Digitally Enhanced Methods for the Diagnosis and Monitoring of Treatment Responses in Actinic Keratoses: A New Avenue in Personalized Skin Care
Abstract
Simple Summary
Abstract
1. Introduction
Background and Motivation
2. AK—Classification Systems and Clinical, Histopathological, and Therapeutic Aspects
2.1. Clinical and Histopathological Aspects
2.2. Therapeutic Aspects
2.2.1. Topical Treatments
2.2.2. Physician-Managed Treatments
| Treatment Category | Treatment Type | Mechanism of Action | Application and Efficacy |
|---|---|---|---|
| Topical treatments | Imiquimod | Immune-response modifier, TLR-7 agonist | 5%, 3.75%, 2.5% strengths for face/scalp. Lower concentrations reduce adverse reactions and allow broader application [34,35]. |
| 5-Fluorouracil (5-FU) | Antimetabolite, inhibits thymidylate synthase | Applied 0.5% to 5%, 1–2 times/day for 2–12 weeks. Up to 96% clearance with 5% cream [40,41]. | |
| Diclofenac | NSAID, inhibits cyclooxygenase 2 | 3% gel with 2.5% hyaluronic acid. Twice daily for 60–90 days. Up to 58% clearance post-treatment [42]. | |
| Piroxicam | NSAID, cyclooxygenase-1 inhibitor | 0.8% formulation with sunscreen, twice daily for six months. 55% complete response rate [43]. | |
| Tirbanibulin | Inhibitor of Src kinase | FDA-approved for topical treatment of AK on face or scalp [49,50]. | |
| Ingenol Mebutate | Dual-action, PKC agonist | Destroys lesions within 2–3 days [53,54]. | |
| Resiquimod | Immune-response modifier, TLR7, and TLR8 agonist | Phase II study showed 77.1–90.3% clearance [56]. | |
| Physician-Managed Treatments | Cryosurgery | Liquid nitrogen application | Targets specific lesions, success rate 39–76%. Enhanced efficacy with topical treatments [58]. |
| Photodynamic Therapy (PDT) | 5-ALA or 5-MAL as photosensitizers | c-PDT and dl-PDT. 69–93% clearance on face/scalp, 44–80% on forearms/hands [65,66]. | |
| Chemical Peels | TCA with Jessner’s solution, or 5-FU with glycolic acid | Varied efficacy, less effective than c-PDT in some cases [72]. | |
| Laser Treatments | Removes epidermis and papillary dermis | Fully ablative carbon dioxide laser more effective than fractional ablative [73]. | |
| Surgical Excision | Biopsy for diagnostic confirmation | Recommended for uncertain diagnosis or treatment-resistant lesions [74]. |
3. Non-Invasive Evaluation of AK
3.1. Dermoscopy of AK
3.2. RCM Diagnosis of AK
| AK Grade | Clinical Appearance [22] | Dermoscopic Appearance [77] | In Vivo RCM Appearance [96] | Histopathological Appearance [106] |
|---|---|---|---|---|
| 1 | Barely palpable AK (more evident on palpation than visual examination) | Pseudo-network red pattern | Focal areas with atypical honeycomb pattern at the stratum spinosum level | Focal keratinocyte atypia in the lower third of the epidermis |
| 2 | Moderately thick AK (easily observable visually and on palpation) | Keratotic follicular openings on an erythematous background | Diffuse keratinocyte atypia involving the spinous and granular layers; marked keratinocyte atypia with varying sizes and shapes | Focal keratinocyte atypia in at least the lower third of the epidermis; focal hyperkeratosis alternating with orthokeratosis and parakeratosis; prominent acanthosis and keratinocyte buds in the upper papillary dermis |
| 3 | Thick AK with hyperkeratotic appearance | White-yellowish astructural zones | Disorganized honeycomb pattern characterized by the presence of pleomorphic keratinocytes and irregular interkeratinocyte connections | Diffuse atypical keratinocyte proliferation involving the entire thickness of the epidermis; parakeratosis, acanthosis, papillomatosis, involvement of annex structures |
3.3. Optical Coherence Tomography (OCT) and AK
3.4. Ultrasound and High Frequency Ultrasound Imaging in AK
4. Field Cancerization and the Role of Non-Invasive Imaging Techniques in Diagnosis and Treatment Follow-Up
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heaphy, M.R., Jr.; Ackerman, A.B. The Nature of Solar Keratosis: A Critical Review in Historical Perspective. J. Am. Acad. Dermatol. 2000, 43, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The Natural History of Actinic Keratosis: A Systematic Review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, C. Update on Actinic Keratosis in Clinical Trial Experience with Imiquimod: Update on Actinic Keratosis. Br. J. Dermatol. 2007, 157, 32–33. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Mones, J.M. Solar (Actinic) Keratosis Is Squamous Cell Carcinoma: Solar (Actinic) Keratosis Is Squamous Cell Carcinoma. Br. J. Dermatol. 2006, 155, 9–22. [Google Scholar] [CrossRef]
- Christensen, S.R. Recent Advances in Field Cancerization and Management of Multiple Cutaneous Squamous Cell Carcinomas. F1000Research 2018, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Warszawik-Hendzel, O.; Olszewska, M.; Rakowska, A.; Sikora, M.; Hendzel, P.; Rudnicka, L. Cardiovascular Drug Use and Risk of Actinic Keratosis: A Case-Control Study. Dermatol. Ther. 2020, 10, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, M.C.; Altomare, G.; Benati, E.; Borgia, F.; Broganelli, P.; Carbone, A.; Chimenti, S.; Donato, S.; Girolomoni, G.; Micali, G.; et al. Prevalence and Risk Factors of Actinic Keratosis in Patients Attending Italian Dermatology Clinics. Eur. J. Dermatol. 2017, 27, 599–608. [Google Scholar] [CrossRef]
- Prezioso, C.; Brazzini, G.; Passerini, S.; Di Fabio, C.; Cosio, T.; Bernardini, S.; Campione, E.; Moens, U.; Pietropaolo, V.; Ciotti, M. Prevalence of MCPyV, HPyV6, HPyV7 and TSPyV in Actinic Keratosis Biopsy Specimens. Viruses 2022, 14, 427. [Google Scholar] [CrossRef]
- De Oliveira, E.C.V.; da Motta, V.R.V.; Pantoja, P.C.; Ilha, C.S.d.O.; Magalhães, R.F.; Galadari, H.; Leonardi, G.R. Actinic Keratosis—Review for Clinical Practice. Int. J. Dermatol. 2019, 58, 400–407. [Google Scholar] [CrossRef]
- Bernard, P.; Dupuy, A.; Sasco, A.; Brun, P.; Duru, G.; Nicoloyannis, N.; Grob, J.-J. Basal Cell Carcinomas and Actinic Keratoses Seen in Dermatological Practice in France: A Cross-Sectional Survey. Dermatology 2008, 216, 194–199. [Google Scholar] [CrossRef]
- Eder, J.; Prillinger, K.; Korn, A.; Geroldinger, A.; Trautinger, F. Prevalence of Actinic Keratosis among Dermatology Outpatients in Austria. Br. J. Dermatol. 2014, 171, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Flohil, S.C.; van der Leest, R.J.T.; Dowlatshahi, E.A.; Hofman, A.; de Vries, E.; Nijsten, T. Prevalence of Actinic Keratosis and Its Risk Factors in the General Population: The Rotterdam Study. J. Investig. Dermatol. 2013, 133, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Dirschka, T.; Gupta, G.; Micali, G.; Stockfleth, E.; Basset-Séguin, N.; Del Marmol, V.; Dummer, R.; Jemec, G.B.E.; Malvehy, J.; Peris, K.; et al. Real-World Approach to Actinic Keratosis Management: Practical Treatment Algorithm for Office-Based Dermatology. J. Dermatol. Treat. 2017, 28, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.A.; Tomenson, J.A.; Bothwell, J.; Friedmann, P.S. Prevalence of Solar Damage and Actinic Keratosis in a Merseyside Population. Br. J. Dermatol. 2000, 142, 1154–1159. [Google Scholar] [CrossRef]
- Yantsos, V.A.; Conrad, N.; Zabawski, E.; Cockerell, C.J. Incipient Intraepidermal Cutaneous Squamous Cell Carcinoma: A Proposal for Reclassifying and Grading Solar (Actinic) Keratoses. Semin. Cutan. Med. Surg. 1999, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Lisa Abernethy, M.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E., Jr. A Double-Blind, Vehicle-Controlled Study Evaluating Masoprocol Cream in the Treatment of Actinic Keratoses on the Head and Neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Kahl, P.; Majores, M.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Actinic Keratosis: Correlation between Clinical and Histological Classification Systems. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, S.; Jansen, M.H.E.; Nelemans, P.J.; Kessels, J.P.H.M.; Arits, A.H.M.M.; de Rooij, M.J.M.; Essers, B.A.B.; Quaedvlieg, P.J.F.; Kelleners-Smeets, N.W.J.; Mosterd, K. Risk of Invasive Cutaneous Squamous Cell Carcinoma after Different Treatments for Actinic Keratosis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 634. [Google Scholar] [CrossRef]
- Zalaudek, I.; Piana, S.; Moscarella, E.; Longo, C.; Zendri, E.; Castagnetti, F.; Pellacani, G.; Lallas, A.; Argenziano, G. Morphologic Grading and Treatment of Facial Actinic Keratosis. Clin. Dermatol. 2014, 32, 80–87. [Google Scholar] [CrossRef]
- Kamstrup, M.R.; Gniadecki, R.; Skovgaard, G.L. Putative Cancer Stem Cells in Cutaneous Malignancies. Exp. Dermatol. 2007, 16, 297–301. [Google Scholar] [CrossRef]
- Berman, B.; Perez, O.A.; Zell, D. Immunological Strategies to Fight Skin Cancer. Ski. Ther. Lett. 2006, 11, 1–7. [Google Scholar]
- Röwert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic Keratosis Is an Early in Situ Squamous Cell Carcinoma: A Proposal for Reclassification. Br. J. Dermatol. 2007, 156, 8–12. [Google Scholar] [CrossRef]
- Rongioletti, F. Actinic Keratoses: What Classification Is Useful to Predict the Risk of Progression? PROs and Cons. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 983–984. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Figueras, M.T. From Actinic Keratosis to Squamous Cell Carcinoma: Pathophysiology Revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Figueras, M.T.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic Keratosis with Atypical Basal Cells (AK I) Is the Most Common Lesion Associated with Invasive Squamous Cell Carcinoma of the Skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Figueras, M.T.; Saenz-Sardà, X.; Vargas, P.; Thompson, C.T.; Carrato, C.; Puig, L.; Ferrándiz, C.; Ariza, A. The Depth of Follicular Extension in Actinic Keratosis Correlates with the Depth of Invasion in Squamous Cell Carcinoma: Implication for Clinical Treatment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Philipp-Dormston, W.G.; Battistella, M.; Boussemart, L.; Di Stefani, A.; Broganelli, P.; Thoms, K.-M. Patient-Centered Management of Actinic Keratosis. Results of a Multi-Center Clinical Consensus Analyzing Non-Melanoma Skin Cancer Patient Profiles and Field-Treatment Strategies. J. Dermatol. Treat. 2020, 31, 576–582. [Google Scholar] [CrossRef]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of Care for the Management of Actinic Keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Cornejo, C.M.; Jambusaria-Pahlajani, A.; Willenbrink, T.J.; Schmults, C.D.; Arron, S.T.; Ruiz, E.S. Field Cancerization: Treatment. J. Am. Acad. Dermatol. 2020, 83, 719–730. [Google Scholar] [CrossRef]
- Dréno, B.; Cerio, R.; Dirschka, T.; Nart, I.; Lear, J.; Peris, K.; Casas, A.; Kaleci, S.; Pellacani, G.; Progressing Evidence in AK (PEAK) Working Group. A Novel Actinic Keratosis Field Assessment Scale for Grading Actinic Keratosis Disease Severity. Acta Derm. Venereol. 2017, 97, 1108–1113. [Google Scholar] [CrossRef]
- Figueras Nart, I.; Cerio, R.; Dirschka, T.; Dréno, B.; Lear, J.T.; Pellacani, G.; Peris, K.; Ruiz de Casas, A.; Progressing Evidence in AK (PEAK) Working Group. Defining the Actinic Keratosis Field: A Literature Review and Discussion. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Ferrandiz, C.; Grob, J.J.; Leigh, I.; Pehamberger, H.; Kerl, H. Development of a Treatment Algorithm for Actinic Keratoses: A European Consensus. Eur. J. Dermatol. 2008, 18, 651–659. [Google Scholar] [PubMed]
- McIntyre, W.J.; Downs, M.R.; Bedwell, S.A. Treatment Options for Actinic Keratoses. Am. Fam. Physician 2007, 76, 667–676. [Google Scholar]
- Bubna, A. Imiquimod—Its Role in the Treatment of Cutaneous Malignancies. Indian J. Pharmacol. 2015, 47, 354. [Google Scholar] [CrossRef]
- Swanson, N.; Smith, C.C.; Kaur, M.; Goldenberg, G. Imiquimod 2.5% and 3.75% for the Treatment of Actinic Keratoses: Two Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies. J. Drugs Dermatol. 2014, 13, 1278–1282. [Google Scholar]
- Alomar, A.; Bichel, J.; McRae, S. Vehicle-Controlled, Randomized, Double-Blind Study to Assess Safety and Efficacy of Imiquimod 5% Cream Applied Once Daily 3 Days per Week in One or Two Courses of Treatment of Actinic Keratoses on the Head: Imiquimod 5% Cream—Safety and Efficacy Study. Br. J. Dermatol. 2007, 157, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Korman, N.; Moy, R.; Ling, M.; Matheson, R.; Smith, S.; McKane, S.; Lee, J.H. Dosing with 5% Imiquimod Cream 3 Times per Week for the Treatment of Actinic Keratosis: Results of Two Phase 3, Randomized, Double-Blind, Parallel-Group, Vehicle-Controlled Trials. Arch. Dermatol. 2005, 141, 467–473. [Google Scholar] [CrossRef]
- Serra-Guillén, C.; Nagore, E.; Llombart, B.; Sanmartín, O.; Requena, C.; Calomarde, L.; Guillén, C. Tratamiento con imiquimod al 5% durante 12 días para las queratosis actínicas: Estudio de la eficacia y la reacción local. Actas Dermosifiliogr. 2018, 109, 248–253. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Schwartz, R.A. Topical Pharmacotherapy for Skin Cancer. J. Am. Acad. Dermatol. 2014, 70, 979.e1–979.e12. [Google Scholar] [CrossRef]
- Peris, K.; Calzavara-Pinton, P.G.; Neri, L.; Girolomoni, G.; Malara, G.; Parodi, A.; Piaserico, S.; Rossi, R.; Pellacani, G. Italian Expert Consensus for the Management of Actinic Keratosis in Immunocompetent Patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1077–1084. [Google Scholar] [CrossRef]
- Ezzedine, K.; Painchault, C.; Brignone, M. Use of Complete Clearance for Assessing Treatment Efficacy for 5-Fluorouracil Interventions in Actinic Keratoses: How Baseline Lesion Count Can Impact This Outcome. J. Mark. Access Health Policy 2020, 8, 1829884. [Google Scholar] [CrossRef]
- Nelson, C.; Rigel, D.; Smith, S.; Swanson, N.; Wolf, J. Phase IV, Open-Label Assessment of the Treatment of Actinic Keratosis with 3.0% Diclofenac Sodium Topical Gel (Solaraze). J. Drugs Dermatol. 2004, 3, 401–407. [Google Scholar] [PubMed]
- Kempers, S.; DuBois, J.; Forman, S.; Poon, A.; Cutler, E.; Wang, H.; Cutler, D.; Fang, J.; Kwan, R. Tirbanibulin Ointment 1% as a Novel Treatment for Actinic Keratosis: Phase 1 and 2 Results. J. Drugs Dermatol. 2020, 19, 1093–1100. [Google Scholar] [CrossRef]
- Ianhez, M.; Fleury Junior, L.F.F.; Miot, H.A.; Bagatin, E. Retinoids for Prevention and Treatment of Actinic Keratosis. An. Bras. Dermatol. 2013, 88, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Peck, G.L. Topical Tretinoin in Actinic Keratosis and Basal Cell Carcinoma. J. Am. Acad. Dermatol. 1986, 15, 829–835. [Google Scholar] [CrossRef]
- Kang, S.; Goldfarb, M.T.; Weiss, J.S.; Metz, R.D.; Hamilton, T.A.; Voorhees, J.J.; Griffiths, C.E.M. Assessment of Adapalene Gel for the Treatment of Actinic Keratoses and Lentigines: A Randomized Trial. J. Am. Acad. Dermatol. 2003, 49, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, S.; Sharma, V.K. Successful Treatment of Multiple Premalignant and Malignant Lesions in Arsenical Keratosis with a Combination of Acitretin and Intralesional 5-fluorouracil. J. Dermatol. 2003, 30, 730–734. [Google Scholar] [CrossRef]
- Galitzer, B.I. Photodynamic Therapy for Actinic Keratoses of the Upper Extremities Using 10% Aminolevulinic Acid Gel, Red Light, and Adapalene Pretreatment. J. Clin. Aesthet. Dermatol. 2021, 14, 19. [Google Scholar]
- Markham, A.; Duggan, S. Tirbanibulin: First Approval. Drugs 2021, 81, 509–513. [Google Scholar] [CrossRef]
- Blauvelt, A.; Kempers, S.; Lain, E.; Schlesinger, T.; Tyring, S.; Forman, S.; Ablon, G.; Martin, G.; Wang, H.; Cutler, D.L.; et al. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N. Engl. J. Med. 2021, 384, 512–520. [Google Scholar] [CrossRef]
- Schlesinger, T.; Stockfleth, E.; Grada, A.; Berman, B. Tirbanibulin for Actinic Keratosis: Insights into the Mechanism of Action. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2495–2506. [Google Scholar] [CrossRef]
- Campione, E.; Rivieccio, A.; Gaeta Shumak, R.; Costanza, G.; Cosio, T.; Lambiase, S.; Garofalo, V.; Artosi, F.; Lozzi, F.; Freni, C.; et al. Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses. Pharmaceuticals 2023, 16, 1686. [Google Scholar] [CrossRef]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual Mechanism of Action of Ingenol Mebutate Gel for Topical Treatment of Actinic Keratoses: Rapid Lesion Necrosis Followed by Lesion-Specific Immune Response. J. Am. Acad. Dermatol. 2012, 66, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Dodds, A.; Chia, A.; Shumack, S. Actinic Keratosis: Rationale and Management. Dermatol. Ther. 2014, 4, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Samrao, A.; Cockerell, C.J. Pharmacotherapeutic Management of Actinic Keratosis: Focus on Newer Topical Agents. Am. J. Clin. Dermatol. 2013, 14, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.-M.; Bichel, J.; Ortonne, J.-P.; Stockfleth, E.; Lee, J.; Meng, T.-C. A Phase II Dose-Ranging Study of Topical Resiquimod to Treat Actinic Keratosis. Br. J. Dermatol. 2008, 159, 205–210. [Google Scholar] [CrossRef]
- Noels, E.C.; Hollestein, L.M.; Egmond, S.; Lugtenberg, M.; Nistelrooij, L.P.J.; Bindels, P.J.E.; Lei, J.; Stern, R.S.; Nijsten, T.; Wakkee, M. Healthcare Utilization and Management of Actinic Keratosis in Primary and Secondary Care: A Complementary Database Analysis. Br. J. Dermatol. 2019, 181, 544–553. [Google Scholar] [CrossRef]
- Heppt, M.V.; Steeb, T.; Ruzicka, T.; Berking, C. Cryosurgery Combined with Topical Interventions for Actinic Keratosis: A Systematic Review and Meta-analysis. Br. J. Dermatol. 2019, 180, 740–748. [Google Scholar] [CrossRef]
- Kaufmann, R.; Spelman, L.; Weightman, W.; Reifenberger, J.; Szeimies, R.-M.; Verhaeghe, E.; Kerrouche, N.; Sorba, V.; Villemagne, H.; Rhodes, L.E. Multicentre Intraindividual Randomized Trial of Topical Methyl Aminolaevulinate–Photodynamic Therapy vs. Cryotherapy for Multiple Actinic Keratoses on the Extremities. Br. J. Dermatol. 2008, 158, 994–999. [Google Scholar] [CrossRef]
- Simon, J.-C.; Dominicus, R.; Karl, L.; Rodríguez, R.; Willers, C.; Dirschka, T. A Prospective Randomized Exploratory Study Comparing the Efficacy of Once-daily Topical 0.5% 5-fluorouracil in Combination with 10.0% Salicylic Acid (5-FU/SA) vs. Cryosurgery for the Treatment of Hyperkeratotic Actinic Keratosis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 881–889. [Google Scholar] [CrossRef]
- Zane, C.; Facchinetti, E.; Rossi, M.T.; Specchia, C.; Ortel, B.; Calzavara-Pinton, P. Cryotherapy Is Preferable to Ablative CO2Laser for the Treatment of Isolated Actinic Keratoses of the Face and Scalp: A Randomized Clinical Trial. Br. J. Dermatol. 2014, 170, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Krawtchenko, N.; Roewert-Huber, J.; Ulrich, M.; Mann, I.; Sterry, W.; Stockfleth, E. A Randomised Study of Topical 5% Imiquimod vs. Topical 5-Fluorouracil vs. Cryosurgery in Immunocompetent Patients with Actinic Keratoses: A Comparison of Clinical and Histological Outcomes Including 1-Year Follow-up: IMQ vs. 5-FU vs. CRYO in AK. Br. J. Dermatol. 2007, 157, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Berker, D.; McGregor, J.M.; Mohd Mustapa, M.F.; Exton, L.S.; Hughes, B.R.; McHenry, P.M.; Gibbon, K.; Buckley, D.A.; Nasr, I.; Duarte Williamson, C.E.; et al. British Association of Dermatologists’ Guidelines for the Care of Patients with Actinic Keratosis 2017. Br. J. Dermatol. 2017, 176, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Rubel, D.M.; Spelman, L.; Murrell, D.F.; See, J.-A.; Hewitt, D.; Foley, P.; Bosc, C.; Kerob, D.; Kerrouche, N.; Wulf, H.C.; et al. Daylight Photodynamic Therapy with Methyl Aminolevulinate Cream as a Convenient, Similarly Effective, Nearly Painless Alternative to Conventional Photodynamic Therapy in Actinic Keratosis Treatment: A Randomized Controlled Trial. Br. J. Dermatol. 2014, 171, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Taub, A.F.; Garretson, C.B. A Randomized, Blinded, Bilateral Intraindividual, Vehicle-Controlled Trial of the Use of Photodynamic Therapy with 5-Aminolevulinic Acid and Blue Light for the Treatment of Actinic Keratoses of the Upper Extremities. J. Drugs Dermatol. 2011, 10, 1049–1056. [Google Scholar] [PubMed]
- Dianzani, C.; Conforti, C.; Giuffrida, R.; Corneli, P.; di Meo, N.; Farinazzo, E.; Moret, A.; Magaton Rizzi, G.; Zalaudek, I. Current Therapies for Actinic Keratosis. Int. J. Dermatol. 2020, 59, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Wiegell, S.R.; Haedersdal, M.; Eriksen, P.; Wulf, H.C. Photodynamic Therapy of Actinic Keratoses with 8% and 16% Methyl Aminolaevulinate and Home-Based Daylight Exposure: A Double-Blinded Randomized Clinical Trial. Br. J. Dermatol. 2009, 160, 1308–1314. [Google Scholar] [CrossRef]
- Lerche, C.; Heerfordt, I.; Heydenreich, J.; Wulf, H. Alternatives to Outdoor Daylight Illumination for Photodynamic Therapy—Use of Greenhouses and Artificial Light Sources. Int. J. Mol. Sci. 2016, 17, 309. [Google Scholar] [CrossRef]
- O’Mahoney, P.; Haigh, N.; Wood, K.; Brown, C.T.A.; Ibbotson, S.; Eadie, E. A Novel Light Source with Tuneable Uniformity of Light Distribution for Artificial Daylight Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2018, 23, 144–150. [Google Scholar] [CrossRef]
- Lawrence, N.; Cox, S.E.; Cockerell, C.J.; Freeman, R.G.; Cruz, P.D. A Comparison of the Efficacy and Safety of Jessner’s Solution and 35% Trichloroacetic Acid vs 5% Fluorouracil in the Treatment of Widespread Facial Actinic Keratoses. Arch. Dermatol. 1995, 131, 176–181. [Google Scholar] [CrossRef]
- Witheiler, D.D.; Lawrence, N.; Cox, S.E.; Cruz, C.; Cockerell, C.J.; Freemen, R.G. Long-Term Efficacy and Safety of Jessnerʼs Solution and 35% Trichloroacetic Acid vs 5% Fluorouracil in the Treatment of Widespread Facial Actinic Keratoses. Dermatol. Surg. 1997, 23, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Steeb, T.; Koch, E.A.T.; Wessely, A.; Wiest, L.G.; Schmitz, L.; Berking, C.; Heppt, M.V. Chemical Peelings for the Treatment of Actinic Keratosis: A Systematic Review and Meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.; Shah, M.; Pon, K.; Alavi, A. Laser Resurfacing Monotherapy for the Treatment of Actinic Keratosis. J. Cutan. Med. Surg. 2021, 25, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.; Zhou, S.; Bobotsis, R.; Lynde, C. Comparison of the Treatment Guidelines for Actinic Keratosis: A Critical Appraisal and Review. J. Cutan. Med. Surg. 2017, 21, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Sinz, C.; Tschandl, P.; Rosendahl, C.; Akay, B.N.; Argenziano, G.; Blum, A.; Braun, R.P.; Cabo, H.; Gourhant, J.-Y.; Kreusch, J.; et al. Accuracy of Dermatoscopy for the Diagnosis of Nonpigmented Cancers of the Skin. J. Am. Acad. Dermatol. 2017, 77, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, C.; Tschandl, P.; Cameron, A.; Kittler, H. Diagnostic Accuracy of Dermatoscopy for Melanocytic and Nonmelanocytic Pigmented Lesions. J. Am. Acad. Dermatol. 2011, 64, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, D.; Theofili, M.; Zafeiropoulou, T.; Lallas, A.; Apalla, Z.; Zaras, A.; Liopyris, K.; Pappa, G.; Polychronaki, E.; Kousta, F.; et al. Dermoscopy of Actinic Keratosis: Is There a True Differentiation between Non-Pigmented and Pigmented Lesions? J. Clin. Med. 2023, 12, 1063. [Google Scholar] [CrossRef]
- Huerta-Brogeras, M.; Olmos, O.; Borbujo, J.; Hernández-Núñez, A.; Castaño, E.; Romero-Maté, A.; Martínez-Sánchez, D.; Martínez-Morán, C. Validation of Dermoscopy as a Real-Time Noninvasive Diagnostic Imaging Technique for Actinic Keratosis. Arch. Dermatol. 2012, 148, 1159. [Google Scholar] [CrossRef]
- Draghici, C.; Vajaitu, C.; Solomon, I.; Voiculescu, V.M.; Popa, M.I.; Lupu, M. The Dermoscopic Rainbow Pattern—A Review of the Literature. Acta Dermatovenerol. Croat. 2019, 27, 111–115. [Google Scholar]
- Xiaoqin, Y.; Chan, H.; Long, W.; Yuting, X.; Keyal, U.; Guolong, Z.; Peiru, W.; Xiuli, W. Dermoscopic Monitoring for Treatment and Follow-up of Actinic Keratosis with 5-Aminolaevulinic Acid Photodynamic Therapy. Technol. Cancer Res. Treat. 2018, 17, 153303381882009. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Apalla, Z.; Sotiriou, E.; Papageorgiou, C.; Lazaridou, E.; Vakirlis, S.; Ioannides, D.; Lallas, A. The Limitations of Dermoscopy: False-positive and False-negative Tumours. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Liebman, T.N.; Soriano, R.P.; Dusza, S.W.; Halpern, A.C.; Marghoob, A.A. One-Year Follow-up of Dermoscopy Education on the Ability of Medical Students to Detect Skin Cancer. Dermatology 2013, 226, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, G.; Soyer, H.P.; Argenziano, G.; Talamini, R.; Barbati, R.; Bianchi, L.; Campione, E.; Carboni, I.; Carrozzo, A.M.; Chimenti, M.S.; et al. Diagnosis of Pigmented Skin Lesions by Dermoscopy: Web-Based Training Improves Diagnostic Performance of Non-Experts. Br. J. Dermatol. 2003, 148, 698–702. [Google Scholar] [CrossRef]
- Lallas, A.; Argenziano, G.; Zendri, E.; Moscarella, E.; Longo, C.; Grenzi, L.; Pellacani, G.; Zalaudek, I. Update on Non-Melanoma Skin Cancer and the Value of Dermoscopy in Its Diagnosis and Treatment Monitoring. Expert Rev. Anticancer Ther. 2013, 13, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Stockfleth, E.; Roewert-Huber, J.; Astner, S. Noninvasive Diagnostic Tools for Nonmelanoma Skin Cancer: Noninvasive Diagnostis of Skin Cancer. Br. J. Dermatol. 2007, 157, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Malciu, A.M.; Lupu, M.; Voiculescu, V.M. Artificial Intelligence-Based Approaches to Reflectance Confocal Microscopy Image Analysis in Dermatology. J. Clin. Med. 2022, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Saleh, D.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Patel, L.; Matin, R.N.; O’Sullivan, C.; et al. Reflectance Confocal Microscopy for Diagnosing Keratinocyte Skin Cancers in Adults. Cochrane Libr. 2018, 88–91. [Google Scholar] [CrossRef]
- Lupu, M.; Voiculescu, V.M.; Caruntu, A.; Tebeica, T.; Caruntu, C. Preoperative Evaluation through Dermoscopy and Reflectance Confocal Microscopy of the Lateral Excision Margins for Primary Basal Cell Carcinoma. Diagnostics 2021, 11, 120. [Google Scholar] [CrossRef]
- Rishpon, A.; Kim, N.; Scope, A.; Porges, L.; Oliviero, M.C.; Braun, R.P.; Marghoob, A.A.; Fox, C.A.; Rabinovitz, H.S. Reflectance Confocal Microscopy Criteria for Squamous Cell Carcinomas and Actinic Keratoses. Arch. Dermatol. 2009, 145, 766–772. [Google Scholar] [CrossRef]
- Franceschini, C.; Persechino, F.; Ardigò, M. In Vivo Reflectance Confocal Microscopy in General Dermatology: How to Choose the Right Indication. Dermatol. Pract. Concept. 2020, 10, e2020032. [Google Scholar] [CrossRef]
- Casari, A.; Chester, J.; Pellacani, G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Ulrich, M.; Casari, A.; Prow, T.W.; Cannillo, F.; Benati, E.; Losi, A.; Cesinaro, A.M.; Longo, C.; Argenziano, G.; et al. Grading Keratinocyte Atypia in Actinic Keratosis: A Correlation of Reflectance Confocal Microscopy and Histopathology. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2216–2221. [Google Scholar] [CrossRef]
- Ulrich, M.; Maltusch, A.; Rius-Diaz, F.; Röwert-Huber, J.; González, S.; Sterry, W.; Stockfleth, E.; Astner, S. Clinical Applicability of in Vivo Reflectance Confocal Microscopy for the Diagnosis of Actinic Keratoses. Dermatol. Surg. 2008, 34, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Zalaudek, I.; Welzel, J. Shining into the White. Dermatol. Clin. 2016, 34, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Lange-Asschenfeldt, S.; González, S. In Vivo Reflectance Confocal Microscopy for Early Diagnosis of Nonmelanoma Skin Cancer. Actas Dermosifiliogr. 2012, 103, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Giacomel, J.; Argenziano, G.; Hofmann-Wellenhof, R.; Micantonio, T.; Di Stefani, A.; Oliviero, M.; Rabinovitz, H.; Soyer, H.P.; Peris, K. Dermoscopy of Facial Nonpigmented Actinic Keratosis: Dermoscopy of Facial Nonpigmented AK. Br. J. Dermatol. 2006, 155, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Krueger-Corcoran, D.; Roewert-Huber, J.; Sterry, W.; Stockfleth, E.; Astner, S. Reflectance Confocal Microscopy for Noninvasive Monitoring of Therapy and Detection of Subclinical Actinic Keratoses. Dermatology 2010, 220, 15–24. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Timchik, T.; Hunger, R.E. In Vivo Confocal Microscopy Efficacy Assessment of Daylight Photodynamic Therapy in Actinic Keratosis Patients. Br. J. Dermatol. 2016, 175, 375–381. [Google Scholar] [CrossRef]
- Braghiroli, N.F.; Sugerik, S.; de Freitas, L.A.R.; Oliviero, M.; Rabinovitz, H. The Skin through Reflectance Confocal Microscopy—Historical Background, Technical Principles, and Its Correlation with Histopathology. An. Bras. Dermatol. 2022, 97, 697–703. [Google Scholar] [CrossRef]
- Ishioka, P.; Maia, M.; Rodrigues, S.B.; Lellis, R.F.; Hirata, S.H. In Vivo Confocal Laser Microscopy for Monitoring of Actinic Keratosis Treatment: A Comparison with Histopathologic Assessment after Treatment with Topical 5% 5-fluorouracil. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1155–1163. [Google Scholar] [CrossRef]
- Pasquali, P.; Segurado-Miravalles, G.; Baldi, A.; Vincenzi, B.; Bizzi, S.; Bonavenia, R.; Gonzalez, S. Monitoring Sequential Treatment of Actinic Keratosis Using Post-processed Images: Ingenol Mebutate and Cryosurgery. Photodermatol. Photoimmunol. Photomed. 2019, 35, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Benati, E.; Longhitano, S.; Pampena, R.; Mirra, M.; Raucci, M.; Pellacani, G.; Longo, C. Digital Follow-up by Means of Dermatoscopy and Reflectance Confocal Microscopy of Actinic Keratosis Treated with Imiquimod 3.75% Cream. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Nascimento Cavalleiro Macedo Mota, A.; Piñeiro-Maceira, J.M.; Baptista Barcaui, C. Evaluation of Diagnostic Criteria of Actinic Keratosis through Reflectance Confocal Microscopy. Skin Res. Technol. 2020, 26, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ahlgrimm-Siess, V.; Laimer, M.; Rabinovitz, H.S.; Oliviero, M.; Hofmann-Wellenhof, R.; Marghoob, A.A.; Scope, A. Confocal Microscopy in Skin Cancer. Curr. Dermatol. Rep. 2018, 7, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From Keratinocyte to Cancer: The Pathogenesis and Modeling of Cutaneous Squamous Cell Carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Themstrup, L.; Jemec, G.B. Optical Coherence Tomography in Dermatology. G. Ital. Dermatol. Venereol. 2015, 150, 603–615. [Google Scholar]
- Boone, M.; Jemec, G.B.E.; Del Marmol, V. High-definition Optical Coherence Tomography Enables Visualization of Individual Cells in Healthy Skin: Comparison to Reflectance Confocal Microscopy. Exp. Dermatol. 2012, 21, 740–744. [Google Scholar] [CrossRef]
- Mogensen, M.; Joergensen, T.M.; Nürnberg, B.M.; Morsy, H.A.; Thomsen, J.B.; Thrane, L.; Jemec, G.B.E. Assessment of Optical Coherence Tomography Imaging in the Diagnosis of Non-Melanoma Skin Cancer and Benign Lesions versus Normal Skin: Observer-Blinded Evaluation by Dermatologists and Pathologists. Dermatol. Surg. 2009, 35, 965–972. [Google Scholar] [CrossRef]
- Korde, V.R.; Bonnema, G.T.; Xu, W.; Krishnamurthy, C.; Ranger-Moore, J.; Saboda, K.; Slayton, L.D.; Salasche, S.J.; Warneke, J.A.; Alberts, D.S.; et al. Using Optical Coherence Tomography to Evaluate Skin Sun Damage and Precancer. Lasers Surg. Med. 2007, 39, 687–695. [Google Scholar] [CrossRef]
- Barton, J.K.; Gossage, K.W.; Xu, W.; Ranger-Moore, J.R.; Saboda, K.; Brooks, C.A.; Duckett, L.D.; Salasche, S.J.; Warneke, J.A.; Alberts, D.S. Investigating Sun-Damaged Skin and Actinic Keratosis with Optical Coherence Tomography: A Pilot Study. Technol. Cancer Res. Treat. 2003, 2, 525–535. [Google Scholar] [CrossRef]
- Banzhaf, C.A.; Themstrup, L.; Ring, H.C.; Mogensen, M.; Jemec, G.B.E. Optical Coherence Tomography Imaging of Non-melanoma Skin Cancer Undergoing Imiquimod Therapy. Skin Res. Technol. 2014, 20, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.L.M.; Marneffe, A.; Suppa, M.; Miyamoto, M.; Alarcon, I.; Hofmann-Wellenhof, R.; Malvehy, J.; Pellacani, G.; Del Marmol, V. High-definition Optical Coherence Tomography Algorithm for the Discrimination of Actinic Keratosis from Normal Skin and from Squamous Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Braun-Falco, M.; Laubender, R.P.; Ruzicka, T.; Berking, C. Actinic Keratosis in the En-Face and Slice Imaging Mode of High-Definition Optical Coherence Tomography and Comparison with Histology: Actinic Keratosis in High-Definition Optical Coherence Tomography. Br. J. Dermatol. 2013, 168, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.; Whang, T.B.; Bard, R.L.; Marmur, E.S. Ultrasound in Dermatology: Principles and Applications. J. Am. Acad. Dermatol. 2012, 67, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Tada, M.; Miki, Y. Echographic Evaluation of Nodular Lesions of the Skin. J. Dermatol. 1983, 10, 221–227. [Google Scholar] [CrossRef]
- Stiller, M.J.; Gropper, C.A.; Shupack, J.L.; Lizzi, F.; Driller, J.; Rorke, M. Diagnostic Ultrasound in Dermatology: Current Uses and Future Potential. Cutis 1994, 53, 44–48. [Google Scholar] [PubMed]
- Cammarota, T.; Pinto, F.; Magliaro, A.; Sarno, A. Current Uses of Diagnostic High-Frequency US in Dermatology. Eur. J. Radiol. 1998, 27, S215–S223. [Google Scholar] [CrossRef]
- Lassau, N. Imaging of Melanoma: Usefulness of Ultrasonography before and after Contrast Injection for Diagnosis and Early Evaluation of Treatment. Clin. Cosmet. Investig. Dermatol. 2011, 4, 1–6. [Google Scholar] [CrossRef][Green Version]
- Schmid-Wendtner, M.-H.; Burgdorf, W. Ultrasound Scanning in Dermatology. Arch. Dermatol. 2005, 141, 217–224. [Google Scholar] [CrossRef]
- Badea, R.; Crişan, M.; Lupşor, M.; Fodor, L. Diagnosis and Characterization of Cutaneous Tumors Using Combined Ultrasonographic Procedures (Conventional and High Resolution Ultrasonography). Med. Ultrason. 2010, 12, 317–322. [Google Scholar]
- Csány, G.; Szalai, K.; Gyöngy, M. A Real-Time Data-Based Scan Conversion Method for Single Element Ultrasound Transducers. Ultrasonics 2019, 93, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Csány, G.; Gergely, L.H.; Kiss, N.; Szalai, K.; Lőrincz, K.; Strobel, L.; Csabai, D.; Hegedüs, I.; Marosán-Vilimszky, P.; Füzesi, K.; et al. Preliminary Clinical Experience with a Novel Optical–Ultrasound Imaging Device on Various Skin Lesions. Diagnostics 2022, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Carreno, L.; Morales, C. Skin cancers: The primary tumor. In Dermatologic Ultrasound with Clinical and Histologic Correlations; Wortsman, X., Ed.; Springer: New York, NY, USA, 2013; pp. 249–282. [Google Scholar]
- Schmid-Wendtner, M.-H.; Dill-Müller, D. Ultrasound Technology in Dermatology. Semin. Cutan. Med. Surg. 2008, 27, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. “Field Cancerization” in Oral Stratified Squamous Epithelium. Clinical Implications of Multicentric Origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Torezan, L.A.R.; Festa-Neto, C. Cutaneous Field Cancerization: Clinical, Histopathological and Therapeutic Aspects. An. Bras. Dermatol. 2013, 88, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.A.; Mao, L.; Hruban, R.H.; Boyle, J.O.; Eby, Y.J.; Koch, W.M.; Goodman, S.N.; Sidransky, D. Molecular Assessment of Histopathological Staging in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 1995, 332, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field Cancerization: Definition, Epidemiology, Risk Factors, and Outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef]
- Dakubo, G.D.; Jakupciak, J.P.; Birch-Machin, M.A.; Parr, R.L. Clinical Implications and Utility of Field Cancerization. Cancer Cell Int. 2007, 7, 2. [Google Scholar] [CrossRef]
- Orte Cano, C.; Suppa, M.; del Marmol, V. Where Artificial Intelligence Can Take Us in the Management and Understanding of Cancerization Fields. Cancers 2023, 15, 5264. [Google Scholar] [CrossRef]
- Morton, S.; Muir, J. Field Cancerization in the Skin: Past Errors Repeated. J. Am. Acad. Dermatol. 2021, 85, e41. [Google Scholar] [CrossRef]
- Ulrich, M.; Alarcon, I.; Malvehy, J.; Puig, S. In Vivo Reflectance Confocal Microscopy Characterization of Field-Directed 5-Fluorouracil 0.5%/Salicylic Acid 10% in Actinic Keratosis. Dermatology 2015, 230, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Agozzino, M.; Russo, T.; Franceschini, C.; Mazzilli, S.; Garofalo, V.; Campione, E.; Bianchi, L.; Milani, M.; Argenziano, G. Effects of Topical Piroxicam and Sun Filters in Actinic Keratosis Evolution and Field Cancerization: A Two-Center, Assessor-Blinded, Clinical, Confocal Microscopy and Dermoscopy Evaluation Trial. Curr. Med. Res. Opin. 2019, 35, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
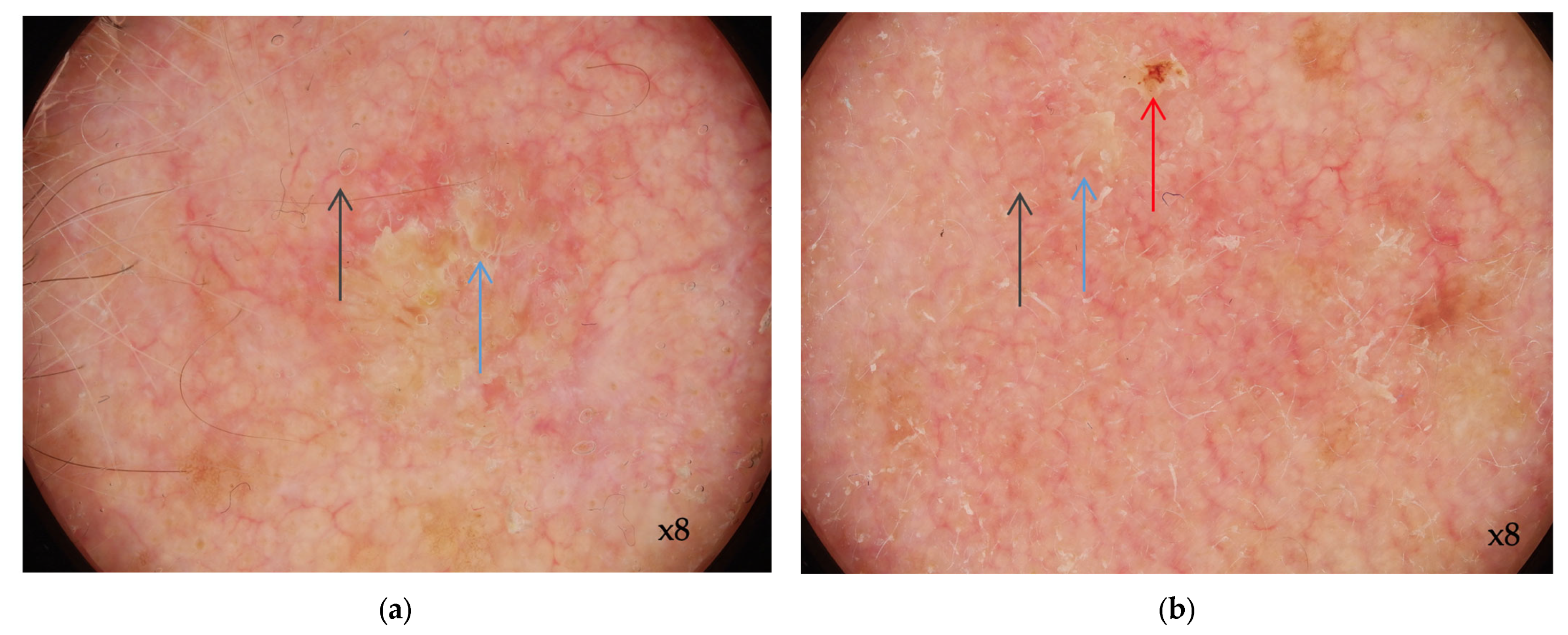
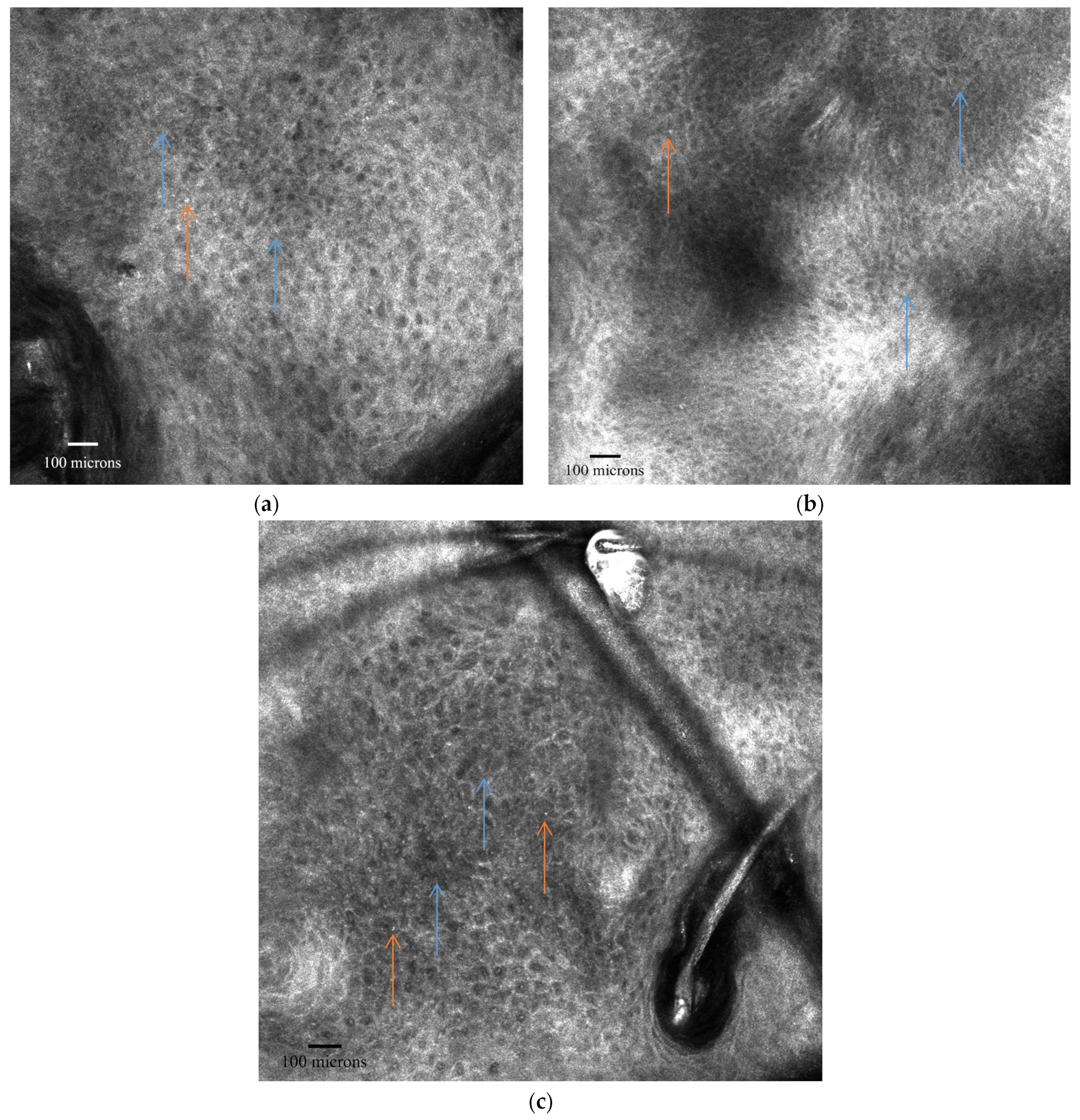


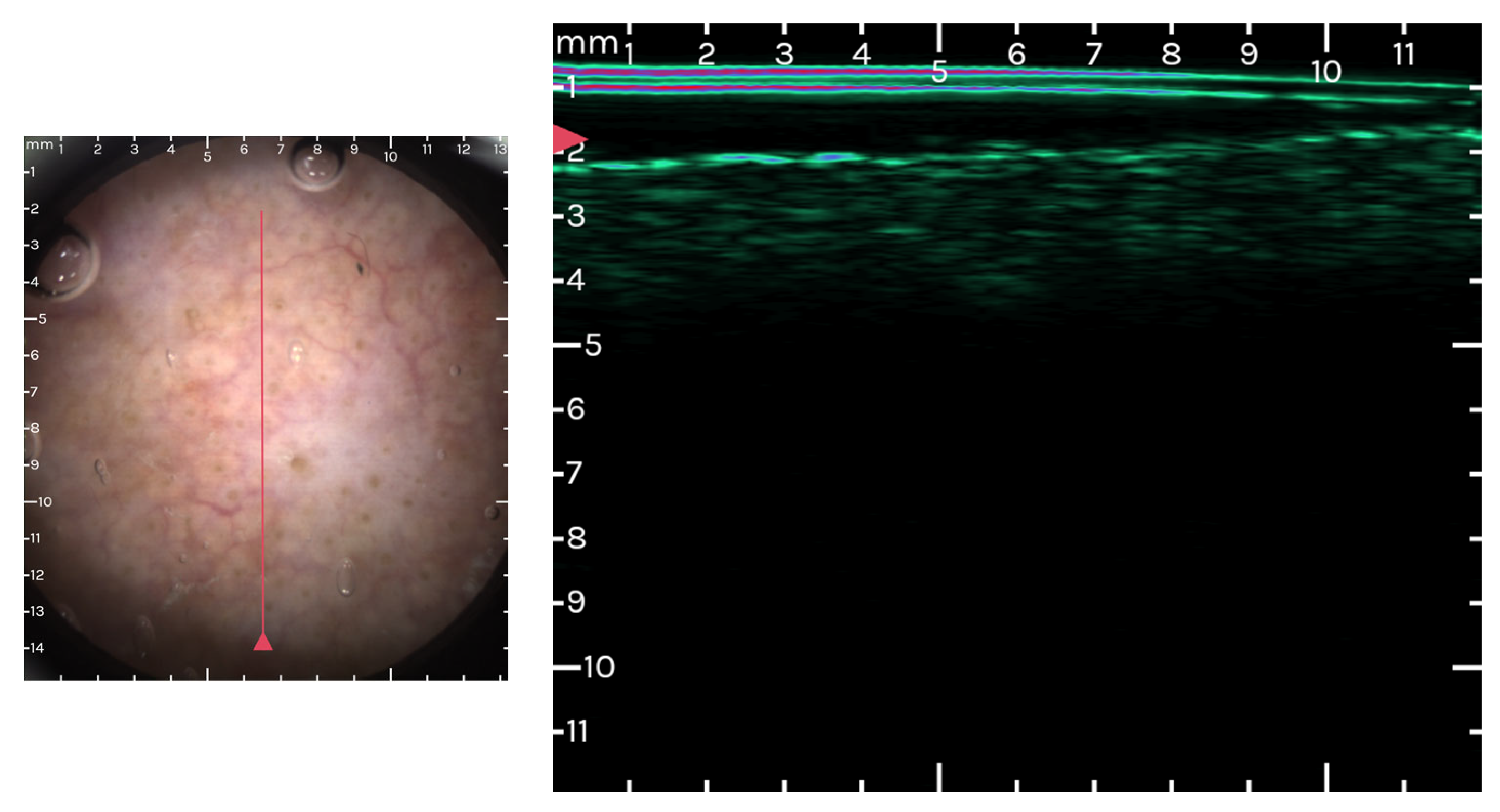
| Progression Stage | Description | Clinical Correlation |
|---|---|---|
| AK I | Initial phase of in situ SCC | Grade 1 — slightly palpable AK lesion (better felt than seen) |
| AK II | Intermediate phase of in situ SCC | Grade 2 — moderately thick AK lesion (easily felt and seen) |
| AK III | Advanced phase of in situ SCC | Grade 3 — Very thick, hyperkeratotic, and/or obvious AK lesion |
| Parameter | Description |
|---|---|
| I (induration/inflammation) | Presence of hardening or inflammation |
| D (diameter > 1 cm) | Lesion diameter larger than 1 cm |
| R (rapid enlargement) | Quick increase in lesion size |
| B (bleeding) | Occurrence of bleeding |
| E (erythema) | Redness of the skin |
| U (ulceration) | Formation of ulcers |
| Dermoscopic Criteria | Description | Diagnostic |
|---|---|---|
| Erythema | areas of redness without a specific structure, lacking any areas of lighter pigmentation or distinct shape | AK I |
| Red pseudo-network | red regions without a defined structure, interlaced with small, round white spots that create a network-like pattern; these small white spots represent the openings of hair follicles | AK I |
| Strawberry pattern | red areas lacking a distinct structure, intermingled with round, target-like formations featuring an inner yellow and outer white ring that resemble strawberries; these target-like areas are associated with hair follicle openings filled with keratin | AK II |
| Rosettes | four white dots positioned in a diamond shape, creating a pattern like a four-leaf clover | AK I-II |
| Surface Scale | opaque areas ranging in color from yellow to light brown, lacking a defined structure and exhibiting a scaly or keratotic appearance, which occupy only a small portion of the tumor’s surface | AK III (in situ SCC) |
| Targetoid hair follicles with whitish halo | circular formations of varying sizes, featuring a center that is yellow to light brown and structureless, surrounded by a white, structureless outer ring; this pattern is indicative of keratotic plugs within the hair follicle openings in the skin | AK II SCC |
| Red starburst | red lines or hairpin-shaped vessels, lacking distinct structure and arranged radially, surrounding a yellowish white, structureless, scaly center, creating a pattern reminiscent of a starburst | AK II AK III (in situ SCC) |
| Erosions | irregularly spaced small areas ranging in color from orange to red and red-brown, without a defined structure; these areas are indicative of superficial bleeding and are typically found in conjunction with yellow, opaque structures | AK III (in situ SCC) SCC |
| Linear, wavy vessels | red structures that are linear or slightly curved, irregular in shape, size, and distribution | NPAK |
| Dotted vessels Glomerular vessels | closely packed, small red dots with a complex morphology, larger than typical dotted vessels and frequently grouped in clusters, representing a variation of the dotted vessel theme | AK III (in situ SCC) |
| Hairpin vessels | twisting and bending vascular loops, often encircled by a whitish halo, typically observed in keratinizing tumors | SCC |
| Rainbow pattern | lines with multiple colors (from blue to red) | AK SCC BCC |
| Skin Layer | RCM Features of AK |
|---|---|
| Stratum Corneum | Superficial disruption with large single keratinocytes Nucleated cells (parakeratosis) Small bright cells with dark centers (neutrophils) |
| Stratum Granulosum/Spinosum | Atypical honeycomb pattern: variations in cell size and morphology Broadened honeycomb pattern: areas with broadened and blurred intercellular connections Loss of regular epidermal architecture |
| Dermis | Slightly dilated blood vessels Solar elastosis: moderately refractive lace-like material adjacent to collagen bundles |
| AK Grade | Description |
|---|---|
| Grade 1 | Focal areas of atypical, honeycombed pattern at the stratum spinosum level, mixed with areas of typical honeycombed pattern. |
| Grade 2 | Diffuse atypia of keratinocytes in both the stratum spinosum and granulosum, with marked variation in cell sizes and shapes. |
| Grade 3 | Markedly atypical, honeycombed pattern with partial disruption of normal epidermal layers (disarranged pattern), wide variability in keratinocyte size and shape, and irregular intercellular connections. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soare, C.; Cozma, E.C.; Celarel, A.M.; Rosca, A.M.; Lupu, M.; Voiculescu, V.M. Digitally Enhanced Methods for the Diagnosis and Monitoring of Treatment Responses in Actinic Keratoses: A New Avenue in Personalized Skin Care. Cancers 2024, 16, 484. https://doi.org/10.3390/cancers16030484
Soare C, Cozma EC, Celarel AM, Rosca AM, Lupu M, Voiculescu VM. Digitally Enhanced Methods for the Diagnosis and Monitoring of Treatment Responses in Actinic Keratoses: A New Avenue in Personalized Skin Care. Cancers. 2024; 16(3):484. https://doi.org/10.3390/cancers16030484
Chicago/Turabian StyleSoare, Cristina, Elena Codruta Cozma, Ana Maria Celarel, Ana Maria Rosca, Mihai Lupu, and Vlad Mihai Voiculescu. 2024. "Digitally Enhanced Methods for the Diagnosis and Monitoring of Treatment Responses in Actinic Keratoses: A New Avenue in Personalized Skin Care" Cancers 16, no. 3: 484. https://doi.org/10.3390/cancers16030484
APA StyleSoare, C., Cozma, E. C., Celarel, A. M., Rosca, A. M., Lupu, M., & Voiculescu, V. M. (2024). Digitally Enhanced Methods for the Diagnosis and Monitoring of Treatment Responses in Actinic Keratoses: A New Avenue in Personalized Skin Care. Cancers, 16(3), 484. https://doi.org/10.3390/cancers16030484






