Isolation of Plasma Extracellular Vesicles for High-Depth Analysis of Proteomic Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Recruitment
2.2. Mass Spectrometry
2.3. Processing of Mass Spectrometry-Derived Proteomic Data
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Protein Compartmentalization
2.6. PSMA-PET Scan Imaging
2.7. Statistical Analysis
3. Results
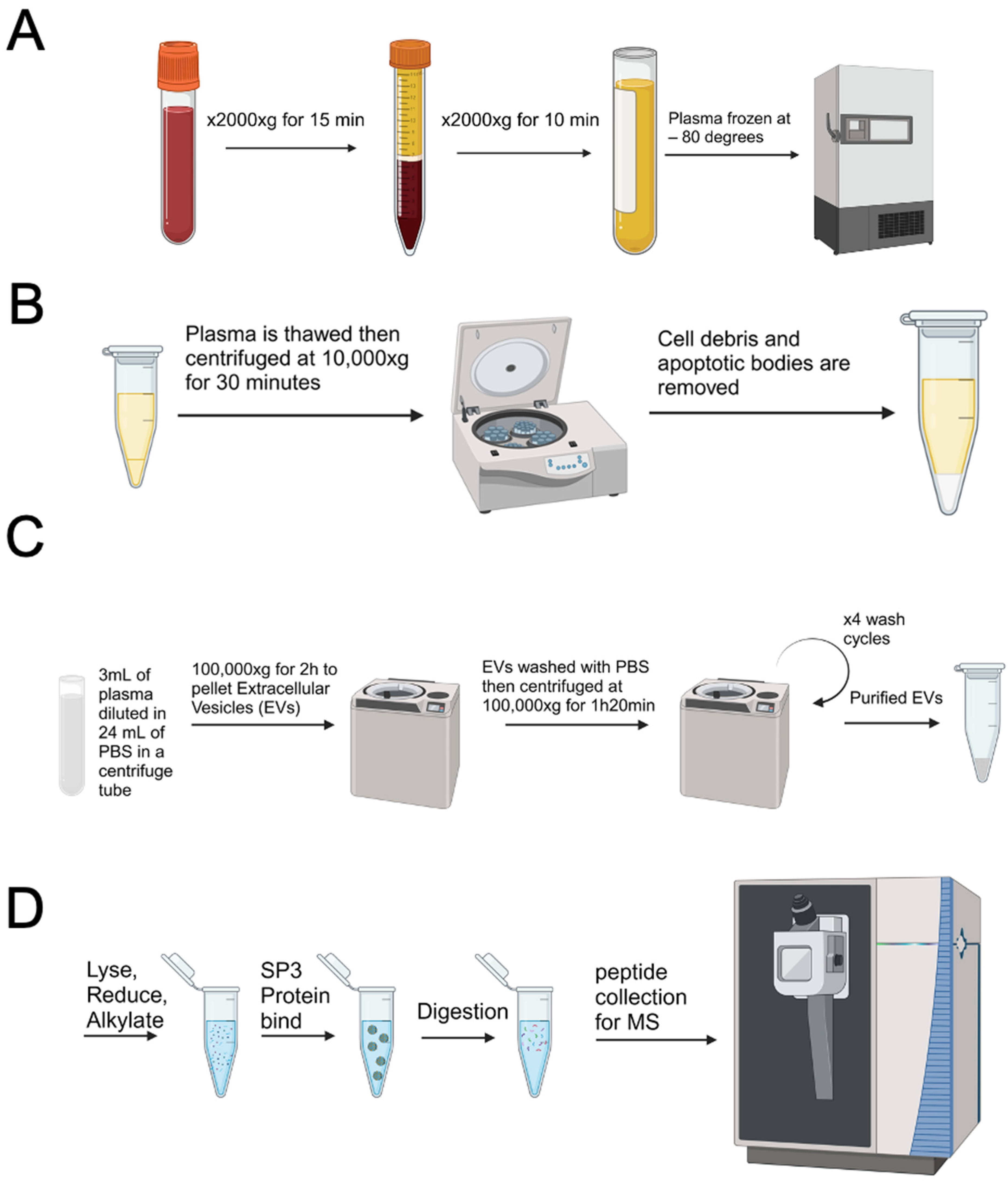
3.1. Plasma Extracellular Vesicle Isolation and Characterization from Metastatic Castration Resistant Prostate Cancer Patients
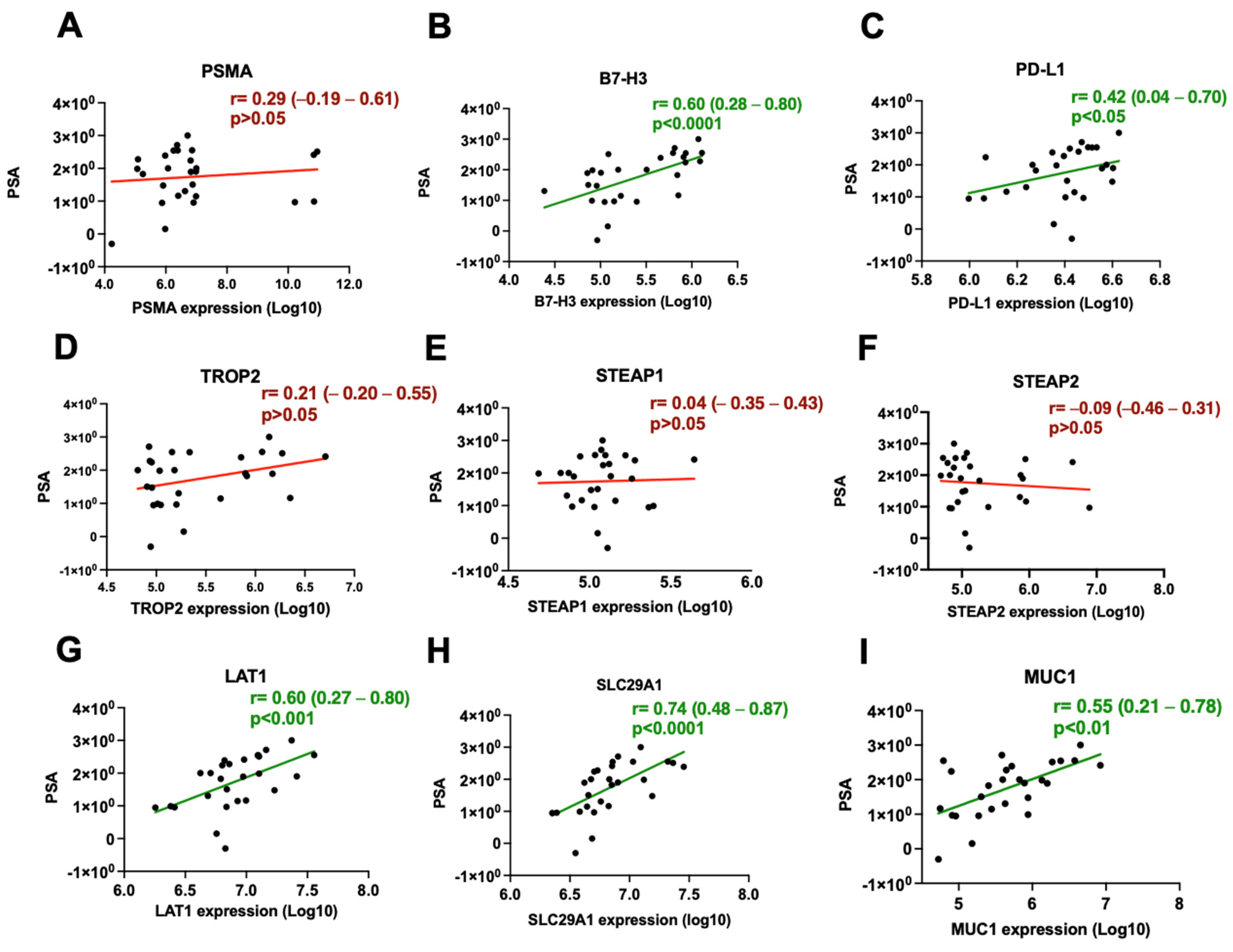
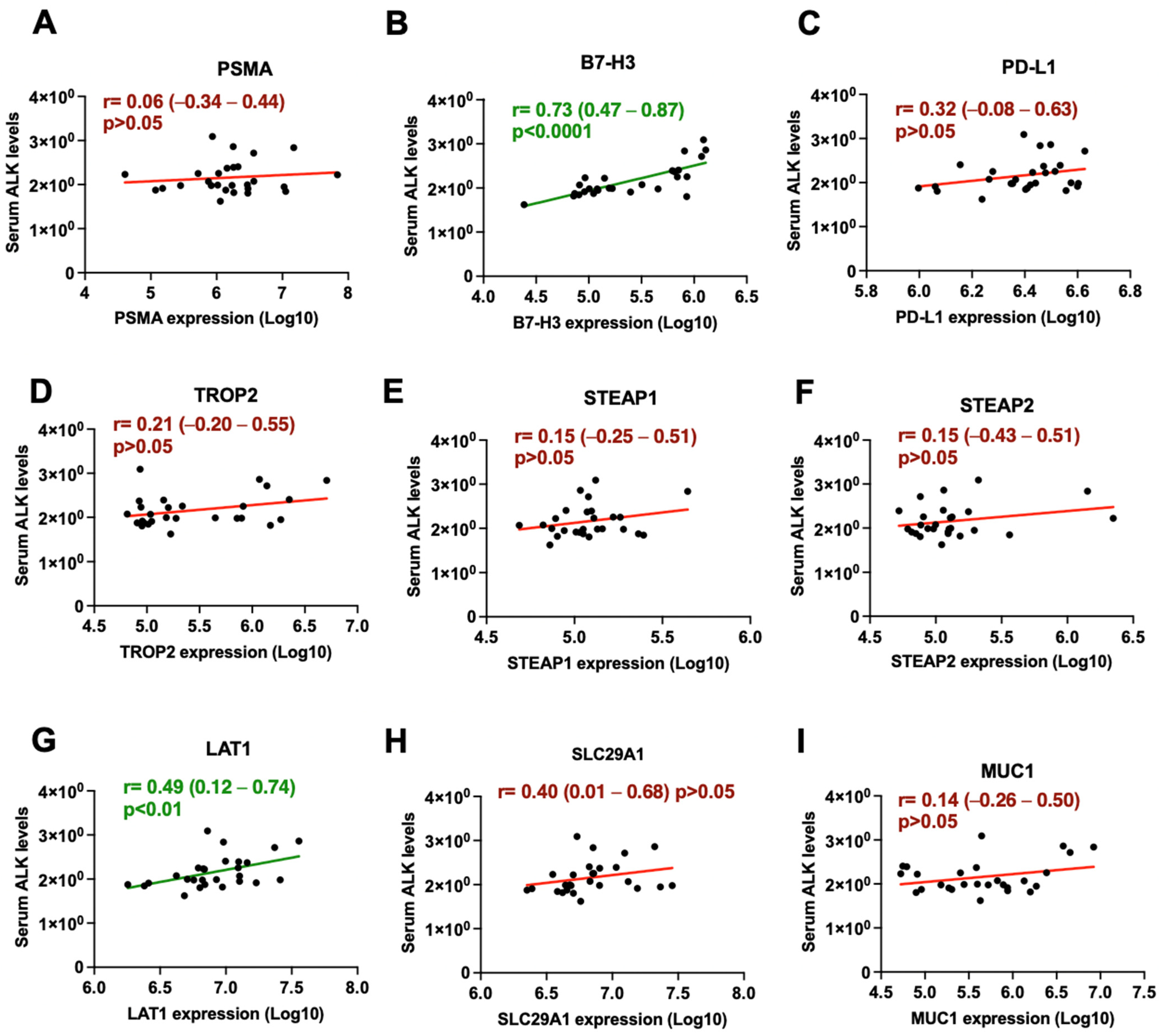
3.2. B7-H3 and LAT1 Proteins Correlate with Serum PSA Levels
3.3. EV-Derived LAT1 Protein Expression and Bone Metastatic Burden
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; de Szczepaniak, J.; Fléchon, A.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Kelleher, E.; Bowles, D.W.; Sokolova, A.O.; Hohl, R.J.; et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 2020, 38, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Sternberg, C.N.; Morris, M.J.; Hirmand, M.; Goh, J.; et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- Hotta, M.; Gafita, A.; Murthy, V.; Benz, M.R.; Sonni, I.; Burger, I.A.; Eiber, M.; Emmett, L.; Farolfi, A.; Fendler, W.P.; et al. PSMA PET tumor-to-salivary gland ratio to predict response to [177Lu]PSMA radioligand therapy: An international multicenter retrospective study. J. Nucl. Med. 2023, 64, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Shore, N.D.; Heidegger, I.; Smith, M.R.; Tombal, B.; Saad, F. Metastatic hormone-sensitive prostate cancer and combination treatment outcomes: A review. JAMA Oncol. 2024, 10, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.S. Metastatic prostate cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef]
- Gartrell, B.A.; Coleman, R.; Efstathiou, E.; Fizazi, K.; Logothetis, C.J.; Smith, M.R.; Sonpavde, G.; Sartor, O.; Saad, F. Metastatic prostate cancer and the bone: Significance and therapeutic options. Eur. Urol. 2015, 68, 850–858. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Chen, M.; Yang, L.; Li, Z.; et al. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Tang, H.; Yu, D.; Zhang, J.; Wang, M.; Fu, M.; Qian, Y.; Zhang, X.; Ji, R.; Gu, J.; Zhang, X. The new advance of exosome-based liquid biopsy for cancer diagnosis. J. Nanobiotechnol. 2024, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, P.; Marzan, A.L.; Mathivanan, S. Introduction to the community of extracellular vesicles. Subcell. Biochem. 2021, 97, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Babajani, A.; Abdollahpour-Alitappeh, M.; Ahmadi, N.; Rezaei-Tavirani, M. Exosomes and cancer: From molecular mechanisms to clinical applications. Med. Oncol. 2021, 38, 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option. Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Kowkabany, G.; Bao, Y. Nanoparticle Tracking Analysis: An Effective Tool to Characterize Extracellular Vesicles. Molecules 2024, 29, 4672. [Google Scholar] [CrossRef] [PubMed]
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert, O.T.; Wollscheid, B. The in silico human surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; de Candia, P.; Di Vizio, D.; Falcon-Perez, J.M.; Gabrielsson, S.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, E.; Muro, A.; Sgueglia, G.; Massaro, C.; Dell’Aversana, C.; Altucci, L. Exosomal MicroRNAs: Comprehensive Methods from Exosome Isolation to miRNA Extraction and Purity Analysis. Methods Mol. Biol. 2023, 2595, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Abhange, K.; Kitata, R.B.; Zhang, J.; Wang, Y.T.; Gaffrey, M.J.; Liu, T.; Gunchick, V.; Khaykin, V.; Sahai, V.; Cuneo, K.C.; et al. In-Depth Proteome Profiling of Small Extracellular Vesicles Isolated from Cancer Cell Lines and Patient Serum. J. Proteome Res. 2024, 23, 386–396. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, E.; De Marzo, A.M.; Lotan, T.L.; Wang, H.; Chan, S.; Lim, S.J.; Ji, H.; Allaf, M.E.; Chapman, C.; Moore, P.A.; et al. Neoadjuvant enoblituzumab in localized prostate cancer: A single-arm, phase 2 trial. Nat. Med. 2023, 29, 888–897. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Day, A.; Bergom, H.E.; Tape, S.; Baca, S.C.; Sychev, Z.E.; Larson, G.; Bozicevich, A.; Drake, J.M.; Zorko, N.; et al. Integrative molecular analyses define correlates of high B7-H3 expression in metastatic castrate-resistant prostate cancer. NPJ Precis. Oncol. 2022, 6, 80. [Google Scholar] [CrossRef]
- Haffner, M.C.; Guner, G.; Taheri, D.; Netto, G.J.; Palsgrove, D.N.; Zheng, Q.; Guedes, L.B.; Kim, K.; Tsai, H.; Esopi, D.M.; et al. Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am. J. Pathol. 2018, 188, 1478–1485. [Google Scholar] [CrossRef]
- Xu, M.; Evans, L.; Bizzaro, C.L.; Quaglia, F.; Verrillo, C.E.; Li, L.; Stieglmaier, J.; Schiewer, M.J.; Languino, L.R.; Kelly, W.K.; et al. STEAP1–4 (Six-Transmembrane Epithelial Antigen of the Prostate 1–4) and Their Clinical Implications for Prostate Cancer. Cancers 2022, 14, 4034. [Google Scholar] [CrossRef]
- Ajkunic, A.; Sayar, E.; Roudier, M.P.; Patel, R.A.; Coleman, I.M.; De Sarkar, N.; Hanratty, B.; Adil, M.; Zhao, J.; Zaidi, S.; et al. Assessment of TROP2, CEACAM5 and DLL3 in metastatic prostate cancer: Expression landscape and molecular correlates. NPJ Precis. Oncol. 2024, 8, 104. [Google Scholar] [CrossRef]
- Cormerais, Y.; Giuliano, S.; LeFloch, R.; Front, B.; Durivault, J.; Tambutté, E.; Massard, P.A.; de la Ballina, L.R.; Endou, H.; Wempe, M.F.; et al. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016, 76, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sakamoto, S.; Wei, J.; Pae, S.; Saito, S.; Sazuka, T.; Imamura, Y.; Anzai, N.; Ichikawa, T. Contribution of the L-Type Amino Acid Transporter Family in the Diagnosis and Treatment of Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 6178. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W.; et al. Immune checkpoint of B7-H3 in cancer: From immunology to clinical immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.; Zhao, Y.; Kim, J.J.; Li, H.; Meng, C.; Chen, F.; Zhang, J.; Mak, D.H.; Van, V.; et al. Immune checkpoint B7-H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies. Sci. Transl. Med. 2023, 15, eadf6724. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Figueiredo, I.; Gurel, B.; Neeb, A.; Seed, G.; Crespo, M.; Carreira, S.; Rekowski, J.; Buroni, L.; Welti, J.; et al. B7-H3 as a Therapeutic Target in Advanced Prostate Cancer. Eur. Urol. 2023, 83, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.D.; Lozada, J.R.; Zorko, N.A.; Elliott, A.; Makovec, A.; Radovich, M.; Heath, E.I.; Agarwal, N.; McKay, R.R.; Garje, R.; et al. Pan-Cancer Interrogation of B7-H3 (CD276) as an Actionable Therapeutic Target Across Human Malignancies. Cancer Res. Commun. 2024, 4, 1369–1379. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Q.; Zhang, H.; Wang, Y.; Zhang, K.; Mu, H.; Fu, F.; Zhang, X.; Wang, Y.; Miao, L. Development of a Specifically Labeled 89Zr Antibody for the Noninvasive Imaging of Tumors Overexpressing B7-H3. Mol. Pharm. 2024, 21, 5205–5216. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Buonerba, L.; Ingenito, C.; Crocetto, F.; Buonerba, C.; Libroia, A.; Sciarra, A.; Ragone, G.; Sanseverino, R.; Iaccarino, S.; et al. Clinical Characteristics of Metastatic Prostate Cancer Patients Infected with COVID-19 in South Italy. Oncology 2020, 98, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: Interim results from a multicenter phase I/II trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef]
- Häfliger, P.; Charles, R.P. The L-Type Amino Acid Transporter LAT1—An Emerging Target in Cancer. Int. J. Mol. Sci. 2019, 20, 2428. [Google Scholar] [CrossRef]


| Total Number of mCRPC Patients | 27 |
|---|---|
| Age at diagnosis | |
| Median (min–max) | 72 (42–92) |
| Age at enrollment | 74 (44–94) |
| PSA at diagnosis (ng/mL) | |
| Median (min–max) | 44 (2.6–8970) |
| Race | |
| White | 25 (92.6) |
| Black | 2 (7.4) |
| Grade group (%) | |
| 1 | 0 (0.0) |
| 2 | 2 (7.4) |
| 3 | 3 (11.1) |
| 4 | 5 (18.5) |
| 5 | 12 (44.4) |
| unknown | 6 (22.2) |
| Baseline Serum PSA (ng/mL) | |
| Median (min–max) | 70 (0.5–1000) |
| Baseline Serum ALK levels (U/L) | |
| Median (min–max) | 99 (44–1237) |
| Site of disease at enrollment—no. (%) | |
| Bone | 27 (100.0) |
| Lymph node | 17 (63.0) |
| Visceral | 6 (22.2) |
| Previous local therapies—no. (%) | |
| RP | 7 (25.9) |
| Radiation Therapy | 4 (14.8) |
| None | 18 (66.7) |
| Number of prior systemic therapies for mCRPC | |
| One | 3 (11.1) |
| Two | 8 (29.6) |
| Three or more | 16 (59.3) |
| Types of previous systemic therapies—no. (%) | |
| Luteinizing Hormone Releasing Hormone (LHRH) agonist or Antagonist | 27 (100.0) |
| Androgen receptor pathway inhibitors (ARPIs) | |
| Abiraterone | 19 (70.4) |
| Enzalutamide | 17 (63.0) |
| Apalutamide | 3 (11.1) |
| Darolutamide | 2 (7.4) |
| Chemotherapy | |
| Docetaxel | 21 (77.8) |
| Cabazitaxel | 6 (22.2) |
| Carboplatin | 4 (14.8) |
| Other | |
| Radium-223 | 1 (3.7) |
| Olaparib | 1 (3.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafa, A.T.; Ludwig, M.; Tuncer, O.; Kollitz, L.; Gustafson, A.; Boytim, E.; Luo, C.; Sabal, B.; Steinberger, D.; Zhao, Y.; et al. Isolation of Plasma Extracellular Vesicles for High-Depth Analysis of Proteomic Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients. Cancers 2024, 16, 4261. https://doi.org/10.3390/cancers16244261
Arafa AT, Ludwig M, Tuncer O, Kollitz L, Gustafson A, Boytim E, Luo C, Sabal B, Steinberger D, Zhao Y, et al. Isolation of Plasma Extracellular Vesicles for High-Depth Analysis of Proteomic Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients. Cancers. 2024; 16(24):4261. https://doi.org/10.3390/cancers16244261
Chicago/Turabian StyleArafa, Ali T., Megan Ludwig, Onur Tuncer, Lily Kollitz, Ava Gustafson, Ella Boytim, Christine Luo, Barbara Sabal, Daniel Steinberger, Yingchun Zhao, and et al. 2024. "Isolation of Plasma Extracellular Vesicles for High-Depth Analysis of Proteomic Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients" Cancers 16, no. 24: 4261. https://doi.org/10.3390/cancers16244261
APA StyleArafa, A. T., Ludwig, M., Tuncer, O., Kollitz, L., Gustafson, A., Boytim, E., Luo, C., Sabal, B., Steinberger, D., Zhao, Y., Dehm, S. M., Cayci, Z., Hwang, J., Villalta, P. W., Antonarakis, E. S., & Drake, J. M. (2024). Isolation of Plasma Extracellular Vesicles for High-Depth Analysis of Proteomic Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients. Cancers, 16(24), 4261. https://doi.org/10.3390/cancers16244261








