Reversal of Endothelial Cell Anergy by T Cell-Engaging Bispecific Antibodies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Isolation, Cell Lines and Culture Conditions
2.2. Cloning of bsAb
2.3. Production and Purification of bsAb
2.4. In Vitro Biological Activity of Bispecific Antibodies
2.5. Flow Cytometry
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Transendothelial Migration (TEM) Assay
2.7.1. Two-Layer Model
2.7.2. Three-Layer Model and Tumor Spheroid Generation
2.8. In Vitro Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
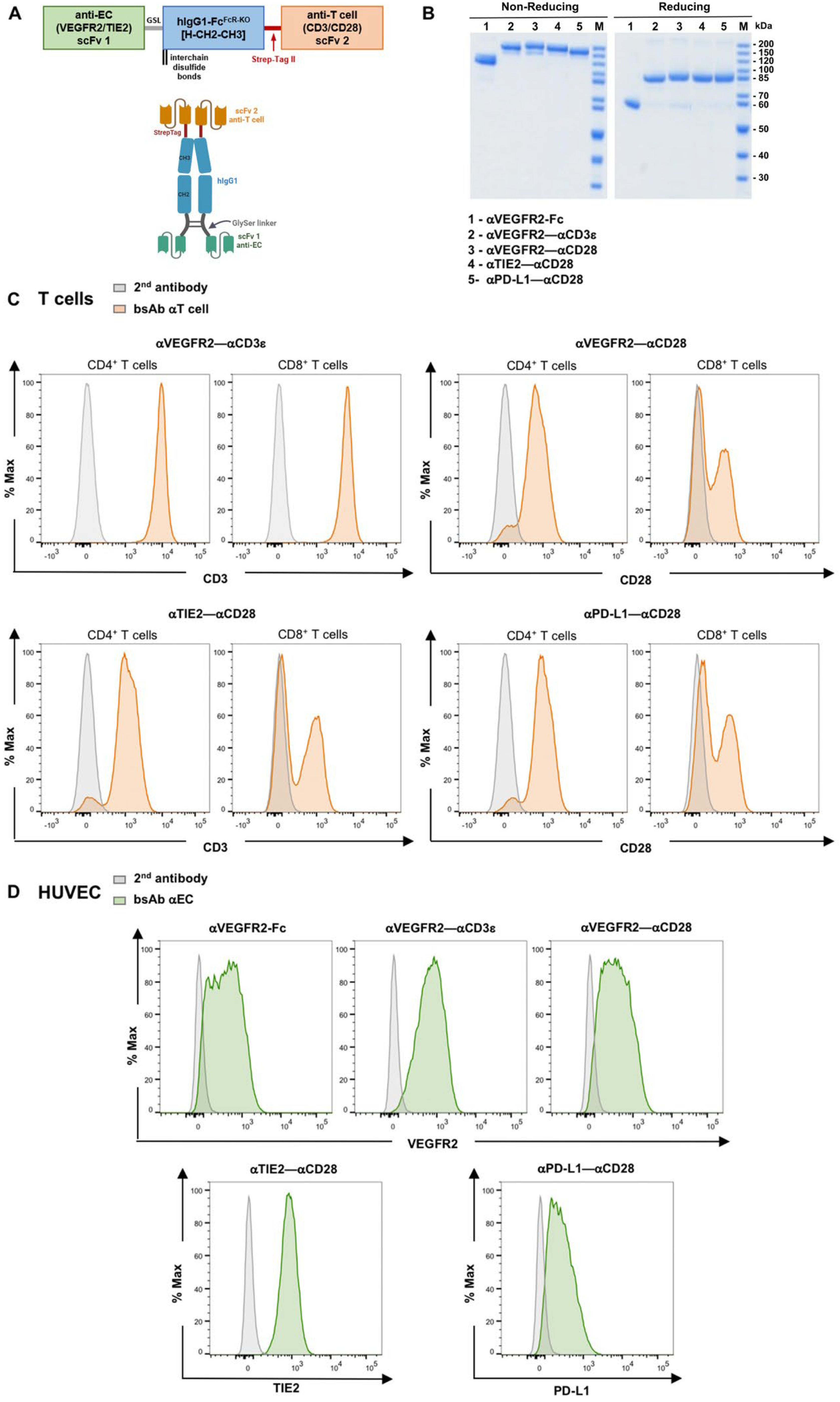
3.1. Novel Anti-EC–Anti-T Cell Bispecific Antibodies
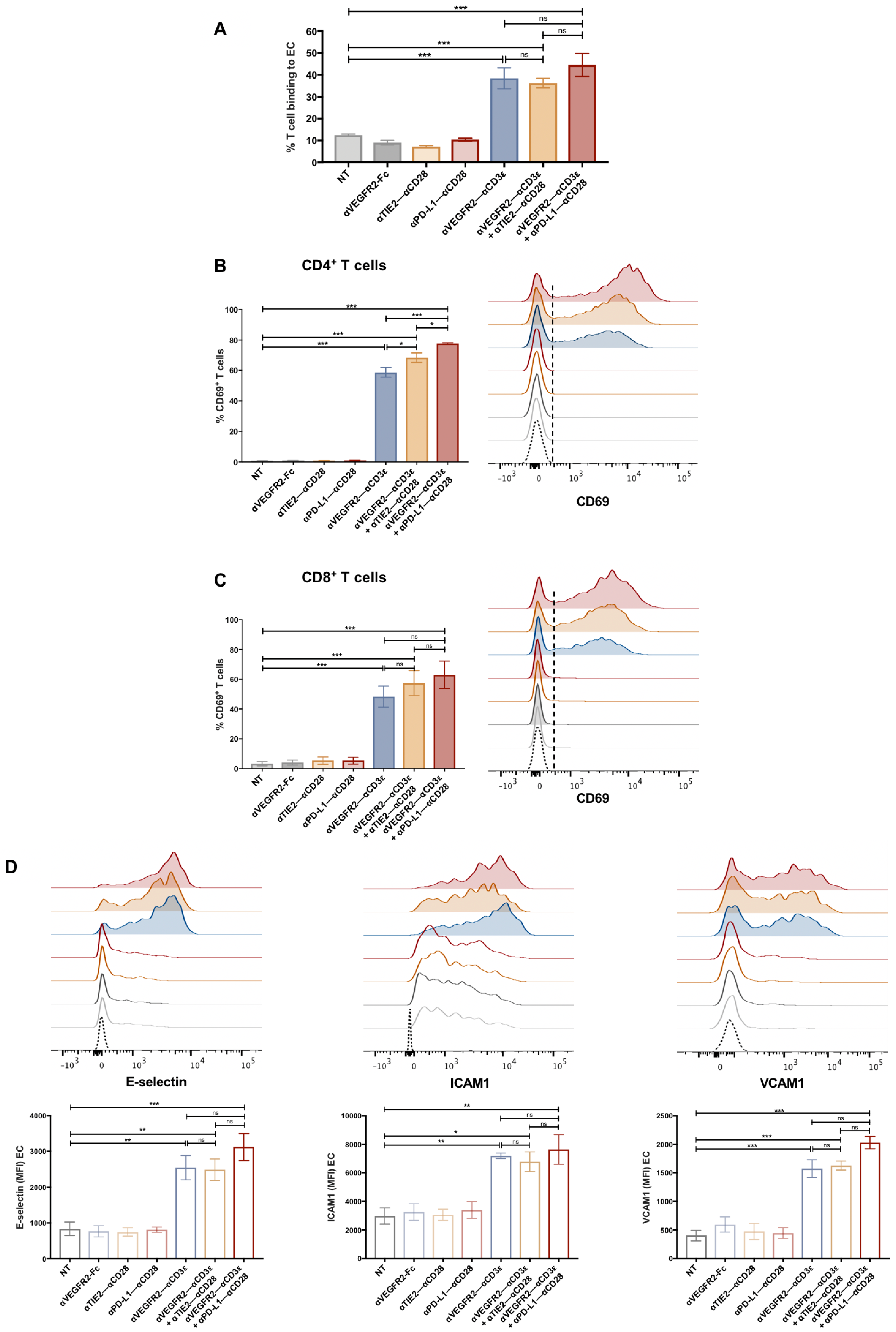
3.2. T Cell Activation by bsAb-Mediated Targeting to Endothelial Cells
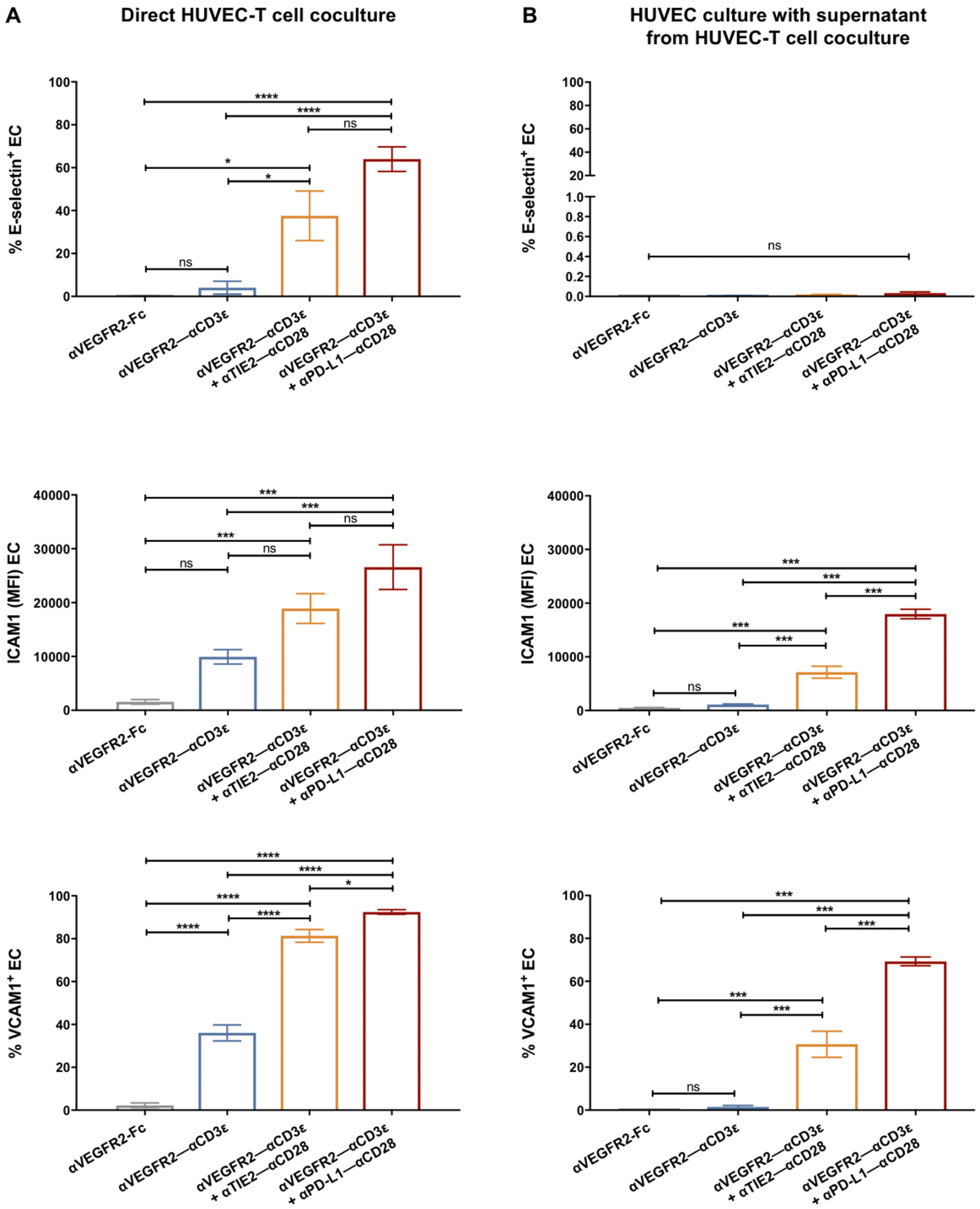
3.3. Endothelial Cell Activation in Cocultures with T Cells and bsAb
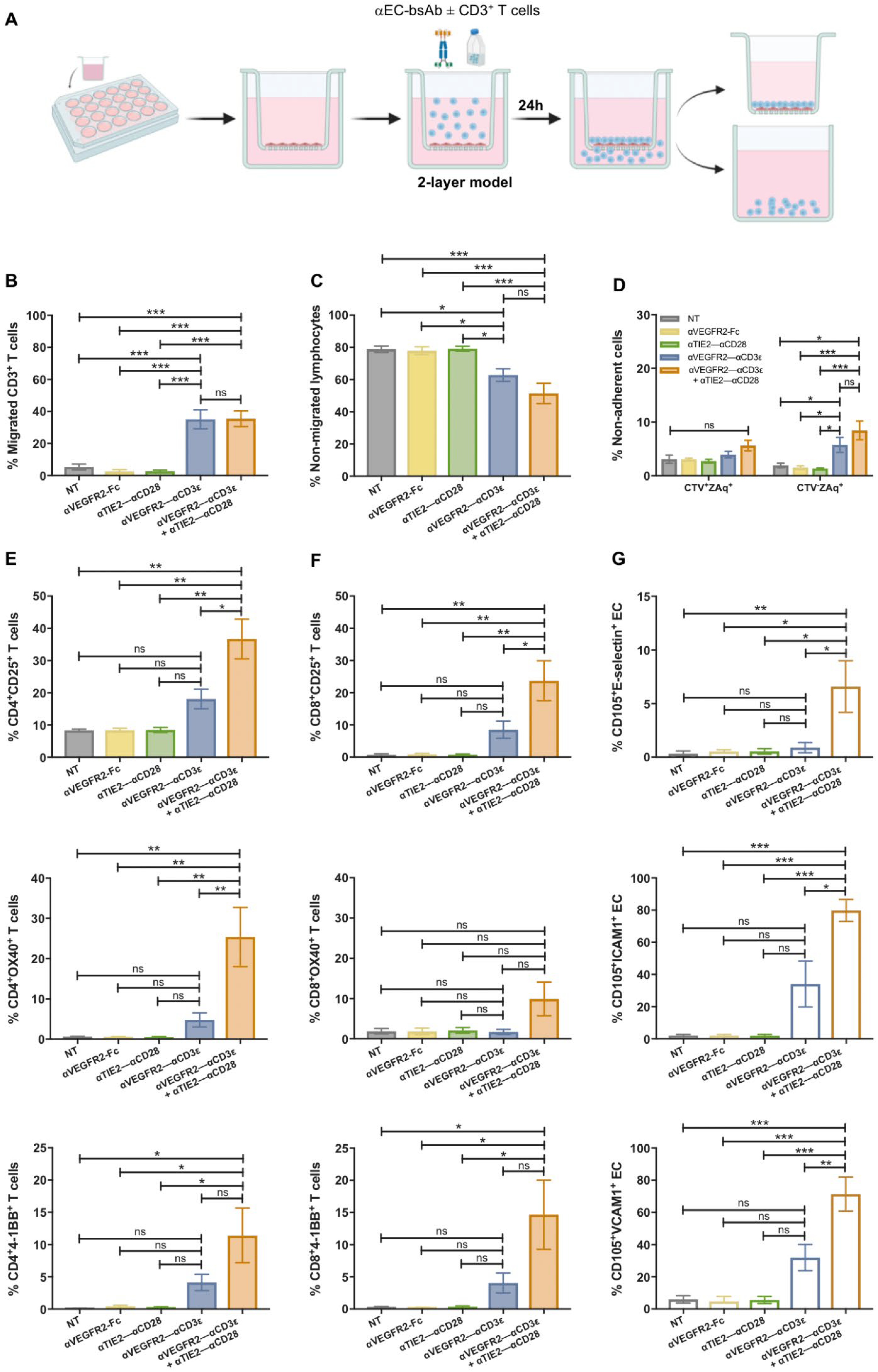
3.4. T Cell Transendothelial Migration Is Increased in the Presence of bsAb
3.5. Tumor Cell Cytotoxicity of Transmigrated T Cells After (Co)stimulation on Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Damen, C.A.; Blijham, G.H.; Groenewegen, G. Tumor Angiogenesis Is Accompanied by a Decreased Inflammatory Response of Tumor-Associated Endothelium. Blood 1996, 88, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-Angiogenic Agents—Overcoming Tumour Endothelial Cell Anergy and Improving Immunotherapy Outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, A.E.M.; oude Egbrink, M.G.A.; Kuijpers, M.J.E.; van der Niet, S.T.; Heijnen, V.V.T.; Steege, J.C.A.B.; Wagstaff, J.; Griffioen, A.W. Tumor Angiogenesis Modulates Leukocyte-Vessel Wall Interactions in Vivo by Reducing Endothelial Adhesion Molecule Expression1. Cancer Res. 2003, 63, 2322–2329. [Google Scholar] [PubMed]
- Klein, D. The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef]
- De Sanctis, F.; Ugel, S.; Facciponte, J.; Facciabene, A. The Dark Side of Tumor-Associated Endothelial Cells. Semin. Immunol. 2018, 35, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; Griffioen, C.J.; Huinen, Z.R.; Sopesens, N.G.; Schulz, R.; Jenkins, S.V.; Dings, R.P.M.; Groenendijk, F.H.; Huijbers, E.J.M.; et al. Proinflammatory Activity of VEGF-Targeted Treatment through Reversal of Tumor Endothelial Cell Anergy. Angiogenesis 2023, 26, 279–293. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of Anti-Angiogenic Therapy and Immune Checkpoint Blockade Normalizes Vascular-Immune Crosstalk to Potentiate Cancer Immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Vafopoulou, P.; Kourti, M. Anti-Angiogenic Drugs in Cancer Therapeutics: A Review of the Latest Preclinical and Clinical Studies of Anti-Angiogenic Agents with Anticancer Potential. J. Cancer Metastasis Treat. 2022, 8, 18. [Google Scholar] [CrossRef]
- Montemagno, C.; Pagès, G. Resistance to Anti-Angiogenic Therapies: A Mechanism Depending on the Time of Exposure to the Drugs. Front. Cell Dev. Biol. 2020, 8, 584. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist Antibodies to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) as Anti-Angiogenic Agents. Pharmacol. Ther. 2016, 164, 204–225. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.; Cuthbert, G.A.; Homer-Vanniasinkam, S.; Muench, S.P.; Ponnambalam, S.; Harrison, M.A. Structural Basis for Vascular Endothelial Growth Factor Receptor Activation and Implications for Disease Therapy. Biomolecules 2020, 10, 1673. [Google Scholar] [CrossRef]
- Huijbers, E.J.M.; Khan, K.A.; Kerbel, R.S.; Griffioen, A.W. Tumors Resurrect an Embryonic Vascular Program to Escape Immunity. Sci. Immunol. 2022, 7, eabm6388. [Google Scholar] [CrossRef]
- Wilkus, K.; Brodaczewska, K.; Kajdasz, A.; Kieda, C. Distinctive Properties of Endothelial Cells from Tumor and Normal Tissue in Human Breast Cancer. Int. J. Mol. Sci. 2021, 22, 8862. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.L.; Cohen, R.B.; Eadens, M.; Gore, L.; Camidge, D.R.; Diab, S.; Leong, S.; O’Bryant, C.; Chow, L.Q.M.; Serkova, N.J.; et al. Phase I Pharmacologic and Biologic Study of Ramucirumab (IMC-1121B), a Fully Human Immunoglobulin G1 Monoclonal Antibody Targeting the Vascular Endothelial Growth Factor Receptor-2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 780–787. [Google Scholar] [CrossRef]
- Arrieta, O.; Zatarain-Barrón, Z.L.; Cardona, A.F.; Carmona, A.; Lopez-Mejia, M. Ramucirumab in the Treatment of Non-Small Cell Lung Cancer. Expert Opin. Drug Saf. 2017, 16, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J. Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr. Oncol. Rep. 2011, 13, 97–102. [Google Scholar] [CrossRef]
- Warwas, K.M.; Meyer, M.; Gonçalves, M.; Moldenhauer, G.; Bulbuc, N.; Knabe, S.; Luckner-Minden, C.; Ziegelmeier, C.; Heussel, C.P.; Zörnig, I.; et al. Co-Stimulatory Bispecific Antibodies Induce Enhanced T Cell Activation and Tumor Cell Killing in Breast Cancer Models. Front. Immunol. 2021, 12, 3240. [Google Scholar] [CrossRef]
- Leong, A.; Kim, M. The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer. Int. J. Mol. Sci. 2020, 21, 8689. [Google Scholar] [CrossRef]
- Kaipainen, A.; Vlaykova, T.; Hatva, E.; Böhling, T.; Jekunen, A.; Pyrhönen, S.; Alitalo, K. Enhanced Expression of the Tie Receptor Tyrosine Kinase Mesenger RNA in the Vascular Endothelium of Metastatic Melanomas. Cancer Res. 1994, 54, 6571–6577. [Google Scholar] [PubMed]
- Mazanet, M.M.; Hughes, C.C.W. B7-H1 Is Expressed by Human Endothelial Cells and Suppresses T Cell Cytokine Synthesis. J. Immunol. 2002, 169, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Rodig, N.; Ryan, T.; Allen, J.A.; Pang, H.; Grabie, N.; Chernova, T.; Greenfield, E.A.; Liang, S.C.; Sharpe, A.H.; Lichtman, A.H.; et al. Endothelial Expression of PD-L1 and PD-L2 down-Regulates CD8+ T Cell Activation and Cytolysis. Eur. J. Immunol. 2003, 33, 3117–3126. [Google Scholar] [CrossRef]
- Piao, W.; Li, L.; Saxena, V.; Iyyathurai, J.; Lakhan, R.; Zhang, Y.; Lape, I.T.; Paluskievicz, C.; Hippen, K.L.; Lee, Y.; et al. PD-L1 Signaling Selectively Regulates T Cell Lymphatic Transendothelial Migration. Nat. Commun. 2022, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Rood, P.M.L.; Calafat, J.; Von Dem Borne, A.E.G.K.; Gerritsen, W.R.; Van Der Schoot, C.E. Immortalisation of Human Bone Marrow Endothelial Cells: Characterisation of New Cell Lines. Eur. J. Clin. Investig. 2000, 30, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, Y.; Kiseljak, D.; Baldi, L.; Hacker, D.L.; Wurm, F.M. A Simple High-Yielding Process for Transient Gene Expression in CHO Cells. J. Biotechnol. 2011, 153, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, Y.; Kiseljak, D.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Reduced Glutamine Concentration Improves Protein Production in Growth-Arrested CHO-DG44 and HEK-293E Cells. Biotechnol. Lett. 2012, 34, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Wulhfard, S.; Baldi, L.; Hacker, D.; Wurm, F. Valproic Acid Enhances Recombinant MRNA and Protein Levels in Transiently Transfected Chinese Hamster Ovary Cells. J. Biotechnol. 2010, 148, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Wulhfard, S.; Bouchet, S.; Cevey, J.; De Jesus, M.; Hacker, D.L.; Wurm, F.M. Mild Hypothermia Improves Transient Gene Expression Yields Several Fold in Chinese Hamster Ovary Cells. Biotechnol. Prog. 2008, 24, 458–465. [Google Scholar] [CrossRef]
- Arosa, F.A. CD8+CD28− T Cells: Certainties and Uncertainties of a Prevalent Human T-Cell Subset. Immunol. Cell Biol. 2002, 80, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Diskin, B.; Adam, S.; Cassini, M.F.; Sanchez, G.; Liria, M.; Aykut, B.; Buttar, C.; Li, E.; Sundberg, B.; Salas, R.D.; et al. PD-L1 Engagement on T Cells Promotes Self-Tolerance and Suppression of Neighboring Macrophages and Effector T Cells in Cancer. Nat. Immunol. 2020, 21, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Onishi, H.; Wada, J.; Yamasaki, A.; Tanaka, H.; Nakano, K.; Morisaki, T.; Katano, M. VEGFR2 Is Selectively Expressed by FOXP3high CD4+ Treg. Eur. J. Immunol. 2010, 40, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Gimbrone, M.A.; Lapierre, L.A.; Mendrick, D.L.; Fiers, W.; Rothlein, R.; Springer, T.A. Overlapping Patterns of Activation of Human Endothelial Cells by Interleukin 1, Tumor Necrosis Factor, and Immune Interferon. J. Immunol. 1986, 137, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Swerlick, R.A.; Lee, K.H.; Li, L.J.; Sepp, N.T.; Caughman, S.W.; Lawley, T.J. Regulation of Vascular Cell Adhesion Molecule 1 on Human Dermal Microvascular Endothelial Cells. J. Immunol. 1992, 149, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Goveia, J.; Rohlenova, K.; Taverna, F.; Treps, L.; Conradi, L.-C.; Pircher, A.; Geldhof, V.; de Rooij, L.P.M.H.; Kalucka, J.; Sokol, L.; et al. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell 2020, 37, 21–36.e13. [Google Scholar] [CrossRef] [PubMed]
- Kalucka, J.; de Rooij, L.P.M.H.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.-A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef]
- Xie, Y.; He, L.; Lugano, R.; Zhang, Y.; Cao, H.; He, Q.; Chao, M.; Liu, B.; Cao, Q.; Wang, J.; et al. Key Molecular Alterations in Endothelial Cells in Human Glioblastoma Uncovered through Single-Cell RNA Sequencing. JCI Insight 2021, 6, e150861. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.M.; Yang, L.; Garcia-Cardena, G.; Luscinskas, F.W. Endothelial-Dependent Mechanisms of Leukocyte Recruitment to the Vascular Wall. Circ. Res. 2007, 101, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of Leukocyte Transendothelial Migration. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune Surveillance in the Skin: Mechanisms and Clinical Consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lee, Y.; Liu, W.; Krausz, T.; Chong, A.; Schreiber, H.; Fu, Y.X. Intratumor Depletion of CD4+ Cells Unmasks Tumor Immunogenicity Leading to the Rejection of Late-Stage Tumors. J. Exp. Med. 2005, 201, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Peske, J.D.; Woods, A.B.; Engelhard, V.H. Control of CD8 T-Cell Infiltration into Tumors by Vasculature and Microenvironment. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 128, pp. 263–307. ISBN 9780128023167. [Google Scholar]
- Lanitis, E.; Irving, M.; Coukos, G. Targeting the Tumor Vasculature to Enhance T Cell Activity. Curr. Opin. Immunol. 2015, 33, 55–63. [Google Scholar] [CrossRef]
- Lanitis, E.; Dangaj, D.; Irving, M.; Coukos, G. Mechanisms Regulating T-Cell Infiltration and Activity in Solid Tumors. Ann. Oncol. 2017, 28, xii18–xii32. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, J. Present and Future of Cancer Immunotherapy: A Tumor Microenvironmental Perspective (Review). Oncol. Lett. 2018, 16, 4105–4113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Kopacek, A.; Böldicke, T.; Lergenmüller, S.; Berthold, F.; Jensen, M.; Müller, P.P.; Grosse-Hovest, L. Construction, Expression and Binding Specificity of Bispecific CD3 × VEGFR-2 and CD3 × NCAM Antibodies in the Single Chain and Diabody Format. Adv. Biosci. Biotechnol. 2013, 4, 654–664. [Google Scholar] [CrossRef][Green Version]
- Zhong, L.; Shi, W.; Gan, L.; Liu, X.; Huo, Y.; Wu, P.; Zhang, Z.; Wu, T.; Peng, H.; Huang, Y.; et al. Human Endoglin-CD3 Bispecific T Cell Engager Antibody Induces Anti-Tumor Effect in Vivo. Theranostics 2021, 11, 6393–6406. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Damen, C.A.; Blijham, G.H.; Groenewegen, G. Endoglin/CD 105 May Not Be an Optimal Tumor Endothelial Treatment Target. Breast Cancer Res. Treat. 1996, 39, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Wang, Y.; Pan, M.; Wang, M.; Zhang, J. A VEGFR2–MICA Bispecific Antibody Activates Tumor-Infiltrating Lymphocytes and Exhibits Potent Anti-Tumor Efficacy in Mice. Cancer Immunol. Immunother. 2019, 68, 1429–1441. [Google Scholar] [CrossRef]
- Liu, X.; Sun, T.; Ge, Q.; Zhu, J. Construction of Novel Bispecific Single-Domain Antibodies (BiSdAbs) with Potent Antiangiogenic Activities. Pharm. Front. 2020, 2, 64–76. [Google Scholar] [CrossRef]
- Niederman, T.M.J.; Ghogawala, Z.; Carter, B.S.; Tompkins, H.S.; Russell, M.M.; Mulligan, R.C. Antitumor Activity of Cytotoxic T Lymphocytes Engineered to Target Vascular Endothelial Growth Factor Receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 7009–7014. [Google Scholar] [CrossRef]
- Chinnasamy, D.; Yu, Z.; Theoret, M.R.; Zhao, Y.; Shrimali, R.K.; Morgan, R.A.; Feldman, S.A.; Restifo, N.P.; Rosenberg, S.A. Gene Therapy Using Genetically Modified Lymphocytes Targeting VEGFR-2 Inhibits the Growth of Vascularized Syngenic Tumors in Mice. J. Clin. Investig. 2010, 120, 3953–3968. [Google Scholar] [CrossRef]
- Kulemzin, S.V.; Gorchakov, A.A.; Chikaev, A.N.; Kuznetsova, V.V.; Volkova, O.Y.; Matvienko, D.A.; Petukhov, A.V.; Zaritskey, A.Y.; Taranin, A. V VEGFR2-Specific FnCAR Effectively Redirects the Cytotoxic Activity of T Cells and YT NK Cells. Oncotarget 2018, 9, 9021–9029. [Google Scholar] [CrossRef] [PubMed]
- Hajari Taheri, F.; Hassani, M.; Sharifzadeh, Z.; Behdani, M.; Arashkia, A.; Abolhassani, M. T Cell Engineered with a Novel Nanobody-based Chimeric Antigen Receptor against VEGFR2 as a Candidate for Tumor Immunotherapy. IUBMB Life 2019, 71, 1259–1267. [Google Scholar] [CrossRef]
- Lanitis, E.; Kosti, P.; Ronet, C.; Cribioli, E.; Rota, G.; Spill, A.; Reichenbach, P.; Zoete, V.; Dangaj Laniti, D.; Coukos, G.; et al. VEGFR-2 Redirected CAR-T Cells Are Functionally Impaired by Soluble VEGF-A Competition for Receptor Binding. J. Immunother. Cancer 2021, 9, e002151. [Google Scholar] [CrossRef]
- Chinnasamy, D.; Yu, Z.; Kerkar, S.P.; Zhang, L.; Morgan, R.A.; Restifo, N.P.; Rosenberg, S.A. Local Delivery of Lnterleukin-12 Using T Cells Targeting VEGF Receptor-2 Eradicates Multiple Vascularized Tumors in Mice. Clin. Cancer Res. 2012, 18, 1672–1683. [Google Scholar] [CrossRef]
- Lanitis, E.; Rota, G.; Kosti, P.; Ronet, C.; Spill, A.; Seijo, B.; Romero, P.; Dangaj, D.; Coukos, G.; Irving, M. Optimized Gene Engineering of Murine CAR-T Cells Reveals the Beneficial Effects of IL-15 Coexpression. J. Exp. Med. 2021, 218, e20192203. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Rivera, A.; Tao, L.; Zhang, X. Genetically Modified T Cells Targeting Neovasculature Efficiently Destroy Tumor Blood Vessels, Shrink Established Solid Tumors and Increase Nanoparticle Delivery. Int. J. Cancer 2013, 133, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.P.; Kim, S.; Motz, G.T.; Alatzoglou, D.; Li, C.; Irving, M.; Powell, D.J.; Coukos, G. T Cells Bearing a Chimeric Antigen Receptor against Prostate-Specific Membrane Antigen Mediate Vascular Disruption and Result in Tumor Regression. Cancer Immunol. Res. 2015, 3, 68–84. [Google Scholar] [CrossRef]
- Xie, Y.J.; Dougan, M.; Jailkhani, N.; Ingram, J.; Fang, T.; Kummer, L.; Momin, N.; Pishesha, N.; Rickelt, S.; Hynes, R.O.; et al. Nanobody-Based CAR T Cells That Target the Tumor Microenvironment Inhibit the Growth of Solid Tumors in Immunocompetent Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 7624–7631. [Google Scholar] [CrossRef] [PubMed]
- Quitt, O.; Luo, S.; Meyer, M.; Xie, Z.; Golsaz-Shirazi, F.; Loffredo-Verde, E.; Festag, J.; Bockmann, J.H.; Zhao, L.; Stadler, D.; et al. T-Cell Engager Antibodies Enable T Cells to Control HBV Infection and to Target HBsAg-Positive Hepatoma in Mice. J. Hepatol. 2021, 75, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Tuijnman, W.B.; Van Wichen, D.F.; Schuurman, H.-J. Tissue Distribution of Human IgG Fc Receptors CD16, CD32 and CD64: An Immunohistochemical Study. APMIS 1993, 101, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus Paclitaxel versus Placebo plus Paclitaxel in Patients with Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma (RAINBOW): A Double-Blind, Randomised Phase 3 Trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Obermannová, R.; Van Cutsem, E.; Yoshino, T.; Bodoky, G.; Prausová, J.; Garcia-Carbonero, R.; Ciuleanu, T.; Garcia Alfonso, P.; Portnoy, D.; Cohn, A.; et al. Subgroup Analysis in RAISE: A Randomized, Double-Blind Phase III Study of Irinotecan, Folinic Acid, and 5-Fluorouracil (FOLFIRI) plus Ramucirumab or Placebo in Patients with Metastatic Colorectal Carcinoma Progression. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 2082–2090. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Singh, A.D.; Parmar, S. Ramucirumab (Cyramza): A Breakthrough Treatment for Gastric Cancer. Pharm. Ther. 2015, 40, 430. [Google Scholar] [PubMed] [PubMed Central]
- Basu, A.; Hoerning, A.; Datta, D.; Edelbauer, M.; Stack, M.P.; Calzadilla, K.; Pal, S.; Briscoe, D.M. Cutting Edge: Vascular Endothelial Growth Factor-Mediated Signaling in Human CD45RO+ CD4+ T Cells Promotes Akt and ERK Activation and Costimulates IFN-γ Production. J. Immunol. 2009, 184, 545–549. [Google Scholar] [CrossRef]
- Ziogas, A.C.; Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Terpos, E.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF Directly Suppresses Activation of T Cells from Ovarian Cancer Patients and Healthy Individuals via VEGF Receptor Type 2. Int. J. Cancer 2012, 130, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Werner Sunderland, M.; Nerviani, A.; Scottà, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 Signaling on Human Memory CD4+ T Cells Induces a Regulatory Phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef]
- Murdoch, C.; Tazzyman, S.; Webster, S.; Lewis, C.E. Expression of Tie-2 by Human Monocytes and Their Responses to Angiopoietin-21. J. Immunol. 2007, 178, 7405–7411. [Google Scholar] [CrossRef]
- Venneri, M.A.; De Palma, M.; Ponzoni, M.; Pucci, F.; Scielzo, C.; Zonari, E.; Mazzieri, R.; Doglioni, C.; Naldini, L. Identification of Proangiogenic TIE2-Expressing Monocytes (TEMs) in Human Peripheral Blood and Cancer. Blood 2007, 109, 5276–5285. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a Natural Antagonist for Tie2 That Disrupts in Vivo Angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Tait, C.R.; Jones, P.F. Angiopoietins in Tumours: The Angiogenic Switch. J. Pathol. 2004, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kim, I.-K.; Han, S.; Park, I.; Kim, C.; Bae, J.; Oh, S.J.; Lee, S.; Kim, J.H.; Woo, D.-C.; et al. Normalization of Tumor Vessels by Tie2 Activation and Ang2 Inhibition Enhances Drug Delivery and Produces a Favorable Tumor Microenvironment. Cancer Cell 2016, 30, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, E.-A.; Kong, E.; Chon, H.; Llaiqui-Condori, M.; Park, C.H.; Park, B.Y.; Kang, N.R.; Yoo, J.-S.; Lee, H.-S.; et al. An Agonistic Anti-Tie2 Antibody Suppresses the Normal-to-Tumor Vascular Transition in the Glioblastoma Invasion Zone. Exp. Mol. Med. 2023, 55, 470–484. [Google Scholar] [CrossRef]
- Thurston, G. Anti-Tie2 Antibodies and Uses Thereof. U.S. Patent 9017670B2, 28 April 2015. [Google Scholar]
- Correnti, C.E.; Laszlo, G.S.; de van der Schueren, W.J.; Godwin, C.D.; Bandaranayake, A.; Busch, M.A.; Gudgeon, C.J.; Bates, O.M.; Olson, J.M.; Mehlin, C.; et al. Simultaneous Multiple Interaction T-Cell Engaging (SMITE) Bispecific Antibodies Overcome Bispecific T-Cell Engager (BiTE) Resistance via CD28 Co-Stimulation. Leukemia 2018, 32, 1239–1243. [Google Scholar] [CrossRef]
- Tada, Y.; Togashi, Y.; Kotani, D.; Kuwata, T.; Sato, E.; Kawazoe, A.; Doi, T.; Wada, H.; Nishikawa, H.; Shitara, K. Targeting VEGFR2 with Ramucirumab Strongly Impacts Effector/Activated Regulatory T Cells and CD8+ T Cells in the Tumor Microenvironment. J. Immunother. Cancer 2018, 6, 106. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Damen, C.A.; Martinotti, S.; Blijham, G.H.; Groenewegen, G. Endothelial Intercellular Adhesion Molecule-1 Expression Is Suppressed in Human Malignancies: The Role of Angiogenic Factors. Cancer Res. 1996, 56, 1111–1117. [Google Scholar] [PubMed]
- Urban, D.; Thanabalasingam, U.; Stibenz, D.; Kaufmann, J.; Meyborg, H.; Fleck, E.; Gräfe, M.; Stawowy, P. CD40/CD40L Interaction Induces E-Selectin Dependent Leukocyte Adhesion to Human Endothelial Cells and Inhibits Endothelial Cell Migration. Biochem. Biophys. Res. Commun. 2011, 404, 448–452. [Google Scholar] [CrossRef]
- Subramaniyan, A.; Maddaly, R. Cytokine Profiling of MCF-7 Cell Line 2D, Progressive 3D, and 3D Revert Cultures. J. Cell. Biochem. 2018, 119, 1309–1312. [Google Scholar] [CrossRef]
- Shimizu, Y.; Newman, W.; Tanaka, Y.; Shaw, S. Lymphocyte Interactions with Endothelial Cells. Immunol. Today 1992, 13, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Marsal, J.; Agace, W.W. Targeting T-Cell Migration in Inflammatory Bowel Disease. J. Intern. Med. 2012, 272, 411–429. [Google Scholar] [CrossRef]
- Mirenda, V.; Jarmin, S.J.; David, R.; Dyson, J.; Scott, D.; Gu, Y.; Lechler, R.I.; Okkenhaug, K.; Marelli-Berg, F.M. Physiologic and Aberrant Regulation of Memory T-Cell Trafficking by the Costimulatory Molecule CD28. Blood 2007, 109, 2968–2977. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Zhu, S.; Wang, H.; Wu, K. Recent Advances and Challenges of Bispecific Antibodies in Solid Tumors. Exp. Hematol. Oncol. 2021, 10, 56. [Google Scholar] [CrossRef]
- Middelburg, J.; Kemper, K.; Engelberts, P.; Labrijn, A.F.; Schuurman, J.; van Hall, T. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers 2021, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, A.; Liu, Q.; Yuan, X.; Xu, H.; Jiao, D.; Pestell, R.G.; Han, X.; Wu, K. Recent Advances of Bispecific Antibodies in Solid Tumors. J. Hematol. Oncol. 2017, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Runcie, K.; Budman, D.R.; John, V.; Seetharamu, N. Bi-Specific and Tri-Specific Antibodies- the next Big Thing in Solid Tumor Therapeutics. Mol. Med. 2018, 24, 50. [Google Scholar] [CrossRef] [PubMed]


| αVEGFR2 scFv–hIgG1-FcFcR-null–αhCD3ε scFv |
| αTIE2 scFv–hIgG1-FcFcR-null–αhCD28 scFv |
| αPD-L1 scFv–hIgG1-FcFcR-null–αhCD28 scFv |
| αVEGFR2 scFv–hIgG1-FcFcR-null–ahCD28 scFv |
| αEpCAM scFv–hIgG1-FcFcR-null–αhCD3ε scFv [20] |
| αEpCAM scFv–hIgG1-FcFcR-null–αhCD28 scFv [20] |
| αVEGFR2 scFv–hIgG1-FcFcR-null (control) |
| αVEGFR2 scFv–mIgG2a-FcFcR-null–hTNF-α |
| αVEGFR2 scFv–mIgG2a-FcFcR-null–hIL-1β |
| αVEGFR2 scFv–mIgG2a-FcFcR-null (control) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.; Warwas, K.M.; Meyer, M.; Schwartz-Albiez, R.; Bulbuc, N.; Zörnig, I.; Jäger, D.; Momburg, F. Reversal of Endothelial Cell Anergy by T Cell-Engaging Bispecific Antibodies. Cancers 2024, 16, 4251. https://doi.org/10.3390/cancers16244251
Gonçalves M, Warwas KM, Meyer M, Schwartz-Albiez R, Bulbuc N, Zörnig I, Jäger D, Momburg F. Reversal of Endothelial Cell Anergy by T Cell-Engaging Bispecific Antibodies. Cancers. 2024; 16(24):4251. https://doi.org/10.3390/cancers16244251
Chicago/Turabian StyleGonçalves, Márcia, Karsten M. Warwas, Marten Meyer, Reinhard Schwartz-Albiez, Nadja Bulbuc, Inka Zörnig, Dirk Jäger, and Frank Momburg. 2024. "Reversal of Endothelial Cell Anergy by T Cell-Engaging Bispecific Antibodies" Cancers 16, no. 24: 4251. https://doi.org/10.3390/cancers16244251
APA StyleGonçalves, M., Warwas, K. M., Meyer, M., Schwartz-Albiez, R., Bulbuc, N., Zörnig, I., Jäger, D., & Momburg, F. (2024). Reversal of Endothelial Cell Anergy by T Cell-Engaging Bispecific Antibodies. Cancers, 16(24), 4251. https://doi.org/10.3390/cancers16244251






