, M.; Ye, T.; Feng, J.; Xu, X.; Yang, H.; Wang, X.; Bao, L.; Li, R.; Xue, B.; et al. Mitochondrial GRIM-19 loss in parietal cells promotes spasmolytic polypeptide-expressing metaplasia through NLR family pyrin domain-containing 3 (NLRP3)-mediated IL-33 activation via a reactive oxygen species (ROS) -NRF2- Heme oxygenase-1(HO-1)-NF-small ka, CyrillicB axis. Free Radic. Biol. Med. 2023, 202, 46–61. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, B.; Kim, K.H.; Cho, S.Y.; Cho, Y.; Park, J.; Lee, Y.; Oh, Y.; Hwang, B.R.; Jang, A.R.; et al. WFDC2 Promotes Spasmolytic Polypeptide-Expressing Metaplasia Through the Up-Regulation of IL33 in Response to Injury. Gastroenterology 2021, 161, 953–967.e15. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; De Salvo, C.; Pastorelli, L.; Rana, N.; Senkfor, H.N.; Petito, V.; Di Martino, L.; Scaldaferri, F.; Gasbarrini, A.; Cominelli, F.; et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc. Natl. Acad. Sci. USA 2018, 115, E9362–E9370. [Google Scholar] [CrossRef]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef]
- Buzzelli, J.N.; Chalinor, H.V.; Pavlic, D.I.; Sutton, P.; Menheniott, T.R.; Giraud, A.S.; Judd, L.M. IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 203–221.e3. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Seok, S.H.; Kim, S.; An, H.W.; Choudhury, A.D.; Woo, S.H.; Oh, J.S.; Kim, J.K.; Voon, D.C.; Kim, D.Y.; et al. A synergistic partnership between IL-33/ST2 and Wnt pathway through Bcl-xL drives gastric cancer stemness and metastasis. Oncogene 2023, 42, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Giuffre, G.; Inferrera, C. Minute and small early gastric carcinoma with special reference to eosinophil infiltration. Histol. Histopathol. 1993, 8, 155–166. [Google Scholar] [PubMed]
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 1999, 103, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.P.; Rosenberg, H.F.; Moqbel, R.; Phipps, S.; Foster, P.S.; Lacy, P.; Kay, A.B.; Rothenberg, M.E. Eosinophils: Biological properties and role in health and disease. Clin. Exp. Allergy 2008, 38, 709–750. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, C.; Wang, X.M.; Pastorelli, L.; Mattioli, B.; Omenetti, S.; Buela, K.A.; Chowdhry, S.; Garg, R.R.; Goodman, W.A.; Rodriguez-Palacios, A.; et al. IL-33 Drives Eosinophil Infiltration and Pathogenic Type 2 Helper T-Cell Immune Responses Leading to Chronic Experimental Ileitis. Am. J. Pathol. 2016, 186, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Fox, J.G.; Otto, G.; Murphy, J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 1990, 99, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Rothenberg, M.E. Gastrointestinal eosinophilia. Immunol. Allergy Clin. N. Am. 2007, 27, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D.; et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 2019, 10, 2735. [Google Scholar] [CrossRef] [PubMed]
- Munitz, A.; Levi-Schaffer, F. Eosinophils: ‘New’ roles for ‘old’ cells. Allergy 2004, 59, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, I.; Levi-Schaffer, F.; Mekori, Y.A. Mast cells: Not only in allergy. Immunol. Allergy Clin. N. Am. 2006, 26, 407–425. [Google Scholar] [CrossRef]
- Lv, Y.; Tian, W.; Teng, Y.; Wang, P.; Zhao, Y.; Li, Z.; Tang, S.; Chen, W.; Xie, R.; Lu, M.; et al. Tumor-infiltrating mast cells stimulate ICOS(+) regulatory T cells through an IL-33 and IL-2 axis to promote gastric cancer progression. J. Adv. Res. 2023, in press. [Google Scholar] [CrossRef]
- Klein Wolterink, R.G.; Serafini, N.; van Nimwegen, M.; Vosshenrich, C.A.; de Bruijn, M.J.; Fonseca Pereira, D.; Veiga Fernandes, H.; Hendriks, R.W.; Di Santo, J.P. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10240–10245. [Google Scholar] [CrossRef]
- Li, D.; Guabiraba, R.; Besnard, A.G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C.; et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432.e11. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Germar, K.; Spits, H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr. Opin. Immunol. 2014, 31, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.R.; Engevik, A.C.; Madorsky, T.; Belmont, E.; Stier, M.T.; Norlander, A.E.; Pilkinton, M.A.; McDonnell, W.J.; Weis, J.A.; Jang, B.; et al. Group 2 Innate Lymphoid Cells Coordinate Damage Response in the Stomach. Gastroenterology 2020, 159, 2077–2091.e8. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.; Vallance, J.E.; Hummel, A.; Alenghat, T.; Rosen, M.J. IL-33 Induces Murine Intestinal Goblet Cell Differentiation Indirectly via Innate Lymphoid Cell IL-13 Secretion. J. Immunol. 2019, 202, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.R.; Douglas, B.; Zullo, K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef]
- O’Keefe, R.N.; Carli, A.L.E.; Baloyan, D.; Chisanga, D.; Shi, W.; Afshar-Sterle, S.; Eissmann, M.F.; Poh, A.R.; Pal, B.; Seillet, C.; et al. A tuft cell-ILC2 signaling circuit provides therapeutic targets to inhibit gastric metaplasia and tumor development. Nat. Commun. 2023, 14, 6872. [Google Scholar] [CrossRef]
- Li, J.; Liao, Y.; Ding, T.; Wang, B.; Yu, X.; Chu, Y.; Xu, J.; Zheng, L. Tumor-infiltrating macrophages express interleukin-25 and predict a favorable prognosis in patients with gastric cancer after radical resection. Oncotarget 2016, 7, 11083–11093. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Privitera, G.; Williams, J.J.; De Salvo, C. The Importance of Th2 Immune Responses in Mediating the Progression of Gastritis-Associated Metaplasia to Gastric Cancer. Cancers 2024, 16, 522. https://doi.org/10.3390/cancers16030522
Privitera G, Williams JJ, De Salvo C. The Importance of Th2 Immune Responses in Mediating the Progression of Gastritis-Associated Metaplasia to Gastric Cancer. Cancers. 2024; 16(3):522. https://doi.org/10.3390/cancers16030522
Chicago/Turabian StylePrivitera, Giuseppe, Joseph J. Williams, and Carlo De Salvo. 2024. "The Importance of Th2 Immune Responses in Mediating the Progression of Gastritis-Associated Metaplasia to Gastric Cancer" Cancers 16, no. 3: 522. https://doi.org/10.3390/cancers16030522
APA StylePrivitera, G., Williams, J. J., & De Salvo, C. (2024). The Importance of Th2 Immune Responses in Mediating the Progression of Gastritis-Associated Metaplasia to Gastric Cancer. Cancers, 16(3), 522. https://doi.org/10.3390/cancers16030522




