The Safety and Suitability of DNA Sequencing of Tissue Biopsies Performed on Patients Referred to a Phase I Unit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
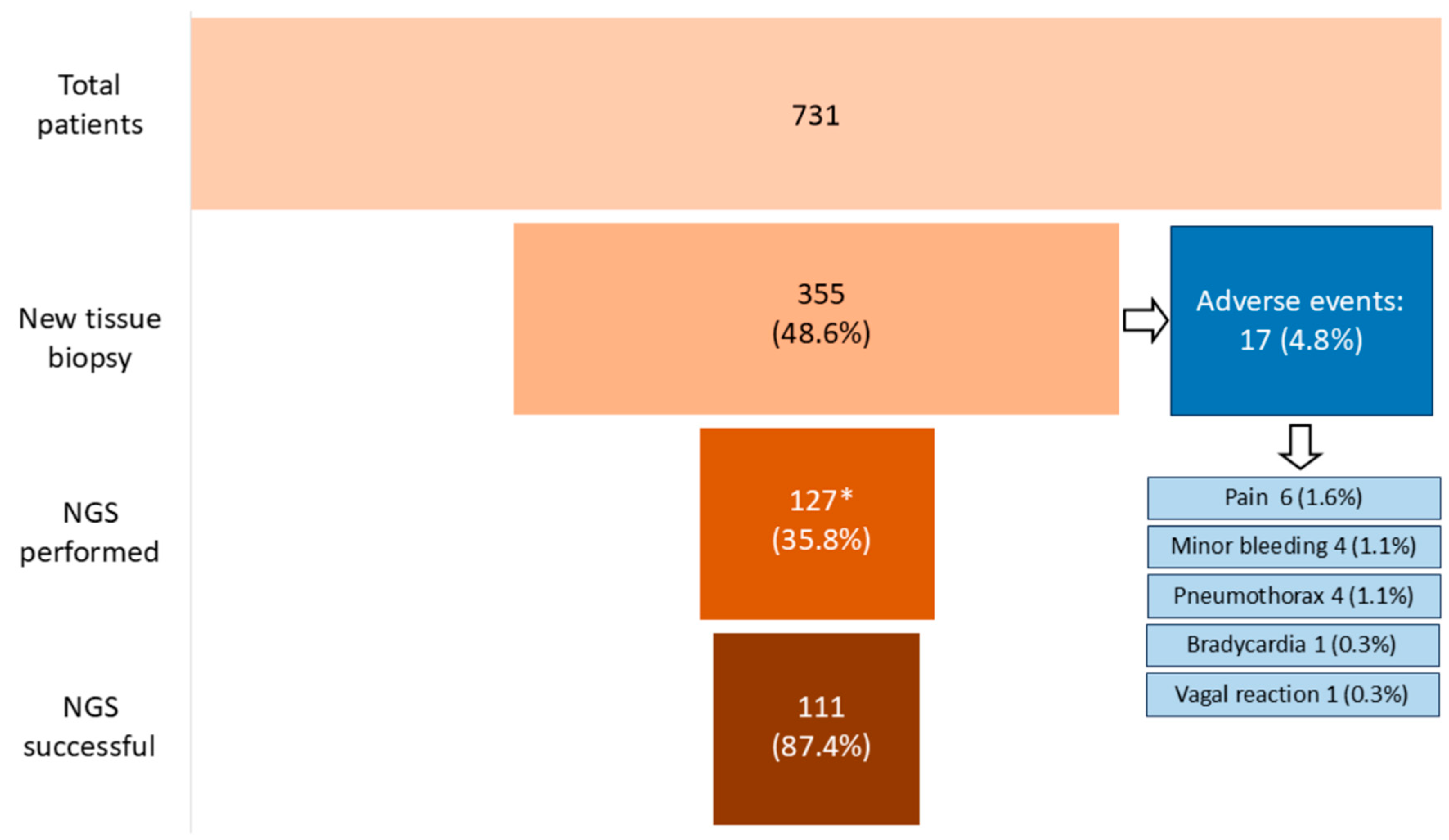
3.1. Characteristics of Patients and Biopsies
3.2. Adequacy of Biopsies for Histological Diagnosis and NGS
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.; Crimini, E.; Belli, C.; Boscolo Bielo, L.; Ascione, L.; Meric-Bernstam, F.; Drilon, A.; Curigliano, G. A demand-offer critical analysis of current drug development. Phase I drugs versus TCGA sequencing data. Eur. J. Cancer 2023, 190, 112958. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kass, N.; Taylor, H.; Fogarty, L.; Sugarman, J.; Goodman, S.N.; Goodwin-Landher, A.; Carducci, M.; Hurwitz, H. Purpose and Benefits of Early Phase Cancer Trials: What Do Oncologists Say? What Do Patients Hear? J. Empir. Res. Hum. Res. Ethic. 2008, 3, 57. [Google Scholar] [CrossRef]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. In American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology Meeting; NIH Public Access: Washington, WA, USA, 2016; Volume 35, p. 185. [Google Scholar] [CrossRef]
- Levit, L.A.; Peppercorn, J.M.; Tam, A.L.; Marron, J.M.; Mathews, D.J.H.; Levit, K.; Roach, N.; Ratain, M.J. Ethical framework for including research biopsies in oncology clinical trials: American Society of Clinical oncology research statement. J. Clin. Oncol. 2019, 37, 2368–2377. [Google Scholar] [CrossRef]
- Crimini, E.; Repetto, M.; Tarantino, P.; Ascione, L.; Antonarelli, G.; Rocco, E.G.; Barberis, M.; Mazzarella, L.; Curigliano, G. Challenges and Obstacles in Applying Therapeutical Indications Formulated in Molecular Tumor Boards. Cancers 2022, 14, 3193. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Gray, R.; Chen, A.; Li, S.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Mitchell, E.P.; Iafrate, A.J.; Sklar, J.; et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) Trial: Lessons for Genomic Trial Design. J. Natl. Cancer Inst. 2020, 112, 1021–1029. [Google Scholar] [CrossRef]
- Araya, A.; Zeng, J.; Johnson, A.; Shufean, M.A.; Rodon, J.; Meric-Bernstam, F.; Bernstam, E.V. Rate of change in investigational treatment options: An analysis of reports from a large precision oncology decision support effort. Int. J. Med. Inform. 2020, 143, 104261. [Google Scholar] [CrossRef]
- Sánchez, N.S.; Kahle, M.P.; Bailey, A.M.; Wathoo, C.; Balaji, K.; Demirhan, M.E.; Yang, D.; Javle, M.; Kaseb, A.; Eng, C.; et al. Identification of Actionable Genomic Alterations Using Circulating Cell-Free DNA. JCO Precis. Oncol. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Johnson, A.; Ng, P.K.; Kahle, M.; Castillo, J.; Amador, B.; Wang, Y.; Zeng, J.; Holla, V.; Vu, T.; Su, F.; et al. Actionability classification of variants of unknown significance correlates with functional effect. NPJ Precis. Oncol. 2023, 7, 67. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Kahle, M.; Vo, H.H.; Baysal, M.A.; Johnson, A.; Meric-Bernstam, F. Molecular tumour boards—Current and future considerations for precision oncology. Nat. Rev. Clin. Oncol. 2023, 20, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.; Crimini, E.; Boscolo Bielo, L.; Guerini-Rocco, E.; Ascione, L.; Bonfanti, A.; Zanzottera, C.; Mazzarella, L.; Ranghiero, A.; Belli, C.; et al. Molecular tumour board at European Institute of Oncology: Report of the first three year activity of an Italian precision oncology experience. Eur. J. Cancer 2023, 183, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Filleron, T.; Kamal, M.; Mosele, F.; Arnedos, M.; Dalenc, F.; Sablin, M.P.; Campone, M.; Bonnefoi, H.; Lefeuvre-Plesse, C.; et al. Genomics to select treatment for patients with metastatic breast cancer. Nature 2022, 610, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Subbiah, V.; Iannotti, N.O.; Gutierrez, M.; Smith, D.C.; Féliz, L.; Lihou, C.F.; Tian, C.; Silverman, I.M.; Ji, T.; Saleh, M. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann. Oncol. 2022, 33, 522–533. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Cho, M.; Ahn, S.; Hong, M.; Bang, H.; Van Vrancken, M.; Kim, S.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; et al. Tissue recommendations for precision cancer therapy using next generation sequencing: A comprehensive single cancer center’s experiences. Oncotarget 2017, 8, 42478–42486. [Google Scholar] [CrossRef]
- Samoyedny, A.; Srinivasan, A.; States, L.; Mosse, Y.P.; Alai, E.; Pawel, B.; Pogoriler, J.; Shellikeri, S.; Vatsky, S.; Acord, M.; et al. Image-Guided Biopsy for Relapsed Neuroblastoma: Focus on Safety, Adequacy for Genetic Sequencing, and Correlation of Tumor Cell Percent with Quantitative Lesion MIBG Uptake. JCO Precis. Oncol. 2021, 5, 275–285. [Google Scholar] [CrossRef]
- Kongkam, P.; Orprayoon, T.; Yooprasert, S.; Sirisub, N.; Klaikaew, N.; Sanpawat, A.; Safa, S.; Ridtitid, W.; Kullavanijaya, P.; Rerknimitr, R. Endoscopic ultrasound guided fine needle biopsy (EUS-FNB) from peritoneal lesions: A prospective cohort pilot study. BMC Gastroenterol. 2021, 21, 400. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.J.; Yoon, S.; Ryoo, B.Y.; Kim, S.W.; Choi, S.H.; Lee, S.M.; Chae, E.J.; Park, Y.; Jang, S.J.; et al. Feasibility, safety, and adequacy of research biopsies for cancer clinical trials at an academic medical center. PLoS ONE 2019, 14, e0221065. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Belle, S.H.; Kleiner, D.E.; Smith, C.; Kelley, S.S.; Rosenthal, P.; Fontana, R.J. Safety and yield of percutaneous liver biopsy in adults and children with chronic hepatitis B: Results from a prospective, multicenter study. Hepatol. Commun. 2023, 7, e0116. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y.; Kanogawa, N.; Ogasawara, S.; Ogawa, K.; Ishino, T.; Nakagawa, M.; Fujiwara, K.; Unozawa, H.; Iwanaga, T.; Sakuma, T.; et al. Liver biopsy technique in the era of genomic cancer therapies: A single-center retrospective analysis. Int. J. Clin. Oncol. 2022, 27, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- van der Poorten, D.; Kwok, A.; Lam, T.; Ridley, L.; Jones, D.B.; Ngu, M.C.; Lee, A.U. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern. Med. J. 2006, 36, 692–699. [Google Scholar] [CrossRef]
- Chi, H.; Hansen, B.E.; Tang, W.Y.; Schouten, J.N.; Sprengers, D.; Taimr, P.; Janssen, H.L.; de Knegt, R.J. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur. J. Gastroenterol. Hepatol. 2017, 29, 36–41. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- FoundationOne CDx|Foundation Medicine N.D. Available online: https://www.foundationmedicine.com/test/foundationone-cdx (accessed on 29 March 2024).
- Di Chiaro, P.; Nacci, L.; Arco, F.; Brandini, S.; Polletti, S.; Palamidessi, A.; Donati, B.; Soriani, C.; Gualdrini, F.; Frigè, G.; et al. Mapping functional to morphological variation reveals the basis of regional extracellular matrix subversion and nerve invasion in pancreatic cancer. Cancer Cell 2024, 42, 662–681.e10. [Google Scholar] [CrossRef] [PubMed]
- Eso, Y.; Kou, T.; Nagai, H.; Kim, Y.H.; Kanai, M.; Matsumoto, S.; Mishima, M.; Arasawa, S.; Iguchi, E.; Nakamura, F.; et al. Utility of ultrasound-guided liver tumor biopsy for next-generation sequencing-based clinical sequencing. Hepatol. Res. 2019, 49, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for Validation of Next-Generation Sequencing—Based Oncology Panels: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2017, 19, 341. [Google Scholar] [CrossRef]
- Yip, S.; Christofides, A.; Banerji, S.; Downes, M.R.; Izevbaye, I.; Lo, B.; MacMillan, A.; McCuaig, J.; Stockley, T.; Yousef, G.M.; et al. A Canadian guideline on the use of next-generation sequencing in oncology. Curr. Oncol. 2019, 26, e241–e254. [Google Scholar] [CrossRef]
- Compton, C.C.; Robb, J.A.; Anderson, M.W.; Berry, A.B.; Birdsong, G.G.; Bloom, K.J.; Branton, P.A.; Crothers, J.W.; Cushman-Vokoun, A.M.; Hicks, D.G.; et al. Preanalytics and Precision Pathology: Pathology Practices to Ensure Molecular Integrity of Cancer Patient Biospecimens for Precision Medicine. Arch. Pathol. Lab. Med. 2019, 143, 1346–1363. [Google Scholar] [CrossRef]

| Characteristics | Number of Patients n (%) | |
|---|---|---|
| Gender | Female, 280 (79%) | |
| Male, 75 (21%) | ||
| Median age | 56 years (range: 20–83 years) | |
| Tumor type | Breast cancer, 190 (53.5%) Skin cutaneous melanoma, 24 (6.8%) Carcinoma of unknown primary, 21 (5.8%) Lung adenocarcinoma, 15 (4.2%) Cholangiocarcinoma, 14 (3.9%) Stomach adenocarcinoma, 12 (3.4%) Ovarian cancer, 12 (3.4%) Head and neck squamous cell carcinoma, 11 (3.1%) Mesothelioma, 7 (2.0%) Pancreatic adenocarcinoma, 6 (1.7%) Colorectal adenocarcinoma, 5 (1.4%) Urothelial cancer, 4 (1.1%) Lung squamous cell carcinoma, 4 (1.1%) Hepatocellular carcinoma, 3 (0.8%) Endometrial carcinoma, 3 (0.8%) Cervical squamous cell carcinoma, 2 (0.6%) Sarcoma, 1 (0.3%) Miscellanea, 21 (5.9%) | |
| Anticoagulant or antiplatelet therapy | Anticoagulant agents, 29 (8.2%) | LMWH, 27 (7.6%) |
| NOAC, 2 (0.6%) | ||
| Antiplatelets agents, 18 (5.1%) | ||
| No anticoagulant nor antiplatelets agents, 308 (86.7%) | ||
| Test | Foundation One™ CDx [33] | GerSom [34] |
|---|---|---|
| FFPE | Allowed | Allowed |
| Decalcification | Not allowed | Unspecified |
| Sample size | Block + 1 H&E slide or 10 USS (5 µm thickness) + 1 H&E | Block or 3 USS (5 µm thickness) |
| Surface area or volume | 25 mm2 (block) or 1 mm3 (slides) | Unspecified |
| Tumor content | Optimum: >30% TN Minimum: 20% TN | Unspecified |
| Number of genes tested | 324 | 467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, A.; Crimini, E.; Criscitiello, C.; Belli, C.; Scafetta, R.; Scalia, R.; Castellano, G.; Giordano, E.; Katrini, J.; Ascione, L.; et al. The Safety and Suitability of DNA Sequencing of Tissue Biopsies Performed on Patients Referred to a Phase I Unit. Cancers 2024, 16, 4252. https://doi.org/10.3390/cancers16244252
Esposito A, Crimini E, Criscitiello C, Belli C, Scafetta R, Scalia R, Castellano G, Giordano E, Katrini J, Ascione L, et al. The Safety and Suitability of DNA Sequencing of Tissue Biopsies Performed on Patients Referred to a Phase I Unit. Cancers. 2024; 16(24):4252. https://doi.org/10.3390/cancers16244252
Chicago/Turabian StyleEsposito, Angela, Edoardo Crimini, Carmen Criscitiello, Carmen Belli, Roberta Scafetta, Raimondo Scalia, Grazia Castellano, Elisa Giordano, Jalissa Katrini, Liliana Ascione, and et al. 2024. "The Safety and Suitability of DNA Sequencing of Tissue Biopsies Performed on Patients Referred to a Phase I Unit" Cancers 16, no. 24: 4252. https://doi.org/10.3390/cancers16244252
APA StyleEsposito, A., Crimini, E., Criscitiello, C., Belli, C., Scafetta, R., Scalia, R., Castellano, G., Giordano, E., Katrini, J., Ascione, L., Boscolo Bielo, L., Repetto, M., Marra, A., Trapani, D., Varano, G. M., Maiettini, D., Della Vigna, P., Orsi, F., Guerini Rocco, E., ... Curigliano, G. (2024). The Safety and Suitability of DNA Sequencing of Tissue Biopsies Performed on Patients Referred to a Phase I Unit. Cancers, 16(24), 4252. https://doi.org/10.3390/cancers16244252