Efficacy of Neoadjuvant Hypofractionated Chemoradiotherapy in Elderly Patients with Locally Advanced Rectal Cancer: A Single-Center Retrospective Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Neoadjuvant Treatment
2.3. Surgery and Surveillance
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Total neoadjuvant therapy for rectal cancer: Evidence and challenge. Ann. Coloproctol. 2023, 39, 301–306. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Sychev, S.; Ponomarenko, A.; Chernyshov, S.; Alekseev, M.; Mamedli, Z.; Kuzmichev, D.; Polynovskiy, A.; Rybakov, E. Total neoadjuvant therapy in rectal cancer: A network meta-analysis of randomized trials. Ann. Coloproctol. 2023, 39, 289–300. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Crocetti, D.; Maiuri, V.; Parisi, M.; Marampon, F.; Izzo, L.; De Toma, G.; Musio, D.; Tombolini, V. Locally Advanced Rectal Cancer: Treatment Approach in Elderly Patients. Curr. Treat. Options Oncol. 2020, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.X.; Koh, F.H.; Tan, H.L.; Sivarajah, S.S.; Ng, J.L.; Ho, L.M.; Aw, D.K.-L.; Koo, W.-H.; Han, S.; Koo, S.-L.; et al. The impact of short-course total neoadjuvant therapy, long-course chemoradiotherapy, and upfront surgery on the technical difficulty of total mesorectal excision: An observational study with an intraoperative perspective. Ann. Coloproctol. 2024, 40, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.D.; Simunovic, M.; Jhaveri, K.; Kirsch, R.; Brierley, J.; Drolet, S.; Brown, C.; Vos, P.M.; Xiong, W.; MacLean, T.; et al. Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients With “Good Prognosis” Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial. JAMA Oncol. 2019, 5, 961–966. [Google Scholar] [CrossRef]
- Nilsson, P.J.; van Etten, B.; Hospers, G.A.; Påhlman, L.; van de Velde, C.J.; Beets-Tan, R.G.; Blomqvist, L.; Beukema, J.C.; Kapiteijn, E.; Marijnen, C.A.; et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 2013, 13, 279. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, H.S.; Jung, I.; Shin, S.J.; Beom, S.H.; Chang, J.S.; Koom, W.S.; Kim, T.I.; Hur, H.; Min, B.S.; et al. Upfront radical surgery with total mesorectal excision followed by adjuvant FOLFOX chemotherapy for locally advanced rectal cancer (TME-FOLFOX): An open-label, multicenter, phase II randomized controlled trial. Trials 2020, 21, 320. [Google Scholar] [CrossRef]
- Lancellotti, F.; Solinas, L.; Sagnotta, A.; Mancini, S.; Cosentino, L.P.M.; Belardi, A.; Battaglia, B.; Mirri, M.A.; Ciabattoni, A.; Salerno, F.; et al. Short course radiotherapy and delayed surgery for locally advanced rectal cancer in frail patients: Is it a valid option? Eur. J. Surg. Oncol. 2021, 47, 2046–2052. [Google Scholar] [CrossRef]
- Ma, B.; Gao, P.; Song, Y.; Huang, X.; Wang, H.; Xu, Q.; Zhao, S.; Wang, Z. Short-Course Radiotherapy in Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-analysis. Clin. Colorectal Cancer 2018, 17, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, Y.; Liu, K.; Pei, Q.; Zhu, H. Comparing neoadjuvant long-course chemoradiotherapy with short-course radiotherapy in rectal cancer. BMC Gastroenterol. 2021, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Jeong, J.U.; Nam, T.K.; Kim, Y.H.; Song, J.Y.; Yoon, M.S.; Ahn, S.-J.; Cho, S.H. Efficacy of hypofractionated preoperative chemoradiotherapy in rectal cancer. Oncol. Lett. 2023, 25, 263. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Ito, M. Dissection layer selection based on an understanding of pelvic fascial anatomy in transanal total mesorectal excision. Ann. Coloproctol. 2024, 40, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Bae, H.W.; Kim, N.K. Essential knowledge and technical tips for total mesorectal excision and related procedures for rectal cancer. Ann. Coloproctol. 2024, 40, 384–411. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Nassoiy, S.; Christopher, W.; Marcus, R.; Keller, J.; Weiss, J.; Chang, S.; Foshag, L.; Essner, R.; Fischer, T.; Goldfarb, M. Treatment Utilization and Outcomes for Locally Advanced Rectal Cancer in Older Patients. JAMA Surg. 2022, 157, e224456. [Google Scholar] [CrossRef] [PubMed]
- Kammar, P.S.; Garach, N.R.; Masillamany, S.; de’Souza, A.; Ostwal, V.; Saklani, A.P. Downstaging in Advanced Rectal Cancers: A Propensity-Matched Comparison Between Short-Course Radiotherapy Followed by Chemotherapy and Long-Course Chemoradiotherapy. Dis. Colon. Rectum. 2022, 65, 1215–1223. [Google Scholar] [CrossRef]
- Lim, B.L.; Park, I.J.; Ro, J.S.; Kim, Y.I.; Lim, S.B.; Yu, C.S. Oncologic outcomes and associated factors of colon cancer patients aged 70 years and older. Ann. Coloproctol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chi, H.; Kok, S.; Chua, J.M.W.; Huang, X.X.; Zhang, S.; Mah, S.; Foo, L.-X.; Peh, H.-Y.; Lee, H.-B.; et al. Multimodal prerehabilitation for elderly patients with sarcopenia in colorectal surgery. Ann. Coloproctol. 2024, 40, 3–12. [Google Scholar] [CrossRef]
- Huang, C.K.; Shih, C.H.; Kao, Y.S. Elderly Rectal Cancer: An Updated Review. Curr. Oncol. Rep. 2024, 26, 181–190. [Google Scholar] [CrossRef] [PubMed]
- François, E.; De Bari, B.; Ronchin, P.; Nouhaud, E.; Martel-Lafay, I.; Artru, P.; Clavere, P.; Vendrely, V.; Boige, V.; Gargot, D.; et al. Comparison of short course radiotherapy with chemoradiotherapy for locally advanced rectal cancers in the elderly: A multicentre, randomised, non-blinded, phase 3 trial. Eur. J. Cancer 2023, 180, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Swellengrebel, H.; Marijnen, C.; Verwaal, V.; Vincent, A.; Heuff, G.; Gerhards, M.; Geloven, A.A.W.v.; van Tets, W.F.; Verheij, M.; Cats, A. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br. J. Surg. 2011, 98, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Yang, S.Y.; Cho, M.S.; Hur, H.; Min, B.S.; Lee, K.Y.; Kim, N.K. Long-term bowel functional outcomes following anal sphincter-preserving surgery for upper and middle rectal cancer: A single-center longitudinal study. Ann. Coloproctol. 2024, 40, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Tabaja, L.; Sidani, S.M. Management of Radiation Proctitis. Dig. Dis. Sci. 2018, 63, 2180–2188. [Google Scholar] [CrossRef]
- Mohammadianpanah, M.; Tazang, M.; Nguyen, N.P.; Ahmadloo, N.; Omidvari, S.; Mosalaei, A.; Ansari, M.; Nasrollahi, H.; Kadkhodaei, B.; Khanjani, N.; et al. Preventive efficacy of hydrocortisone enema for radiation proctitis in rectal cancer patients undergoing short-course radiotherapy: A phase II randomized placebo-controlled clinical trial. Ann. Coloproctol. 2024, 40, 506–514. [Google Scholar] [CrossRef]
- Knowles, G.; Haigh, R.; McLean, C.; Phillips, H.A.; Dunlop, M.G.; Din, F.V. Long term effect of surgery and radiotherapy for colorectal cancer on defecatory function and quality of life. Eur. J. Oncol. Nurs. 2013, 17, 570–577. [Google Scholar] [CrossRef]
- Cheng, L.J.; Chen, J.H.; Chen, S.Y.; Wei, Z.W.; Yu, L.; Han, S.P.; He, Y.-L.; Wu, Z.-H.; Chen, C.-Q. Distinct Prognosis of High Versus Mid/Low Rectal Cancer: A Propensity Score–Matched Cohort Study. J. Gastrointest. Surg. 2019, 23, 1474–1484. [Google Scholar] [CrossRef]
- Riesco-Martinez, M.C.; Fernandez-Martos, C.; Gravalos-Castro, C.; Espinosa-Olarte, P.; La Salvia, A.; Robles-Diaz, L.; Modrego-Sanchez, A.; Garcia-Carbonero, R. Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers 2020, 12, 3655. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Bao, T.; Cao, Y.; Hu, W.; Deng, J.; Chen, J.; Xiao, T. Efficacy and safety of total neoadjuvant therapy in locally advanced rectal cancer: A meta-analysis. Int. J. Colorectal Dis. 2023, 38, 89. [Google Scholar] [CrossRef] [PubMed]
- Hathout, L.; Maloney-Patel, N.; Malhotra, U.; Wang, S.J.; Chokhavatia, S.; Dalal, I.; Poplin, E.; Jabbour, S.K. Management of locally advanced rectal cancer in the elderly: A critical review and algorithm. J. Gastrointest. Oncol. 2018, 9, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nishimura, S.; Hara, Y.; Oka, N.; Tanaka, H.; Iemura, S.; Akagi, M. Chinical outcomes of patients with primary malignant bone and soft tissue tumor aged 65 years or older. Exp. Ther. Med. 2019, 17, 888–894. [Google Scholar] [PubMed]

| Characteristics | HCRT (n = 30) | LCRT (n = 195) | Upfront Surgery (n = 71) | p Value | |
|---|---|---|---|---|---|
| Sex | Male | 15 (50.0) | 124 (63.6) | 34 (47.9) | 0.303 |
| Female | 15 (50.0) | 71 (36.4) | 37 (52.1) | ||
| Age (years) | 78.0 ± 5.1 | 76.3 ± 4.4 | 76.9 ± 4.5 | 0.129 | |
| ASA score | 1, 2 | 17 (56.7) | 162 (83.1) | 60 (84.5) | 0.002 |
| 3, 4 | 13 (43.3) | 33 (16.9) | 11 (15.5) | ||
| BMI (kg/m2) | <25 | 19 (63.3) | 139 (71.3) | 59 (83.1) | 0.067 |
| ≥25 | 11 (36.7) | 56 (28.7) | 12 (16.9) | ||
| Albumin (g/dL) | <3.5 | 1 (3.3) | 17 (8.7) | 9 (12.7) | 0.311 |
| ≥3.5 | 29 (96.7) | 178 (91.3) | 62 (87.3) | ||
| Hemoglobin (g/dL) | <12 | 14 (46.7) | 97 (49.7) | 26 (36.6) | 0.165 |
| ≥12 | 16 (53.3) | 98 (50.3) | 45 (63.4) | ||
| Pretreatment CEA (ng/mL) | <5 | 14 (46.7) | 103 (52.8) | 47 (66.2) | 0.091 |
| ≥5 | 16 (53.3) | 92 (47.2) | 24 (33.8) | ||
| Tumor location | Mid | 18 (60.0) | 107 (54.9) | 61 (85.9) | <0.001 |
| Low | 12 (40.0) | 88 (45.1) | 10 (14.1) | ||
| Tumor differentiation | WD | 6 (20.0) | 80 (41.0) | 29 (40.8) | 0.135 |
| MD | 21 (70.0) | 108 (55.4) | 40 (56.3) | ||
| PD, mucinous | 3 (10.0) | 7 (3.6) | 2 (2.8) | ||
| Clinical T stage | 1, 2 | 1 (3.3) | 4 (2.1) | 3 (4.2) | 0.277 |
| 3 | 27 (90.0) | 161 (82.6) | 63 (88.7) | ||
| 4 | 2 (6.7) | 30 (15.4) | 5 (7.0) | ||
| Clinical N stage | 0 | 18 (60.0) | 94 (48.2) | 32 (45.1) | 0.459 |
| 1 | 8 (26.7) | 61 (31.3) | 28 (39.4) | ||
| 2 | 4 (13.3) | 40 (20.5) | 11 (15.5) | ||
| HCRT (n = 30) | LCRT (n = 195) | p Value | |
|---|---|---|---|
| Complications | 0.001 | ||
| Yes | 5 (16.7) | 95 (48.7) | |
| No | 25 (83.3) | 100 (51.3) | |
| Types | |||
| Nausea/vomiting | 1 (3.3) | 8 (4.1) | |
| Headache | 0 (0.0) | 9 (4.6) | |
| Bowel habit change | 2 (6.7) | 38 (19.5) | |
| Anorexia | 2 (6.7) | 22 (11.3) | |
| Dermatitis | 0 (0.0) | 10 (5.1) | |
| Dysuria | 1 (3.3) | 16 (8.2) | |
| Anal pain | 2 (6.7) | 43 (22.1) | |
| Abdominal pain | 1 (3.3) | 9 (4.6) | |
| General weakness | 0 (0.0) | 5 (2.6) | |
| Leg edema | 0 (0.0) | 1 (0.5) | |
| CTCAE grade | 0.911 | ||
| 1 | 4 (13.3) | 76 (38.9) | |
| 2 | 1 (3.3) | 16 (8.2) | |
| 3, 4 | 0 (0.0) | 3 (1.0) |
| Characteristic | HCRT (n = 30) | LCRT (n = 195) | p Value | |
|---|---|---|---|---|
| pCR | Yes | 3 (10.0) | 30 (15.4) | 0.002 |
| No | 27 (90.0) | 165 (84.6) | ||
| Pathological T stage | 0, 1 | 3 (10.0) | 42 (21.5) | 0.243 |
| 2 | 7 (23.3) | 39 (20.0) | ||
| 3 | 16 (53.3) | 103 (52.8) | ||
| 4 | 4 (13.3) | 11 (5.6) | ||
| Pathological N stage | 0 | 18 (64.3) | 125 (66.5) | 0.059 |
| 1 | 5 (17.9) | 52 (27.7) | ||
| 2 | 5 (17.9) | 11 (5.9) | ||
| Variables | n | Relapse-Free Survival | Local Recurrence-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|---|---|
| 3-Year RFS (%) | p | 3-Year LRFS (%) | p | 5-Year OS (%) | p | |||
| Sex | Male | 173 | 77.6 | 0.555 | 94.4 | 0.889 | 64.0 | 0.128 |
| Female | 123 | 82.3 | 95.6 | 71.1 | ||||
| Age (years) | <80 | 219 | 80.2 | 0.531 | 94.3 | 0.887 | 71.6 | 0.007 |
| ≥80 | 77 | 77.6 | 96.8 | 53.2 | ||||
| ASA score | 1, 2 | 239 | 79.3 | 0.852 | 93.7 | 0.162 | 68.3 | 0.150 |
| 3, 4 | 57 | 80.9 | 100.0 | 60.7 | ||||
| BMI (kg/m2) | <25 | 217 | 81.6 | 0.379 | 95.1 | 0.685 | 65.9 | 0.872 |
| ≥25 | 79 | 74.2 | 94.4 | 70.1 | ||||
| Albumin (g/dL) | <3.5 | 27 | 87.0 | 0.655 | 100.0 | 0.719 | 43.0 | 0.022 |
| ≥3.5 | 269 | 79.1 | 94.5 | 69.2 | ||||
| Hemoglobin (g/dL) | <12 | 137 | 71.6 | 0.001 | 91.8 | 0.012 | 53.9 | <0.001 |
| ≥12 | 159 | 86.3 | 97.4 | 77.9 | ||||
| Pretreatment CEA (ng/mL) | <5 | 164 | 84.5 | 0.007 | 95.4 | 0.301 | 69.7 | 0.239 |
| ≥5 | 132 | 73.5 | 94.2 | 63.4 | ||||
| Tumor location | Mid | 186 | 83.6 | 0.010 | 98.3 | <0.001 | 69.1 | 0.039 |
| Lower | 110 | 72.9 | 89.0 | 63.2 | ||||
| Tumor differentiation | WD | 114 | 87.2 | <0.001 | 96.2 | <0.001 | 70.5 | 0.003 |
| MD | 168 | 76.6 | 95.5 | 67.6 | ||||
| PD, mucinous | 9 | 42.9 | 71.4 | 33.3 | ||||
| Clinical T stage | 1, 2 | 8 | 87.5 | 0.019 | 87.5 | 0.016 | 71.4 | 0.648 |
| 3 | 251 | 81.8 | 96.5 | 68.4 | ||||
| 4 | 37 | 63.7 | 86.0 | 56.8 | ||||
| Clinical N stage | 0 | 144 | 89.1 | <0.001 | 97.7 | 0.047 | 74.5 | 0.014 |
| 1 | 97 | 73.3 | 94.2 | 66.6 | ||||
| 2 | 55 | 65.9 | 88.5 | 47.4 | ||||
| Adjuvant chemotherapy | (−) | 106 | 80.3 | 0.849 | 95.5 | 0.511 | 49.5 | <0.001 |
| (+) | 190 | 79.2 | 94.6 | 76.6 | ||||
| CRM | (−) | 137 | 87.0 | <0.001 | 96.9 | 0.207 | 72.3 | 0.044 |
| (+) | 44 | 58.8 | 90.0 | 53.5 | ||||
| LVI | (−) | 256 | 83.2 | <0.001 | 95.3 | 0.004 | 62.1 | 0.001 |
| (+) | 40 | 52.5 | 85.0 | 37.5 | ||||
| PNI | (−) | 224 | 84.8 | <0.001 | 96.4 | 0.001 | 62.1 | <0.001 |
| (+) | 72 | 61.1 | 86.1 | 48.6 | ||||
| TRG | ≥2 | 35 | 68.6 | 0.049 | 85.7 | 0.049 | 31.4 | <0.001 |
| 3 | 154 | 76.6 | 92.9 | 59.7 | ||||
| 4 | 33 | 90.9 | 100.0 | 75.8 | ||||
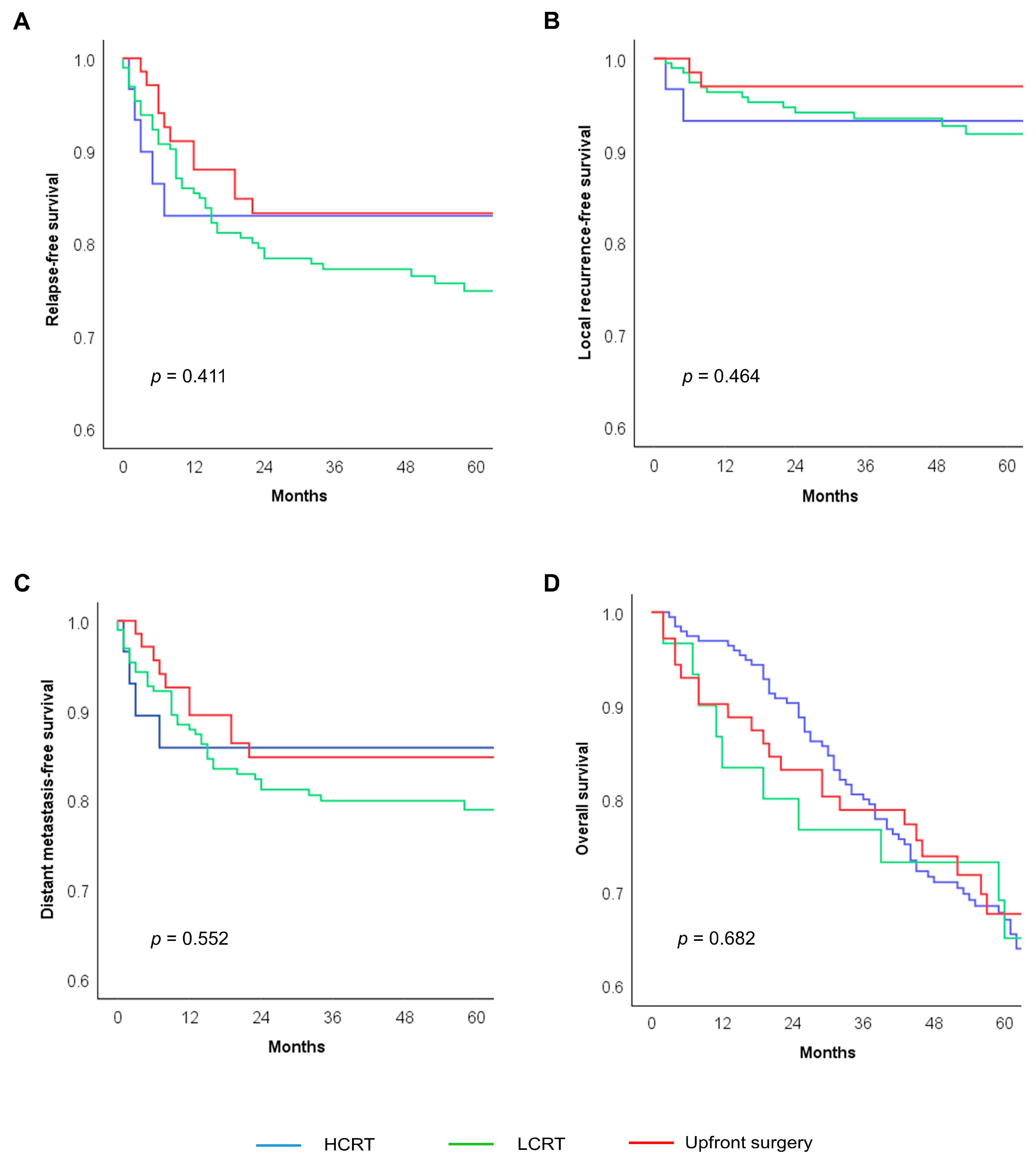
| Treatment strategy | HCRT | 30 | 83.0 | 0.411 | 93.1 | 0.464 | 65.1 | 0.682 |
| LCRT | 195 | 77.2 | 93.2 | 67.0 | ||||
| Upfront surgery | 71 | 83.2 | 93.5 | 67.7 | ||||
| Variables | Relapse-Free Survival (a) | Local Recurrence-Free Survival (b) | Overall Survival (c) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Treatment strategy | |||||||||
| HCRT vs. LCRT | 0.875 | 0.334–2.291 | 0.785 | 1.385 | 0.276–6.947 | 0.692 | 0.962 | 0.491–1.888 | 0.911 |
| Surgery vs. LCRT | 0.650 | 0.315–1.340 | 0.243 | 0.381 | 0.067–2.172 | 0.277 | 0.926 | 0.560–1.532 | 0.765 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Lee, J.; Park, H.-m.; Lee, S.Y.; Kim, C.H.; Kim, H.R. Efficacy of Neoadjuvant Hypofractionated Chemoradiotherapy in Elderly Patients with Locally Advanced Rectal Cancer: A Single-Center Retrospective Analysis. Cancers 2024, 16, 4280. https://doi.org/10.3390/cancers16244280
Kim JS, Lee J, Park H-m, Lee SY, Kim CH, Kim HR. Efficacy of Neoadjuvant Hypofractionated Chemoradiotherapy in Elderly Patients with Locally Advanced Rectal Cancer: A Single-Center Retrospective Analysis. Cancers. 2024; 16(24):4280. https://doi.org/10.3390/cancers16244280
Chicago/Turabian StyleKim, Jae Seung, Jaram Lee, Hyeung-min Park, Soo Young Lee, Chang Hyun Kim, and Hyeong Rok Kim. 2024. "Efficacy of Neoadjuvant Hypofractionated Chemoradiotherapy in Elderly Patients with Locally Advanced Rectal Cancer: A Single-Center Retrospective Analysis" Cancers 16, no. 24: 4280. https://doi.org/10.3390/cancers16244280
APA StyleKim, J. S., Lee, J., Park, H.-m., Lee, S. Y., Kim, C. H., & Kim, H. R. (2024). Efficacy of Neoadjuvant Hypofractionated Chemoradiotherapy in Elderly Patients with Locally Advanced Rectal Cancer: A Single-Center Retrospective Analysis. Cancers, 16(24), 4280. https://doi.org/10.3390/cancers16244280






