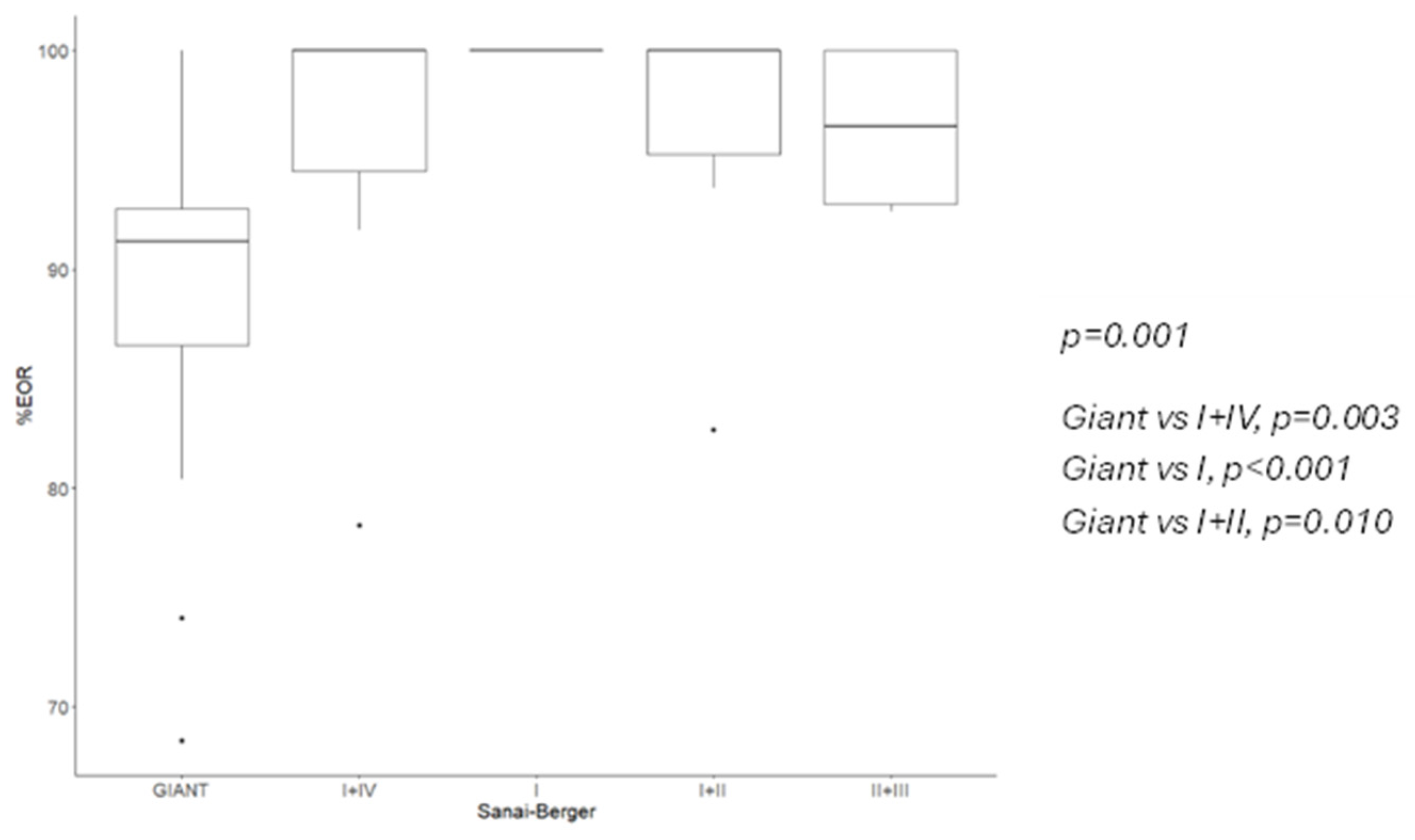
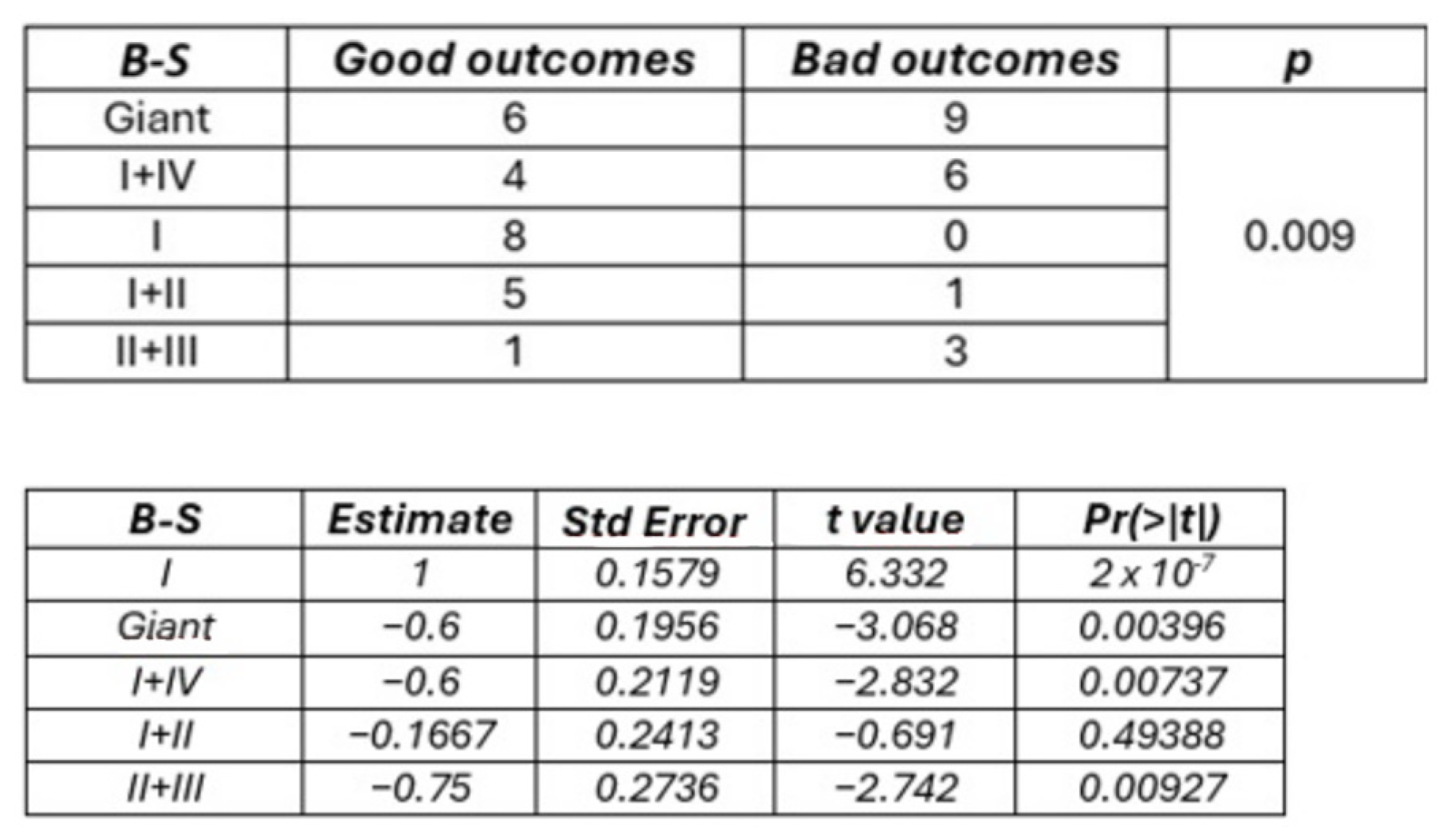
Insular gliomas are distinct entities from both biological and clinical standpoints. In a detailed molecular analysis, Goze et al. compared pure insular gliomas with “para-limbic” gliomas, which, according to the Yasargil classification, extend into fronto-temporal and/or temporopolar regions, with or without the involvement of mesiotemporal structures [
14,
15]. This study found a higher incidence of IDH1/IDH2 mutations in insular gliomas, associated with smaller tumor size at onset and lower malignancy. Although the “triple-negative” subtype was identified in insular gliomas, no significant differences were observed between the two groups [
16,
17,
18]. Insular gliomas typically follow four patterns of progression: they may remain confined to the insular cortex or extend through the anterior, inferior, or superior limiting sulci, each with specific surgical and clinical implications. For instance, spread through the superior sulcus limitans can hinder gross total resection (GTR) due to the risk of damaging the posterior limb of the internal capsule and long insular arteries (LIAs), which supply the corona radiata [
6,
7,
13]. Tamura et al. emphasized that LIA strokes can cause neurological deficits similar to those caused by lenticulostriate arteries (LSAs), underscoring the need to preserve these vessels [
16]. In 2014, Kawaguchi et al. introduced a scoring system that includes factors such as contrast enhancement, LSA involvement, tumor edge morphology, and superior sulcus limitans invasion. This 0–4 score system has clinical and surgical implications; higher scores correspond to higher resection rates and lower neurological complication [
7]. In the well-known Berger–Sanai classification, tumors in zones II and III are less amenable to GTR due to the increased risk of complications like hemiparesis or hemiplegia [
11]. While the Kawaguchi system emphasizes tumor characteristics and proximity to critical structures, the Berger–Sanai classification is more focused on tumor topography, which has indirect surgical and clinical consequences. In this study, we applied both the Berger–Sanai and Kawaguchi classifications to a series of insular glioma patients treated at our center. We achieved radical resection in 53.5% of cases, which is relatively high compared to other series [
19,
20,
21]. Although some recommend a transopercular approach to minimize vessel manipulation and reduce motor deficits [
22,
23,
24,
25,
26], we favored a transylvian approach for its more natural route and improved control of vessels within the cistern. This approach also provided an anatomic perspective, using the M1 and lateral lenticulostriate arteries as depth markers for the surgical cavity. Resection rates varied depending on the classification system used. Berger–Sanai giant tumors had the lowest extent of resection (EOR), as expected, and zone II + III involvement significantly reduced GTR and subtotal resection (STR) rates, aligning with previous studies. For example, while Hervey-Jumper et al. reported a median EOR of 75.5% for zone II tumors [
8], Sufianov et al. achieved an EOR of over 90%. In our series, we did not encounter any pure zone II tumors, but we agree that proximity to the corticospinal tract and LIA involvement can limit resection [
27]. Using the Kawaguchi scoring system, higher scores were associated with higher EOR. Logistic regression showed that grade 1 tumors had a 90.1% lower GTR rate compared to grade 3, though no significant difference was observed between grade 3 and grade 4 tumors. Further analysis revealed that the Berger–Sanai classification had moderate-to-strong predictive power for EOR, consistent with findings from Hervey-Jumper et al. [
8]. Although the Kawaguchi system did not show strong predictive ability, a positive trend was evident, supported by a high Spearman coefficient. The Berger–Sanai classification also significantly predicted GTR, particularly for tumors in zones I and IV, while Kawaguchi grade 1 was associated with a lower GTR rate. Interestingly, histotype did not affect EOR rates, suggesting that maximal safe resection should be pursued even if a high-grade glioma is suspected. Postoperative neurological deterioration occurred in 44.2% of cases, comparable to the 45.5% reported by Biswas et al. (30 out of 66 patients) [
19]. This may be related to ischemic damage or surgical manipulation, as postoperative MRI revealed significant DWI hyperintensity in 32.6% of cases. Moreover, the high incidence of glioblastoma we observed in this series could be a notable factor, as higher-grade tumors may be more likely to involve LSAs, increasing the risk of postoperative neurological worsening. Nevertheless, statistical analysis did not identify it as a confounding factor. Both the Berger–Sanai and Kawaguchi systems were linked to neurological outcomes. Tumors in Berger–Sanai zones II + III were 52.8% more likely to cause immediate postoperative complications than those in zone I. Interestingly, giant tumors were less risky than those in zones II + III, possibly due to the original definition of “giant” tumors. According to Rossi et al., LSA infiltration by the tumor is the only factor associated with postoperative neurological deterioration, and these vessels are easier to control surgically than LIAs [
28]. These findings underscore the importance of Kawaguchi scores in predicting postoperative outcomes, as confirmed by logistic regression models that also linked the Kawaguchi system with DWI changes. At the 6-month follow-up, 9.3% of patients (four cases) showed permanent neurological decline. No significant correlation was found with either classification system, possibly due to the small sample size. Alternatively, postoperative DWI hyperintensities may not always reflect irreversible ischemic damage, or recovery from ischemia may still be possible. Hou et al. reported that while 58.6% of patients had significant DWI hyperintensities postoperatively, only 14.6% were related to core ischemia in the corona radiata or posterior limb of the internal capsule [
29]. Intraoperative neurophysiological monitoring (IONM) is a well-established tool for detecting impending stroke during glioma surgery and allows for a higher resection rate [
30]. Motor-evoked potentials (MEPs) are more sensitive and specific than somatosensory-evoked potentials (SSEPs), with a >50% reduction in MEP amplitude strongly linked to paralysis risk. In our study, we observed a positive trend toward better outcomes among patients without intraoperative changes (OR 4.20), but it did not reach statistical significance, likely due to our study’s small sample size. Regarding classification systems, the Kawaguchi classification correlated with IONM changes, with lower-grade tumors showing higher rates of significant MEP reductions. This may reflect the Kawaguchi system’s focus on the tumor’s relationship to LSAs and the superior extremity of the circular sulcus, making it more directly connected to vessel damage than the Berger–Sanai classification. Our work has two primary limitations. Firstly, the sample size is small, limiting the strength of our conclusions; a multicenter study would be beneficial to increase the sample size. Secondly, the retrospective nature of this study could have introduced selection and data biases.