Contrast-Enhanced Mammography: A Literature Review of Clinical Uses for Cancer Diagnosis and Surgical Oncology
Simple Summary
Abstract
1. Introduction
- (1)
- To understand the fundamentals of CEM including the technique, radiation exposure, and image interpretation;
- (2)
- To familiarize ourselves with the clinical uses for cancer diagnosis including for problem-solving, palpable mass, suspicious microcalcifications, architecture distortion, screening, and CEM-guided biopsy; and
- (3)
- To consider the clinical applications for pre-operative planning and neoadjuvant chemotherapy response assessments.
2. Fundamentals of CEM
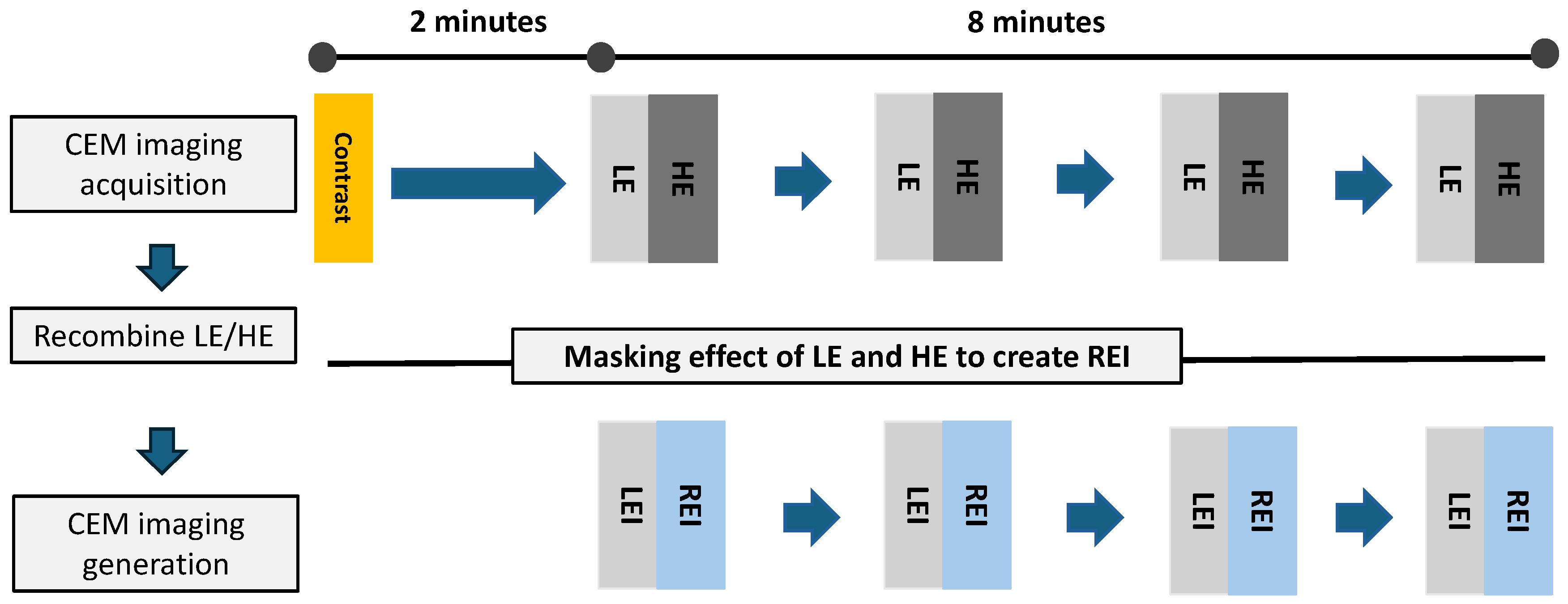
2.1. CEM Technique
2.2. CEM Radiation
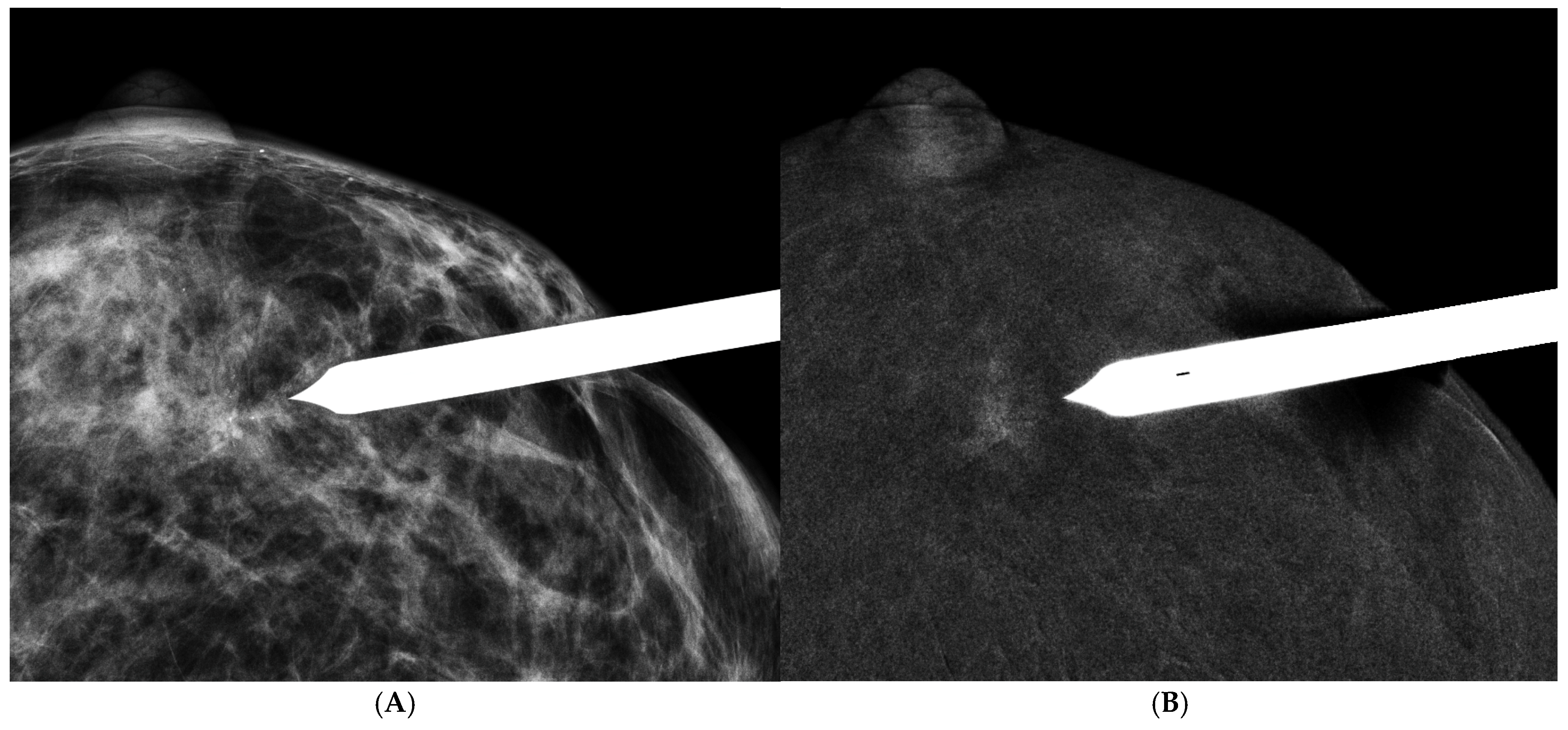
2.3. Image Interpretation
3. CEM for Cancer Diagnosis
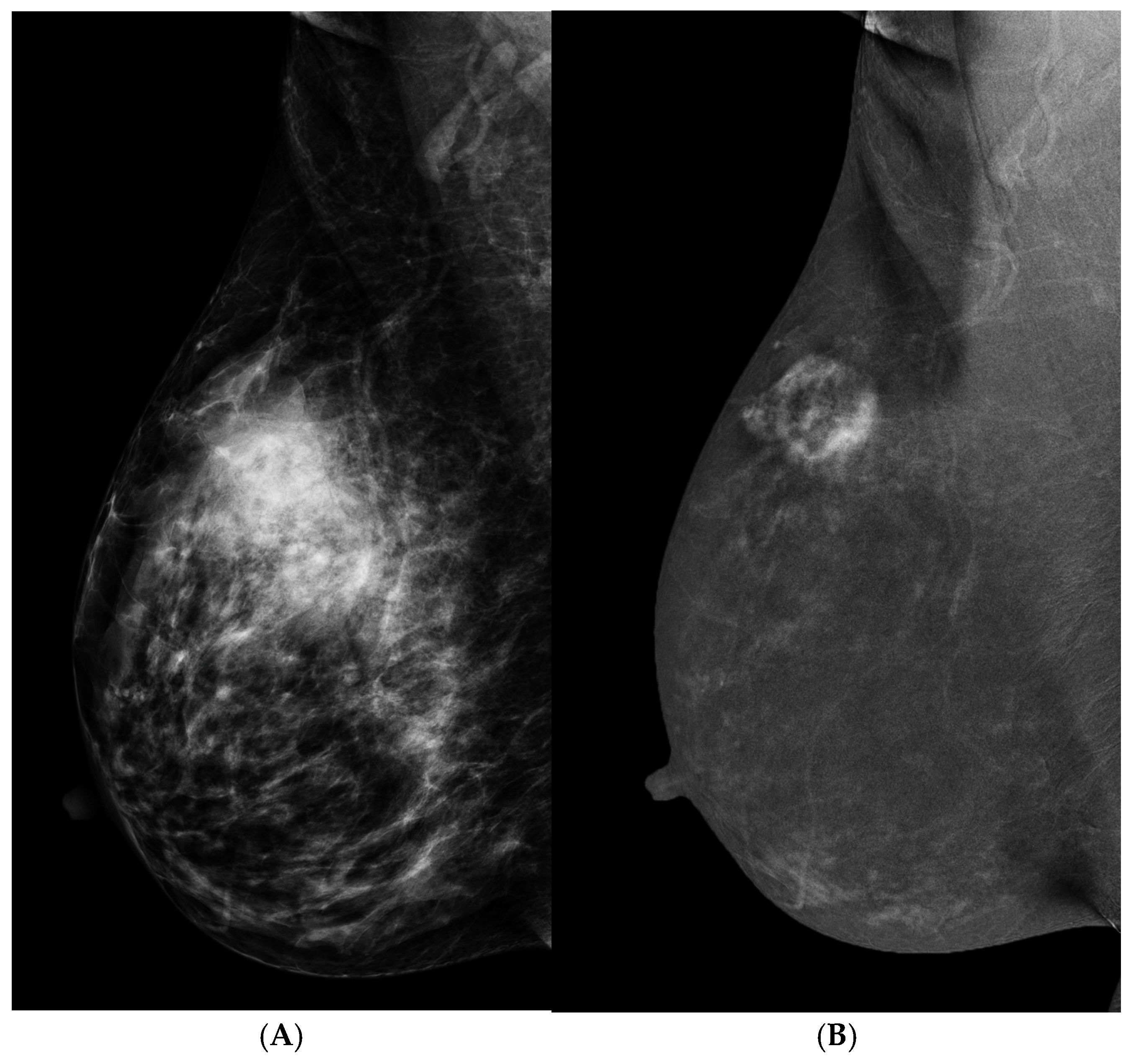
3.1. Problem Solving
3.2. Palpable Mass
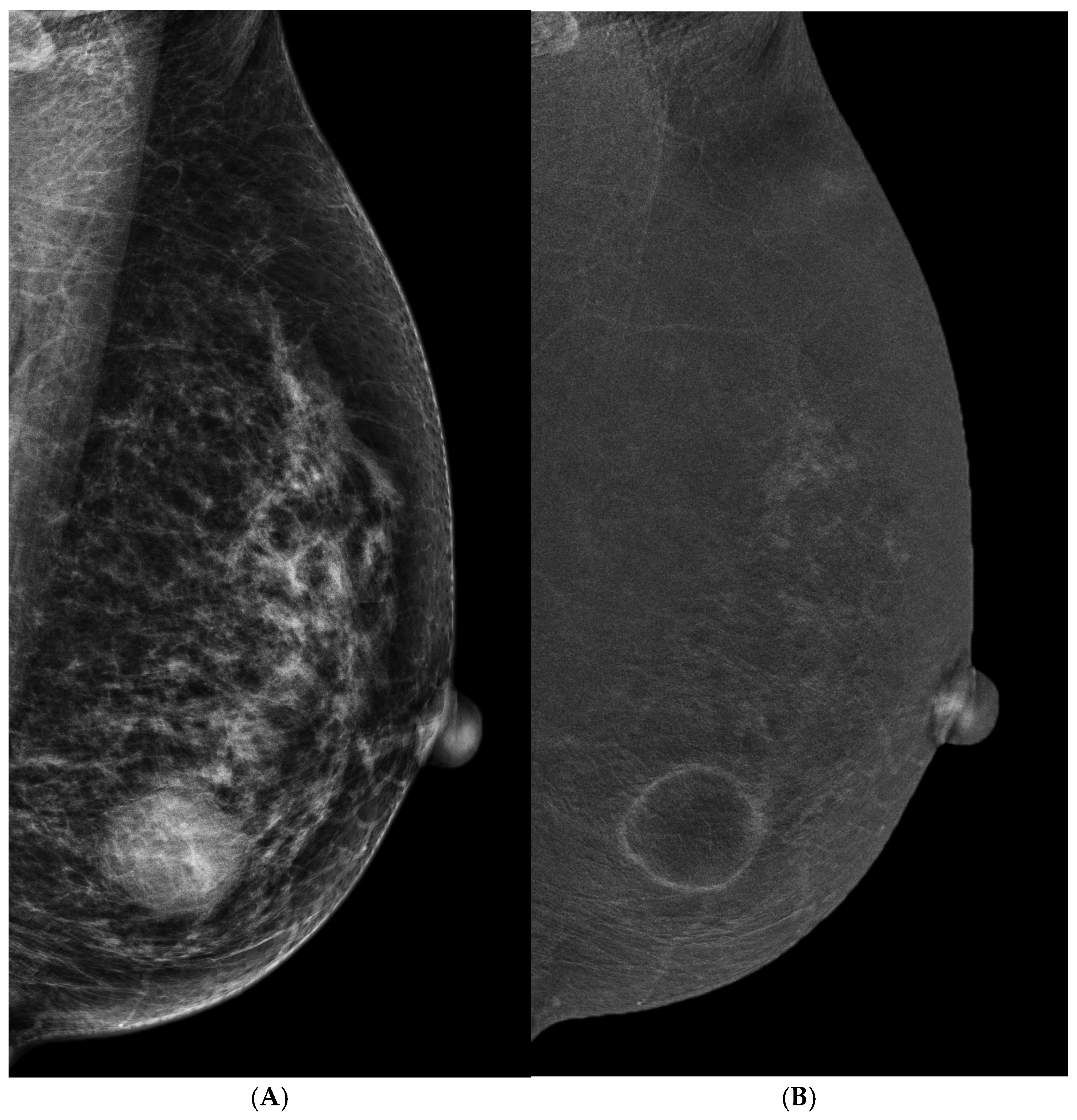
3.3. Suspicious Microcalcifications
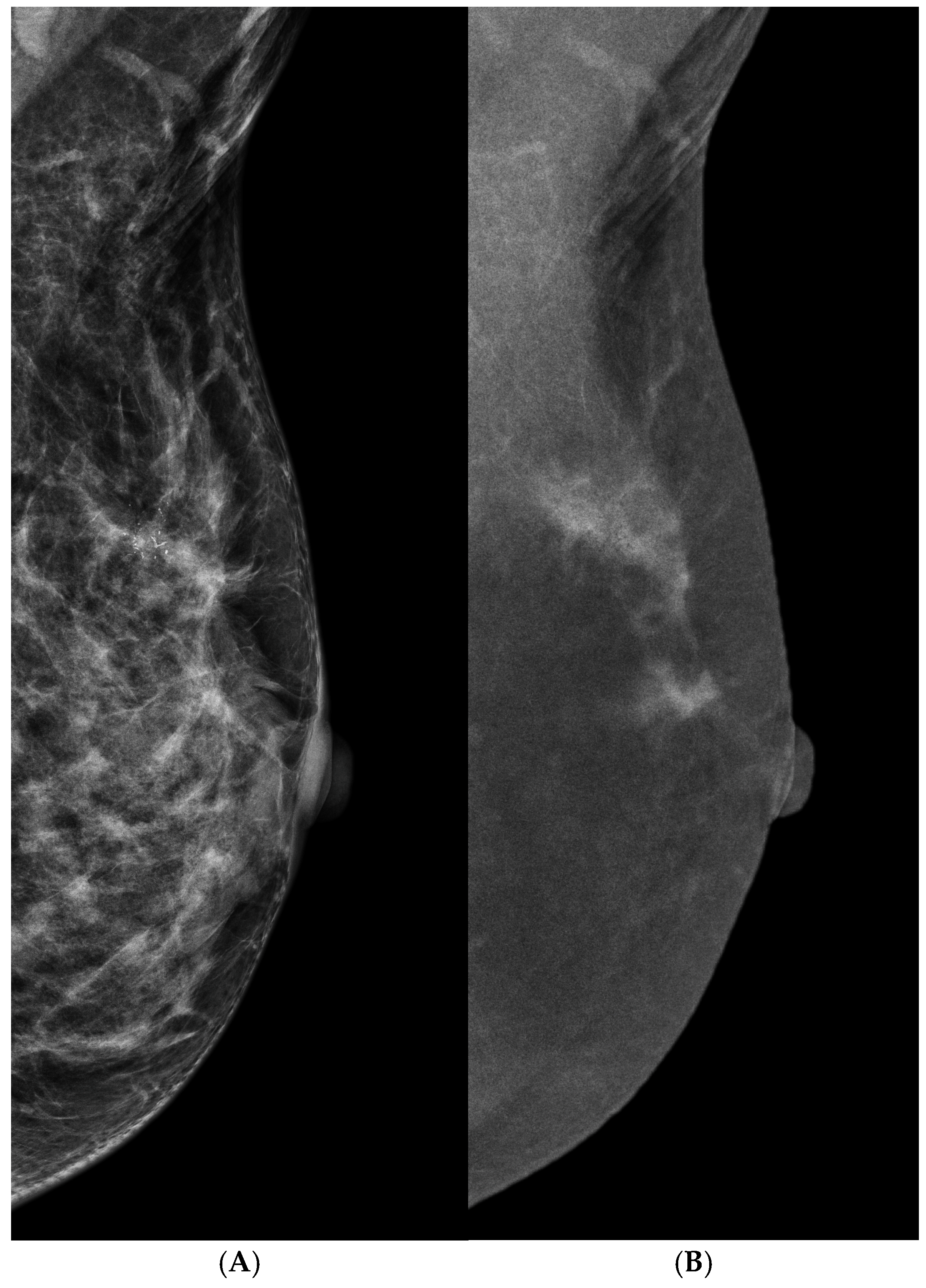
3.4. Architectural Distortion
3.5. Screening
3.6. CEM-Guided Biopsy
4. CEM for Surgical Oncology
4.1. Operation Assessment
4.2. Neoadjuvant Chemotherapy Response Assessment
4.3. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEM | Contrast-enhanced mammography |
| LM | Low-energy mammogram |
| REI | Recombined enhanced image |
| CC | Craniocaudal |
| MLO | Mediolateral oblique |
| DM | Digital mammography |
| AGD | Average glandular dose |
| MRI | Magnetic resonance imaging |
| BI-RADS | Breast Imaging Reporting and Data System |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| CEM-Bx | CEM-guided biopsy |
| AI | Artificial intelligence |
References
- Konstantopoulos, C.; Mehta, T.S.; Brook, A.; Dialani, V.; Mehta, R.; Fein-Zachary, V.; Phillips, J. Cancer Conspicuity on Low-energy Images of Contrast-enhanced Mammography Compared With 2D Mammography. J. Breast Imaging 2022, 4, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Francescone, M.A.; Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Hughes, M.C.; Zheng, J.; Moskowitz, C.; Morris, E.A. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur. J. Radiol. 2014, 83, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.E. Radiation Doses and Risks in Breast Screening. J. Breast Imaging 2020, 2, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Bozzini, A.C.; Pesapane, F.; Rotili, A.; Marinucci, I.; Signorelli, G.; Frassoni, S.; Bagnardi, V.; Origgi, D.; De Marco, P.; et al. Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting. Cancers 2023, 15, 2413. [Google Scholar] [CrossRef]
- Fusco, R.; Raiano, N.; Raiano, C.; Maio, F.; Vallone, P.; Raso, M.M.; Setola, S.V.; Granata, V.; Rubulotta, M.R.; Barretta, M.L.; et al. Evaluation of average glandular dose and investigation of the relationship with compressed breast thickness in dual energy contrast enhanced digital mammography and digital breast tomosynthesis. Eur. J. Radiol. 2020, 126, 108912. [Google Scholar] [CrossRef]
- Bicchierai, G.; Busoni, S.; Tortoli, P.; Bettarini, S.; Naro, F.D.; De Benedetto, D.; Savi, E.; Bellini, C.; Miele, V.; Nori, J. Single Center Evaluation of Comparative Breast Radiation dose of Contrast Enhanced Digital Mammography (CEDM), Digital Mammography (DM) and Digital Breast Tomosynthesis (DBT). Acad. Radiol. 2022, 29, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Compliance Guidance: The Mammography Quality Standards Act Final Regulations Document #1; availability. Food and Drug Administration, HHS. Notice. Fed. Regist. 1999, 64, 13590–13591.
- Sogani, J.; Morris, E.A.; Kaplan, J.B.; D’Alessio, D.; Goldman, D.; Moskowitz, C.S.; Jochelson, M.S. Comparison of Background Parenchymal Enhancement at Contrast-enhanced Spectral Mammography and Breast MR Imaging. Radiology 2017, 282, 63–73. [Google Scholar] [CrossRef]
- Savaridas, S.L.; Taylor, D.B.; Gunawardana, D.; Phillips, M. Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin. Radiol. 2017, 72, 1085.e1–1085.e9. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.E.; Baker, J.A.; Helvie, M.A. Breast cancer deaths averted over 3 decades. Cancer 2019, 125, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, F.; Avendano, D.; Cicero, G.; Garza-Montemayor, M.; Sofia, C.; Venanzi Rullo, E.; Ascenti, G.; Pinker-Domenig, K.; Marino, M.A. High-risk lesions of the breast: Concurrent diagnostic tools and management recommendations. Insights Imaging 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, R.M.; Rossi, P.G.; Battisti, N.M.L.; Cozzi, A.; Magni, V.; Zanardo, M.; Sardanelli, F. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging 2020, 11, 105. [Google Scholar] [CrossRef]
- Ginsburg, O.; Yip, C.H.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126 (Suppl. S10), 2379–2393. [Google Scholar] [CrossRef]
- Broeders, M.; Moss, S.; Nystrom, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E.; et al. The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies. J. Med. Screen. 2012, 19 (Suppl. S1), 14–25. [Google Scholar] [CrossRef]
- Fletcher, S.W.; Elmore, J.G. Clinical practice. Mammographic screening for breast cancer. N. Engl. J. Med. 2003, 348, 1672–1680. [Google Scholar] [CrossRef]
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D’Orsi, C.; et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Helfand, M.; Chan, B.K.; Woolf, S.H. Breast cancer screening: A summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2002, 137, 347–360. [Google Scholar] [CrossRef]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.C.; Lin, Y.C.; Wan, Y.L.; Yeow, K.M.; Huang, P.C.; Lo, Y.F.; Tsai, H.P.; Ueng, S.H.; Chang, C.J. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: Interobserver blind-reading analysis. Eur. Radiol. 2014, 24, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Shalmon, A.; Gotlieb, M.; Neiman, O.H.; Sklair-Levy, M. Contrast-Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breasts. AJR Am. J. Roentgenol. 2018, 211, W267–W274. [Google Scholar] [CrossRef] [PubMed]
- Azzam, H.; Kamal, R.M.; Hanafy, M.M.; Youssef, A.; Hashem, L.M.B. Comparative study between contrast-enhanced mammography, tomosynthesis, and breast ultrasound as complementary techniques to mammography in dense breast parenchyma. Egypt. J. Radiol. Nucl. Med. 2020, 51, 148. [Google Scholar] [CrossRef]
- Rudnicki, W.; Piegza, T.; Rozum-Liszewska, N.; Gorski, M.; Popiela, T.J.; Basta, P.; Heinze, S.; Luczynska, E. The effectiveness of contrast-enhanced spectral mammography and magnetic resonance imaging in dense breasts. Pol. J. Radiol. 2021, 86, e159–e164. [Google Scholar] [CrossRef]
- Moffa, G.; Galati, F.; Maroncelli, R.; Rizzo, V.; Cicciarelli, F.; Pasculli, M.; Pediconi, F. Diagnostic Performance of Contrast-Enhanced Digital Mammography versus Conventional Imaging in Women with Dense Breasts. Diagnostics 2023, 13, 2520. [Google Scholar] [CrossRef]
- Lin, S.T.; Li, H.J.; Li, Y.Z.; Chen, Q.Q.; Ye, J.Y.; Lin, S.; Cai, S.Q.; Sun, J.G. Diagnostic performance of contrast-enhanced mammography for suspicious findings in dense breasts: A systematic review and meta-analysis. Cancer Med. 2024, 13, e7128. [Google Scholar] [CrossRef]
- Ghai, S.; Muradali, D.; Bukhanov, K.; Kulkarni, S. Nonenhancing breast malignancies on MRI: Sonographic and pathologic correlation. AJR Am. J. Roentgenol. 2005, 185, 481–487. [Google Scholar] [CrossRef]
- Lee, C.H.; Phillips, J.; Sung, J.S.; Lewin, J.M.; Newell, M.S. Contrast Enhanced Mammography (A supplement to ACR BI-RADS Mammography 2013); American College of Radiology: Reston, VA, USA, 2022. [Google Scholar]
- Lalji, U.C.; Houben, I.P.; Prevos, R.; Gommers, S.; van Goethem, M.; Vanwetswinkel, S.; Pijnappel, R.; Steeman, R.; Frotscher, C.; Mok, W.; et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: Validation of results in a large multireader, multicase study. Eur. Radiol. 2016, 26, 4371–4379. [Google Scholar] [CrossRef]
- Schmitz, A.M.; Loo, C.E.; Wesseling, J.; Pijnappel, R.M.; Gilhuijs, K.G. Association between rim enhancement of breast cancer on dynamic contrast-enhanced MRI and patient outcome: Impact of subtype. Breast Cancer Res. Treat. 2014, 148, 541–551. [Google Scholar] [CrossRef]
- Wen, C.; Wang, S.; Ma, M.; Xu, Z.; Zeng, F.; Zeng, H.; Liao, X.; He, Z.; Xu, W.; Chen, W. Breast masses with rim enhancement on contrast-enhanced mammography: Morphological and enhancement features for diagnosis and differentiation of benign and malignant. Br. J. Radiol. 2024, 97, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Magni, V.; Zanardo, M.; Schiaffino, S.; Sardanelli, F. Contrast-enhanced Mammography: A Systematic Review and Meta-Analysis of Diagnostic Performance. Radiology 2022, 302, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hao, C.; Pan, Y.; Mao, N.; Wang, X.; Yin, X. Contrast-Enhanced Spectral Mammography Versus Ultrasonography: Diagnostic Performance in Symptomatic Patients with Dense Breasts. Korean J. Radiol. 2020, 21, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Travieso-Aja, M.D.M.; Maldonado-Saluzzi, D.; Naranjo-Santana, P.; Fernandez-Ruiz, C.; Severino-Rondon, W.; Rodriguez Rodriguez, M.; Vega Benitez, V.; Perez-Luzardo, O. Diagnostic performance of contrast-enhanced dual-energy spectral mammography (CESM): A retrospective study involving 644 breast lesions. La Radiol. Medica 2019, 124, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, Z.; Ghaffari, M.; Nakhaie Moghadam, M.; Parooie, F.; Salarzaei, M. Preoperative evaluation of breast cancer: Contrast-enhanced mammography versus contrast-enhanced magnetic resonance imaging: A systematic review and meta-analysis. Breast Dis. 2022, 41, 303–315. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Heuts, E.M.; Moossdorff, M.; van Nijnatten, T.J.A. Contrast enhanced mammography (CEM) versus magnetic resonance imaging (MRI) for staging of breast cancer: The pro CEM perspective. Eur. J. Radiol. 2021, 142, 109883. [Google Scholar] [CrossRef]
- Kettritz, U.; Rotter, K.; Schreer, I.; Murauer, M.; Schulz-Wendtland, R.; Peter, D.; Heywang-Kobrunner, S.H. Stereotactic vacuum-assisted breast biopsy in 2874 patients: A multicenter study. Cancer 2004, 100, 245–251. [Google Scholar] [CrossRef]
- Liberman, L.; Abramson, A.F.; Squires, F.B.; Glassman, J.R.; Morris, E.A.; Dershaw, D.D. The breast imaging reporting and data system: Positive predictive value of mammographic features and final assessment categories. AJR Am. J. Roentgenol. 1998, 171, 35–40. [Google Scholar] [CrossRef]
- Orel, S.G.; Kay, N.; Reynolds, C.; Sullivan, D.C. BI-RADS categorization as a predictor of malignancy. Radiology 1999, 211, 845–850. [Google Scholar] [CrossRef]
- Huang, P.-C.; Lin, Y.-C.; Cheng, H.-Y.; Juan, Y.-H.; Lin, G.; Cheung, Y.-C. Performance of Stereotactic Vacuum-assisted Biopsy on Breast Microcalcifications: Comparison of 7-gauge and 10-gauge Biopsy Needles. J. Radiol. Sci. 2020, 45, 25–31. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Tsai, H.P.; Lo, Y.F.; Ueng, S.H.; Huang, P.C.; Chen, S.C. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: A preliminary analysis. Eur. Radiol. 2016, 26, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.C.; Juan, Y.H.; Lin, Y.C.; Lo, Y.F.; Tsai, H.P.; Ueng, S.H.; Chen, S.C. Dual-Energy Contrast-Enhanced Spectral Mammography: Enhancement Analysis on BI-RADS 4 Non-Mass Microcalcifications in Screened Women. PLoS ONE 2016, 11, e0162740. [Google Scholar] [CrossRef] [PubMed]
- Houben, I.P.; Vanwetswinkel, S.; Kalia, V.; Thywissen, T.; Nelemans, P.J.; Heuts, E.M.; Smidt, M.L.; Meyer-Baese, A.; Wildberger, J.E.; Lobbes, M. Contrast-enhanced spectral mammography in the evaluation of breast suspicious calcifications: Diagnostic accuracy and impact on surgical management. Acta Radiol. 2019, 60, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Cao, K.; Cao, M.; Li, X.T.; Gao, F.; Zhang, F.D.; Yu, Y.Z.; Sun, Y.S. Improving the Diagnostic Accuracy of Breast BI-RADS 4 Microcalcification-Only Lesions Using Contrast-Enhanced Mammography. Clin. Breast Cancer 2021, 21, 256–262.e2. [Google Scholar] [CrossRef]
- Nicosia, L.; Bozzini, A.C.; Signorelli, G.; Palma, S.; Pesapane, F.; Frassoni, S.; Bagnardi, V.; Pizzamiglio, M.; Farina, M.; Trentin, C.; et al. Contrast-Enhanced Spectral Mammography in the Evaluation of Breast Microcalcifications: Controversies and Diagnostic Management. Healthcare 2023, 11, 511. [Google Scholar] [CrossRef]
- Depretto, C.; D’Ascoli, E.; Della Pepa, G.; Irmici, G.; De Berardinis, C.; Ballerini, D.; Bonanomi, A.; Ancona, E.; Ferranti, C.; Scaperrotta, G.P. Assessing the malignancy of suspicious breast microcalcifications: The role of contrast enhanced mammography. La Radiol. Medica 2024, 129, 855–863. [Google Scholar] [CrossRef]
- Bahl, M.; Baker, J.A.; Kinsey, E.N.; Ghate, S.V. Architectural Distortion on Mammography: Correlation With Pathologic Outcomes and Predictors of Malignancy. AJR Am. J. Roentgenol. 2015, 205, 1339–1345. [Google Scholar] [CrossRef]
- Choudhery, S.; Johnson, M.P.; Larson, N.B.; Anderson, T. Malignant Outcomes of Architectural Distortion on Tomosynthesis: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2021, 217, 295–303. [Google Scholar] [CrossRef]
- Walcott-Sapp, S.; Garreau, J.; Johnson, N.; Thomas, K.A. Pathology results of architectural distortion on detected with digital breast tomosynthesis without definite sonographic correlate. Am. J. Surg. 2019, 217, 857–861. [Google Scholar] [CrossRef]
- Patel, B.K.; Naylor, M.E.; Kosiorek, H.E.; Lopez-Alvarez, Y.M.; Miller, A.M.; Pizzitola, V.J.; Pockaj, B.A. Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin. Imaging 2017, 46, 44–52. [Google Scholar] [CrossRef]
- Kriege, M.; Brekelmans, C.T.; Boetes, C.; Besnard, P.E.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Peterse, H.; Tilanus-Linthorst, M.M.; et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Bohm-Velez, M.; Mahoney, M.C.; Evans, W.P.; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Gray, R.J.; Pockaj, B.A. Potential Cost Savings of Contrast-Enhanced Digital Mammography. AJR Am. J. Roentgenol. 2017, 208, W231–W237. [Google Scholar] [CrossRef]
- Sung, J.S.; Lebron, L.; Keating, D.; D’Alessio, D.; Comstock, C.E.; Lee, C.H.; Pike, M.C.; Ayhan, M.; Moskowitz, C.S.; Morris, E.A.; et al. Performance of Dual-Energy Contrast-enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology 2019, 293, 81–88. [Google Scholar] [CrossRef]
- Li, L.; Roth, R.; Germaine, P.; Ren, S.; Lee, M.; Hunter, K.; Tinney, E.; Liao, L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123. [Google Scholar] [CrossRef]
- Lobbes, M.B.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- GE Healthcare Receives FDA Clearance of the Industry’s First Contrast-Enhanced Mammography Solution for Biopsy. Available online: https://www.ge.com/news/press-releases/ge (accessed on 8 October 2020).
- Coffey, K.; Sung, J.; Comstock, C.; Askin, G.; Jochelson, M.S.; Morris, E.A.; D’Alessio, D. Utility of Targeted Ultrasound to Predict Malignancy Among Lesions Detected on Contrast-Enhanced Digital Mammography. AJR Am. J. Roentgenol. 2021, 217, 595–604. [Google Scholar] [CrossRef]
- Alcantara, R.; Posso, M.; Pitarch, M.; Arenas, N.; Ejarque, B.; Iotti, V.; Besutti, G. Contrast-enhanced mammography-guided biopsy: Technical feasibility and first outcomes. Eur. Radiol. 2023, 33, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, A.; Bhaduri, M.; Khan, N.; Nachum, I.B.; Muscedere, G.; Shmuilovich, O.; Lynn, K.; Nano, E.; Blyth, L. Contrast-Enhanced Mammography-Guided Breast Biopsy: Single-Center Experience. AJR Am. J. Roentgenol. 2023, 220, 826–827. [Google Scholar] [CrossRef]
- Tang, Y.C.; Cheung, Y.C. Contrast-enhanced mammography-guided biopsy: Technique and initial outcomes. Quant. Imaging Med. Surg. 2023, 13, 5349–5354. [Google Scholar] [CrossRef]
- James, J. Contrast-enhanced spectral mammography (CESM)-guided breast biopsy as an alternative to MRI-guided biopsy. Br. J. Radiol. 2022, 95, 20211287. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.C.; Kuo, W.L.; Lee, L.Y.; Tang, Y.C. A case report of breast cancer in silicone-injected breasts diagnosed by an emerging technique of contrast-enhanced mammography-guided biopsy. Front. Oncol. 2022, 12, 884576. [Google Scholar] [CrossRef] [PubMed]
- Amir, T.; Hogan, M.P.; Jacobs, S.; Sevilimedu, V.; Sung, J.; Jochelson, M.S. Comparison of False-Positive Versus True-Positive Findings on Contrast-Enhanced Digital Mammography. AJR Am. J. Roentgenol. 2022, 218, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Grazynska, A.; Niewiadomska, A.; Owczarek, A.J.; Winder, M.; Holda, J.; Zwolinska, O.; Barczyk-Gutkowska, A.; Lorek, A.; Kuzbinska, A.; Steinhof-Radwanska, K. BIRADS 4-Is it possible to downgrade lesions that do not enhance on recombinant contrast-enhanced mammography images? Eur. J. Radiol. 2023, 167, 111062. [Google Scholar] [CrossRef] [PubMed]
- Gershon-Cohen, J.; Berger, S.M. Breast cancer with microcalcifications: Diagnostic difficulties. Radiology 1966, 87, 613–622. [Google Scholar] [CrossRef]
- Stomper, P.C.; Geradts, J.; Edge, S.B.; Levine, E.G. Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. AJR Am. J. Roentgenol. 2003, 181, 1679–1684. [Google Scholar] [CrossRef]
- Luparia, A.; Mariscotti, G.; Durando, M.; Ciatto, S.; Bosco, D.; Campanino, P.P.; Castellano, I.; Sapino, A.; Gandini, G. Accuracy of tumour size assessment in the preoperative staging of breast cancer: Comparison of digital mammography, tomosynthesis, ultrasound and MRI. La Radiol. Medica 2013, 118, 1119–1136. [Google Scholar] [CrossRef]
- Katz, B.; Raker, C.; Edmonson, D.; Gass, J.; Stuckey, A.; Rizack, T. Predicting Breast Tumor Size for Pre-operative Planning: Which Imaging Modality is Best? Breast J. 2017, 23, 52–58. [Google Scholar] [CrossRef]
- Pop, C.F.; Stanciu-Pop, C.; Drisis, S.; Radermeker, M.; Vandemerckt, C.; Noterman, D.; Moreau, M.; Larsimont, D.; Nogaret, J.M.; Veys, I. The impact of breast MRI workup on tumor size assessment and surgical planning in patients with early breast cancer. Breast J. 2018, 24, 927–933. [Google Scholar] [CrossRef]
- Houssami, N.; Turner, R.M.; Morrow, M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res. Treat. 2017, 165, 273–283. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Juan, Y.H.; Lo, Y.F.; Lin, Y.C.; Yeh, C.H.; Ueng, S.H. Preoperative assessment of contrast-enhanced spectral mammography of diagnosed breast cancers after sonographic biopsy: Correlation to contrast-enhanced magnetic resonance imaging and 5-year postoperative follow-up. Medicine 2020, 99, e19024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Shao, H.; Li, J.; Yang, Q. Role of contrast-enhanced mammography in the preoperative detection of ductal carcinoma in situ of the breasts: A comparison with low-energy image and magnetic resonance imaging. Eur. Radiol. 2024, 34, 3342–3351. [Google Scholar] [CrossRef] [PubMed]
- Daniaux, M.; Gruber, L.; De Zordo, T.; Geiger-Gritsch, S.; Amort, B.; Santner, W.; Egle, D.; Baltzer, P.A.T. Preoperative staging by multimodal imaging in newly diagnosed breast cancer: Diagnostic performance of contrast-enhanced spectral mammography compared to conventional mammography, ultrasound, and MRI. Eur. J. Radiol. 2023, 163, 110838. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.A.F.; Zeinhom, A.; Hamed, D.A.A.; Ghaly, G.R.M.; Tadros, S.F.K. Contrast-enhanced mammography versus breast MRI in the assessment of multifocal and multicentric breast cancer: A retrospective study. Acta Radiol. 2023, 64, 2868–2880. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.; Chou, C.P.; Chan, C.W.; Buhari, S.A.; Hartman, M.; Tang, S.W.; Ng, C.W.Q.; Pillay, P.; Chua, W.; Jagmohan, P.; et al. Impact of contrast-enhanced mammography in surgical management of breast cancers for women with dense breasts: A dual-center, multi-disciplinary study in Asia. Eur. Radiol. 2022, 32, 8226–8237. [Google Scholar] [CrossRef]
- Avera, E.; Valentic, L.; Bui, L. Current understanding and distinct features of multifocal and multicentric breast cancers. Cancer Rep (Hoboken) 2023, 6, e1851. [Google Scholar] [CrossRef]
- Ustaalioglu, B.O.; Bilici, A.; Kefeli, U.; Seker, M.; Oncel, M.; Gezen, C.; Gumus, M.; Demirelli, F. The importance of multifocal/multicentric tumor on the disease-free survival of breast cancer patients: Single center experience. Am. J. Clin. Oncol. 2012, 35, 580–586. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Danforth, D.N.; Cowan, K.H.; D’Angelo, T.; Steinberg, S.M.; Pierce, L.; Lippman, M.E.; Lichter, A.S.; Glatstein, E.; Okunieff, P. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N. Engl. J. Med. 1995, 332, 907–911. [Google Scholar] [CrossRef]
- Arriagada, R.; Lê, M.G.; Rochard, F.; Contesso, G. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J. Clin. Oncol. 1996, 14, 1558–1564. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- de Boniface, J.; Szulkin, R.; Johansson, A.L.V. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg. 2021, 156, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, P.; Carstensen, S.L.; Ejlertsen, B.; Kroman, N.; Offersen, B.; Bodilsen, A.; Jensen, M.-B. Breast conserving surgery versus mastectomy: Overall and relative survival-a population based study by the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2018, 57, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014, 149, 267–274. [Google Scholar] [CrossRef]
- Gentile, D.; Martorana, F.; Karakatsanis, A.; Caruso, F.; Caruso, M.; Castiglione, G.; Di Grazia, A.; Pane, F.; Rizzo, A.; Vigneri, P.; et al. Predictors of mastectomy in breast cancer patients with complete remission of primary tumor after neoadjuvant therapy: A retrospective study. Eur. J. Surg. Oncol. 2024, 50, 108732. [Google Scholar] [CrossRef]
- Helal, M.H.; Mansour, S.M.; Ahmed, H.A.; Abdel Ghany, A.F.; Kamel, O.F.; Elkholy, N.G. The role of contrast-enhanced spectral mammography in the evaluation of the postoperative breast cancer. Clin. Radiol. 2019, 74, 771–781. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of imaging in neoadjuvant therapy for breast cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
- Kim, E.Y.; Do, S.I.; Yun, J.S.; Park, Y.L.; Park, C.H.; Moon, J.H.; Youn, I.; Choi, Y.J.; Ham, S.Y.; Kook, S.H. Preoperative evaluation of mammographic microcalcifications after neoadjuvant chemotherapy for breast cancer. Clin. Radiol. 2020, 75, 641.e19–641.e27. [Google Scholar] [CrossRef]
- Bernardi, D.; Vatteroni, G.; Acquaviva, A.; Valentini, M.; Sabatino, V.; Bolengo, I.; Pellegrini, M.; Fanto, C.; Trimboli, R.M. Contrast-Enhanced Mammography Versus MRI in the Evaluation of Neoadjuvant Therapy Response in Patients With Breast Cancer: A Prospective Study. AJR Am. J. Roentgenol. 2022, 219, 884–894. [Google Scholar] [CrossRef]
- Kaiyin, M.; Lingling, T.; Leilei, T.; Wenjia, L.; Bin, J. Head-to-head comparison of contrast-enhanced mammography and contrast-enhanced MRI for assessing pathological complete response to neoadjuvant therapy in patients with breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2023, 202, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barra, F.R.; Sobrinho, A.B.; Barra, R.R.; Magalhaes, M.T.; Aguiar, L.R.; de Albuquerque, G.F.L.; Costa, R.P.; Farage, L.; Pratesi, R. Contrast-Enhanced Mammography (CEM) for Detecting Residual Disease after Neoadjuvant Chemotherapy: A Comparison with Breast Magnetic Resonance Imaging (MRI). BioMed Res. Int. 2018, 2018, 8531916. [Google Scholar] [CrossRef] [PubMed]
- Sunen, I.; Isabel Garcia Barrado, A.; Cruz Ciria, S.; Garcia Maroto, J.; Gros Baneres, B.; Garcia Mur, C. Is contrast-enhanced mammography (CEM) an alternative to MRI in assessing the response to primary systemic therapy of breast cancer? Eur. J. Radiol. 2024, 170, 111270. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Hilal, T.; Covington, M.; Zhang, N.; Kosiorek, H.E.; Lobbes, M.; Northfelt, D.W.; Pockaj, B.A. Contrast-Enhanced Spectral Mammography is Comparable to MRI in the Assessment of Residual Breast Cancer Following Neoadjuvant Systemic Therapy. Ann. Surg. Oncol. 2018, 25, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Fusco, R.; Di Bernardo, E.; Petrosino, T.; Barretta, M.L.; Porto, A.; Granata, V.; Di Bonito, M.; Fanizzi, A.; Massafra, R.; et al. Prediction of Breast Cancer Histological Outcome by Radiomics and Artificial Intelligence Analysis in Contrast-Enhanced Mammography. Cancers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Qian, N.; Jiang, W.; Guo, Y.; Zhu, J.; Qiu, J.; Yu, H.; Huang, X. Breast cancer diagnosis from contrast-enhanced mammography using multi-feature fusion neural network. Eur. Radiol. 2024, 34, 917–927. [Google Scholar] [CrossRef]

| Authors [Ref] (Pub Yr) | No. | CEM | Mammography | ||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| Cheung et al. [22] (2014) | 89 | 93 | 68 | 72 | 52 |
| Sorin et al. [23] (2018) | 611 | 91 | 76 | 52 | 91 |
| Azzam et al. [24] (2020) | 37 | 89 | 89 | 83 | 48 |
| Rudnicki et al. [25] (2021) | 121 | 100 | 33 | - | - |
| Moffa et al. [26] (2023) | 110 | 93.5 | 80.9 | 79 | 47 |
| Author [Ref] (Pub Yr) | No. | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Cheung [42] (2016) | 59 | 90.9 | 83.78 | 76.92 | 93.94 |
| Cheung [43] (2016) | 94 | 88.89 | 86.56 | 72.72 | 95.08 |
| Houben [44] (2019) | 147 | 93.8 | 36.6 | 54 | 88.2 |
| Long [45] (2021) | 74 | 77 | 88 | 77 | 88 |
| Nicosia [46] (2023) | 377 | 80 | 85.9 | 70.6 | 91 |
| DM | Sono | CEM | MRI | |
|---|---|---|---|---|
| Performance | ||||
| Imaging mode | Morphology | Morphology | Morpho-functional | Morpho-functional |
| Radiation | Present | Absent | Present | Absent |
| Examination time | 10 min. | 15 min. | 15 min. | 30 min. |
| Contrast medium | No | No | Yes | Yes |
| Cost | Low | Low | Intermediate | High |
| Readily Available | Good | Good | Good | Not good |
| Potential side-effect | No | No | Allergy, Renal function loading | Allergy, Renal function loading, Psychological impact |
| Cancer Diagnosis | ||||
| Mass | Intermediate | Good | Good | Good |
| Non-mass | Intermediate | Intermediate | Good | Good |
| Microcalcification | Excellent | Poor | Excellent | Poor |
| Preferable role | Screening | Palpable mass | Suspicious lesions | Therapeutic assessment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, W.-S.; Tang, Y.-C.; Cheung, Y.-C. Contrast-Enhanced Mammography: A Literature Review of Clinical Uses for Cancer Diagnosis and Surgical Oncology. Cancers 2024, 16, 4143. https://doi.org/10.3390/cancers16244143
Chung W-S, Tang Y-C, Cheung Y-C. Contrast-Enhanced Mammography: A Literature Review of Clinical Uses for Cancer Diagnosis and Surgical Oncology. Cancers. 2024; 16(24):4143. https://doi.org/10.3390/cancers16244143
Chicago/Turabian StyleChung, Wai-Shan, Ya-Chun Tang, and Yun-Chung Cheung. 2024. "Contrast-Enhanced Mammography: A Literature Review of Clinical Uses for Cancer Diagnosis and Surgical Oncology" Cancers 16, no. 24: 4143. https://doi.org/10.3390/cancers16244143
APA StyleChung, W.-S., Tang, Y.-C., & Cheung, Y.-C. (2024). Contrast-Enhanced Mammography: A Literature Review of Clinical Uses for Cancer Diagnosis and Surgical Oncology. Cancers, 16(24), 4143. https://doi.org/10.3390/cancers16244143






