Prognostic Thresholds of Mitotic Count and Ki-67 Labeling Index for Recurrence and Survival in Lung Atypical Carcinoids
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Biomarkers
2.3. Follow-Up
2.4. Statistical Analysis
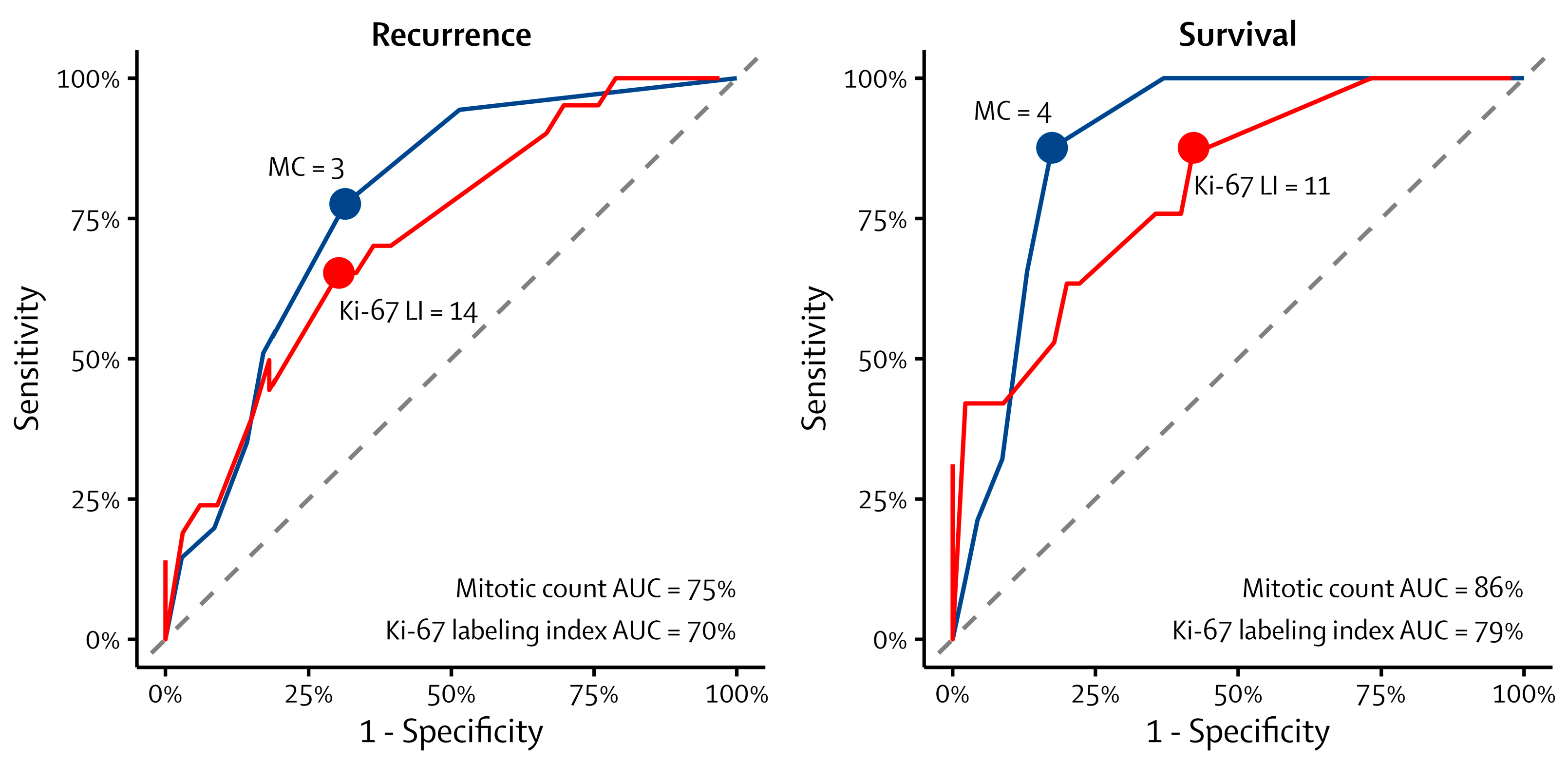
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchio, C.; Gatti, G.; Massa, F.; Bertero, L.; Filosso, P.; Pelosi, G.; Cassoni, P.; Volante, M.; Papotti, M. Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch. 2017, 471, 713–720. [Google Scholar] [CrossRef]
- Rekhtman, N.; Desmeules, P.; Litvak, A.M.; Pietanza, M.C.; Santos-Zabala, M.L.; Ni, A.; Montecalvo, J.; Chang, J.C.; Beras, A.; Preeshagul, I.R.; et al. Stage IV lung carcinoids: Spectrum and evolution of proliferation rate, focusing on variants with elevated proliferation indices. Mod. Pathol. 2019, 32, 1106–1122. [Google Scholar] [CrossRef]
- Kasajima, A.; Konukiewitz, B.; Oka, N.; Suzuki, H.; Sakurada, A.; Okada, Y.; Kameya, T.; Ishikawa, Y.; Sasano, H.; Weichert, W.; et al. Clinicopathological Profiling of Lung Carcinoids with a Ki67 Index >20. Neuroendocrinology 2019, 108, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Alcala, N.; Leblay, N.; Gabriel, A.A.G.; Mangiante, L.; Hervas, D.; Giffon, T.; Sertier, A.S.; Ferrari, A.; Derks, J.; Ghantous, A.; et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat. Commun. 2019, 10, 3407. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Horsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Soldath, P.; Binderup, T.; Kjaer, A.; Federspiel, B.; Langer, S.W.; Knigge, U.; Petersen, R.H. Long-term survival and recurrence after resection of bronchopulmonary carcinoids: A single-center cohort study of 236 patients. Lung Cancer 2021, 156, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zirafa, C.C.; Romano, G.; Sicolo, E.; Cariello, C.; Morganti, R.; Conoscenti, L.; Hung-Key, T.; Davini, F.; Melfi, F. Robotic Surgery for Non-Small Cell Lung Cancer Treatment in High-Risk Patients. J. Clin. Med. 2021, 10, 4408. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P.; International Association for the Study of Lung Cancer (IASLC) Staging Committee. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Grondahl, V.; Binderup, T.; Langer, S.W.; Petersen, R.H.; Nielsen, K.; Kjaer, A.; Federspiel, B.; Knigge, U. Characteristics of 252 patients with bronchopulmonary neuroendocrine tumours treated at the Copenhagen NET Centre of Excellence. Lung Cancer 2019, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fontan, E.M.; Canizares-Carretero, M.A.; Blanco-Ramos, M.; Matilla-Gonzalez, J.M.; Carrasco-Rodriguez, R.; Barreiro-Morandeira, F.; Garcia-Yuste, M.; EMETNE Members, Spanish Society of Pneumology and Thoracic Surgery. Prognostic significance of histopathological factors in survival and recurrence of atypical carcinoid tumours. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 904–910. [Google Scholar] [CrossRef]
- Pelosi, G.; Travis, W.D. The Ki-67 antigen in the new 2021 World Health Organization classification of lung neuroendocrine neoplasms. Pathologica 2021, 113, 377–387. [Google Scholar] [CrossRef]
- Roesel, C.; Welter, S.; Kambartel, K.O.; Weinreich, G.; Krbek, T.; Serke, M.; Ibrahim, M.; Alnajdawi, Y.; Plones, T.; Aigner, C. Prognostic markers in resected large cell neuroendocrine carcinoma: A multicentre retrospective analysis. J. Thorac. Dis. 2020, 12, 466–476. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N = 78 1 |
|---|---|
| Sex | |
| Female | 52 (67) |
| Male | 26 (33) |
| Age | 68 (62–72) |
| Smoking | |
| Current | 17 (22) |
| Former | 30 (38) |
| Never | 31 (40) |
| Tumor location | |
| Central | 32 (41) |
| Peripheral | 46 (59) |
| Surgical resection | |
| Lobectomy | 54 (69) |
| Bilobectomy | 1 (1.3) |
| Sleeve lobectomy | 6 (7.7) |
| Segmentectomy | 7 (9.0) |
| Wedge | 9 (12) |
| Bronchial resection | 1 (1.3) |
| Surgical approach | |
| RATS | 14 (18) |
| VATS | 23 (29) |
| Thoracotomy | 41 (53) |
| Pathological T-category | |
| T1a | 10 (13) |
| T1b | 25 (32) |
| T1c | 11 (14) |
| T2a | 12 (15) |
| T2b | 9 (12) |
| T3 | 9 (12) |
| T4 | 2 (2.6) |
| Pathological N-category | |
| N0 | 55 (72) |
| N1 | 11 (14) |
| N2 | 10 (13) |
| Unknown | 2 |
| Pathological stage | |
| IA | 36 (47) |
| IB | 7 (9.2) |
| IIA | 8 (11) |
| IIB | 12 (16) |
| IIIA | 11 (14) |
| IIIB | 2 (2.6) |
| Unknown | 2 |
| Recurrence | Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | SHR 1 | 95% CI 1 | p-Value | N | SHR 1 | 95% CI 1 | p-Value |
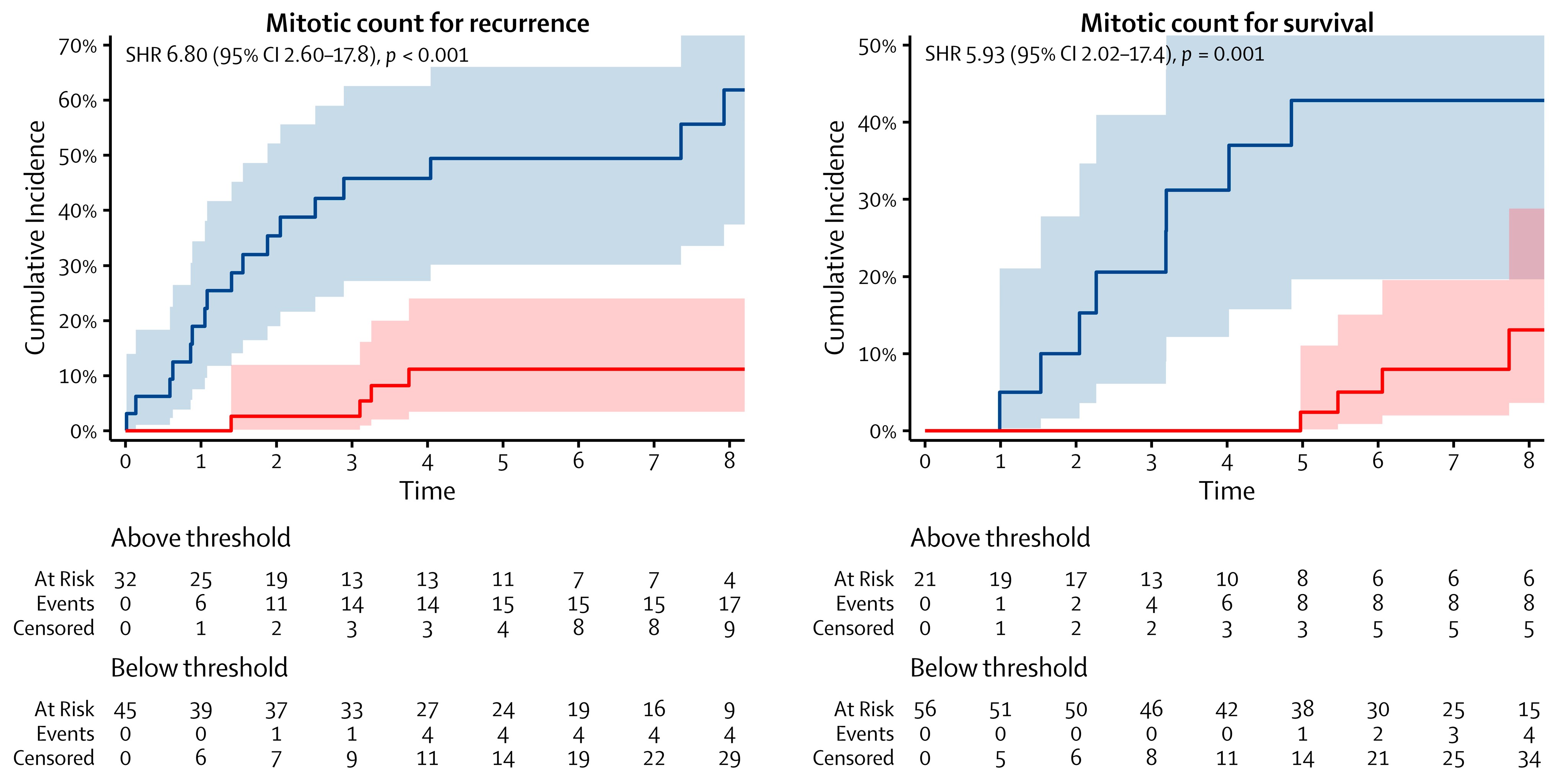
| Mitotic count | ||||||||
| Below threshold | 45 | — | — | 56 | — | — | ||
| Above threshold | 32 | 6.80 | 2.60–17.8 | <0.001 | 21 | 5.93 | 2.02–17.4 | 0.001 |
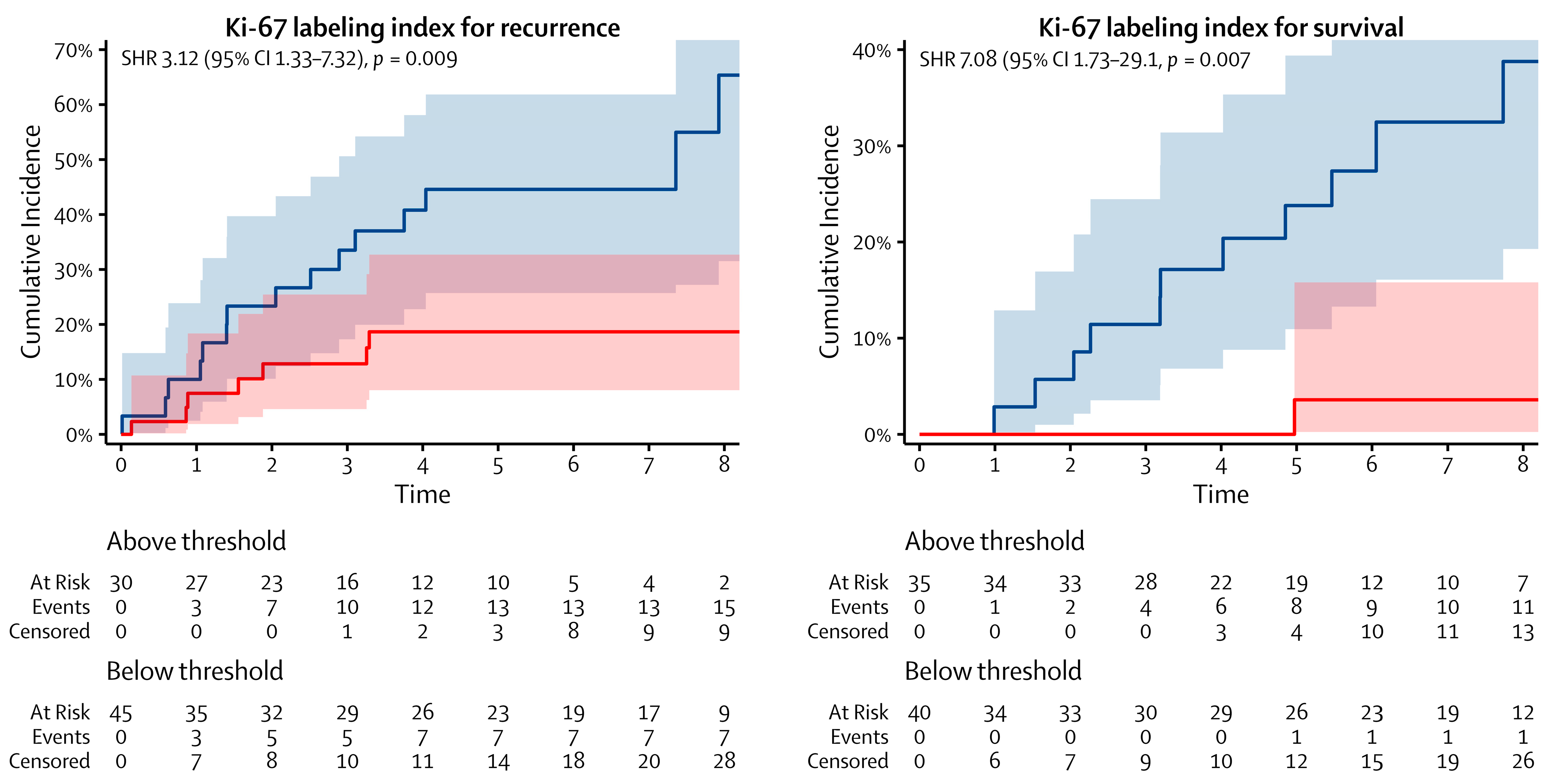
| Ki-67 labeling index | ||||||||
| Below threshold | 45 | — | — | 45 | — | — | ||
| Above threshold | 30 | 3.12 | 1.33–7.32 | 0.009 | 30 | 6.08 | 1.77–20.9 | 0.004 |
| Necrosis | ||||||||
| None | 44 | — | — | 44 | — | — | ||
| Focal | 33 | 3.69 | 1.44–9.46 | 0.007 | 33 | 4.63 | 1.25–17.1 | 0.022 |
| T-category | ||||||||
| T1 | 46 | — | — | 46 | — | — | ||
| T2 | 21 | 1.48 | 0.56–3.91 | 0.43 | 21 | 0.99 | 0.26–3.78 | 0.99 |
| T3 and T4 | 11 | 4.14 | 1.45–11.9 | 0.008 | 11 | 1.80 | 0.53–6.12 | 0.35 |
| N-category | ||||||||
| N0 | 55 | — | — | 55 | — | — | ||
| N1 | 11 | 0.80 | 0.20–3.15 | 0.74 | 11 | 1.41 | 0.28–7.15 | 0.68 |
| N2 | 10 | 6.48 | 2.38–17.7 | <0.001 | 10 | 8.70 | 2.66–28.5 | <0.001 |
| Recurrence | Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | SHR 1 | 95% CI 1 | p-Value | N | SHR 1 | 95% CI 1 | p-Value |
| Mitotic count | ||||||||
| Below threshold | 44 | — | — | 55 | — | — | ||
| Above threshold | 31 | 9.05 | 3.57–22.9 | <0.001 | 20 | 6.65 | 2.36–18.7 | <0.001 |
| Necrosis | ||||||||
| None | 42 | — | — | 42 | — | — | ||
| Focal | 33 | 1.83 | 0.71–4.71 | 0.21 | 33 | 4.42 | 1.00–19.6 | 0.050 |
| T-category | ||||||||
| T1 | 44 | — | — | |||||
| T2 | 20 | 2.15 | 0.76–6.04 | 0.15 | ||||
| T3 and T4 | 11 | 4.64 | 1.72–12.6 | 0.003 | ||||
| N-category | ||||||||
| N0 | 54 | — | — | 54 | — | — | ||
| N1 | 11 | 0.29 | 0.06–1.46 | 0.13 | 11 | 1.14 | 0.23–5.71 | 0.88 |
| N2 | 10 | 8.69 | 2.75–27.5 | <0.001 | 10 | 7.74 | 2.84–21.0 | <0.001 |
| Recurrence | Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | SHR 1 | 95% CI 1 | p-Value | N | SHR 1 | 95% CI 1 | p-Value |
| Ki-67 labeling index | ||||||||
| Below threshold | 43 | — | — | 38 | — | — | ||
| Above threshold | 29 | 5.30 | 2.13–13.2 | <0.001 | 34 | 5.65 | 1.41–22.7 | 0.015 |
| Necrosis | ||||||||
| None | 42 | — | — | 42 | — | — | ||
| Focal | 30 | 1.78 | 0.75–4.21 | 0.19 | 30 | 4.10 | 0.82–20.4 | 0.085 |
| T-category | ||||||||
| T1 | 43 | — | — | |||||
| T2 | 19 | 0.59 | 0.14–2.49 | 0.47 | ||||
| T3 and T4 | 10 | 6.34 | 2.51–16.0 | <0.001 | ||||
| N-category | ||||||||
| N0 | 53 | — | — | 53 | — | — | ||
| N1 | 9 | 1.77 | 0.31–10.1 | 0.52 | 9 | 1.50 | 0.30–7.57 | 0.63 |
| N2 | 10 | 8.87 | 2.83–27.8 | <0.001 | 10 | 8.25 | 2.80–24.3 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldath, P.; Bianchi, D.; Manfredini, B.; Kjaer, A.; Langer, S.W.; Knigge, U.; Melfi, F.; Filosso, P.L.; Petersen, R.H. Prognostic Thresholds of Mitotic Count and Ki-67 Labeling Index for Recurrence and Survival in Lung Atypical Carcinoids. Cancers 2024, 16, 502. https://doi.org/10.3390/cancers16030502
Soldath P, Bianchi D, Manfredini B, Kjaer A, Langer SW, Knigge U, Melfi F, Filosso PL, Petersen RH. Prognostic Thresholds of Mitotic Count and Ki-67 Labeling Index for Recurrence and Survival in Lung Atypical Carcinoids. Cancers. 2024; 16(3):502. https://doi.org/10.3390/cancers16030502
Chicago/Turabian StyleSoldath, Patrick, Daniel Bianchi, Beatrice Manfredini, Andreas Kjaer, Seppo W. Langer, Ulrich Knigge, Franca Melfi, Pier Luigi Filosso, and René Horsleben Petersen. 2024. "Prognostic Thresholds of Mitotic Count and Ki-67 Labeling Index for Recurrence and Survival in Lung Atypical Carcinoids" Cancers 16, no. 3: 502. https://doi.org/10.3390/cancers16030502
APA StyleSoldath, P., Bianchi, D., Manfredini, B., Kjaer, A., Langer, S. W., Knigge, U., Melfi, F., Filosso, P. L., & Petersen, R. H. (2024). Prognostic Thresholds of Mitotic Count and Ki-67 Labeling Index for Recurrence and Survival in Lung Atypical Carcinoids. Cancers, 16(3), 502. https://doi.org/10.3390/cancers16030502








