The Role of Radiotherapy to the Primary Site in Oropharyngeal Cancer with Limited Metastases—An Analysis of a Hospital-Based Registry
Simple Summary
Abstract
1. Introduction
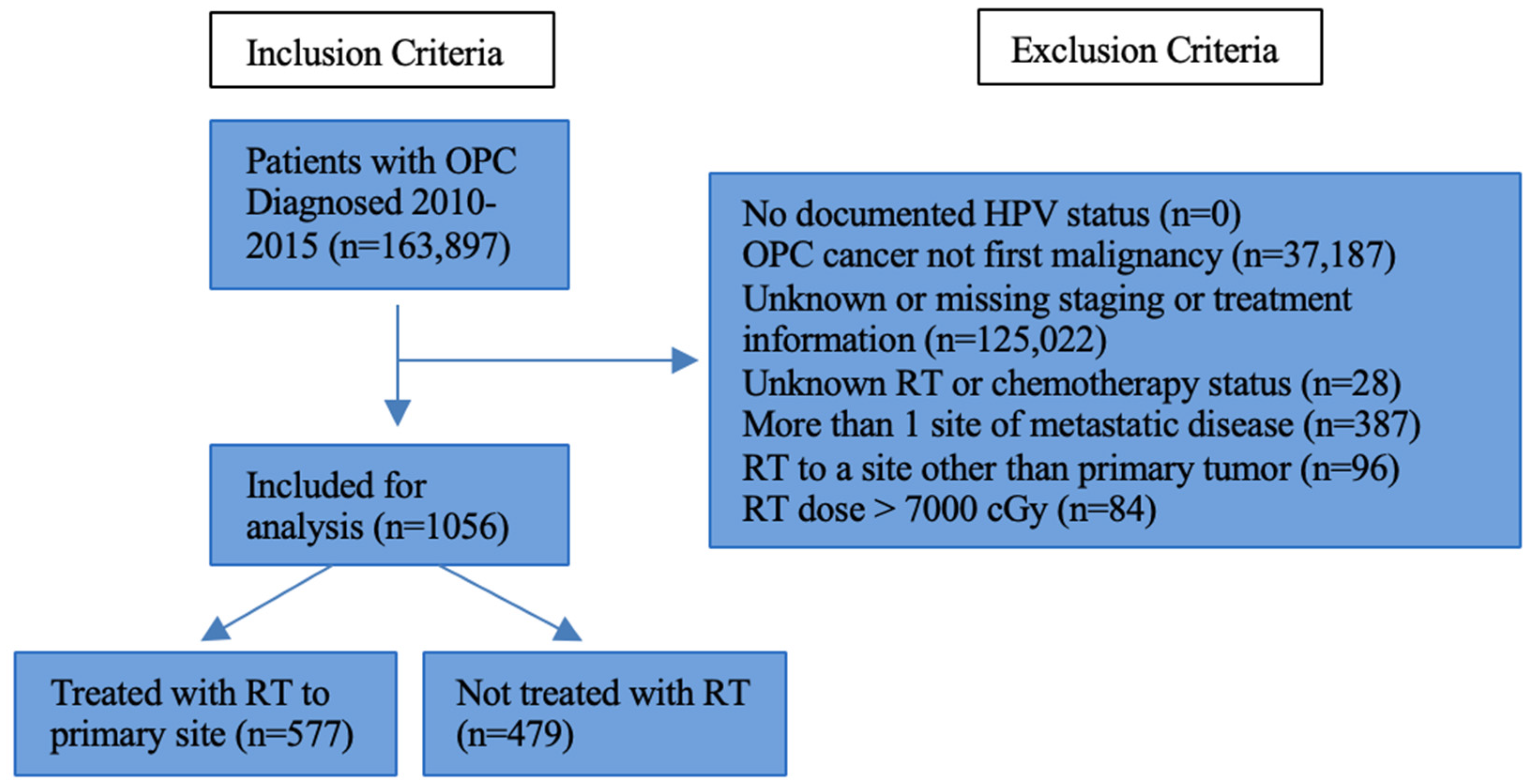
2. Materials and Methods
3. Results
3.1. Cohort Demographics and Clinical Characteristics
3.2. Clinical Characteristics Associated with Survival in Limited Metastatic OPC
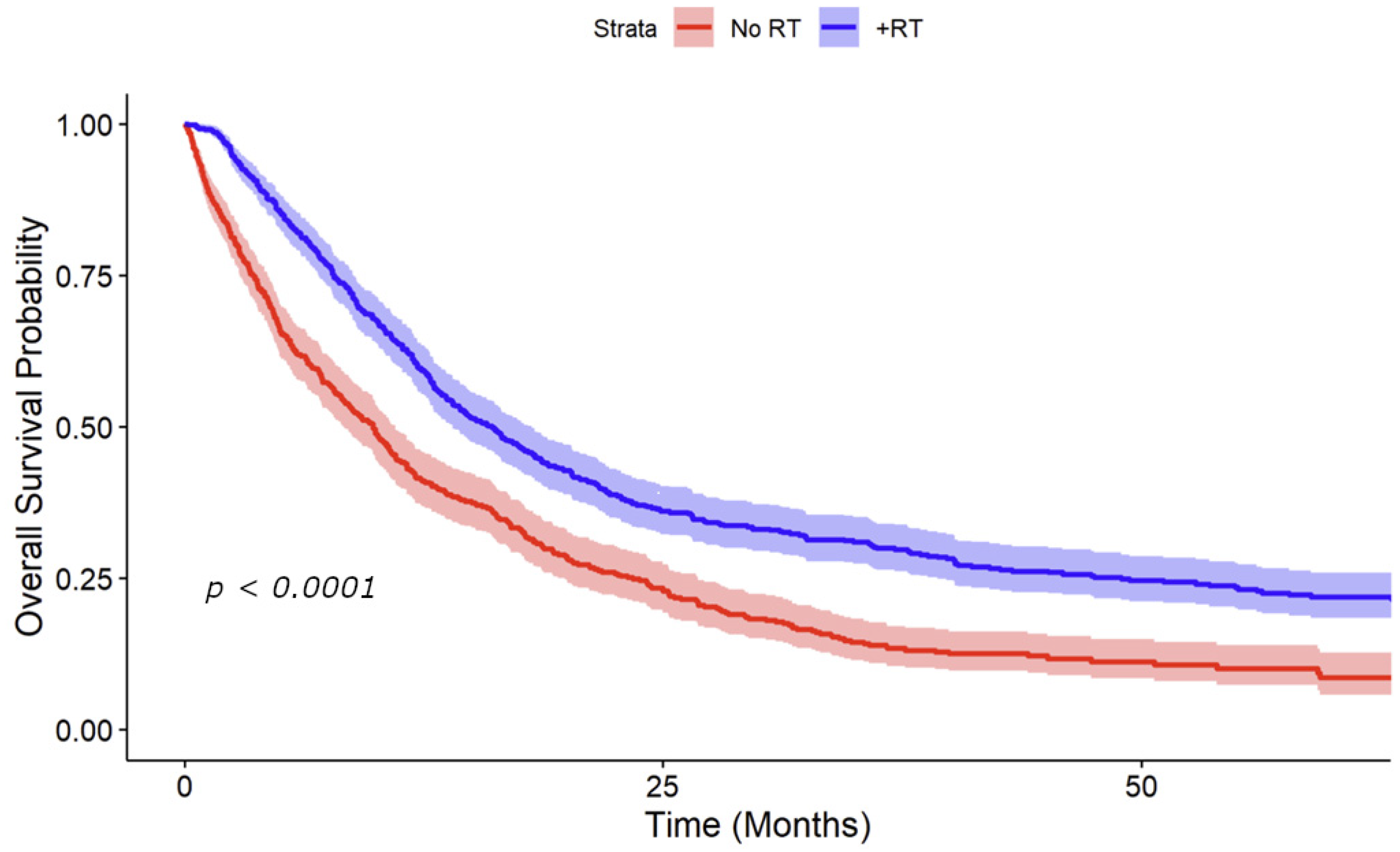
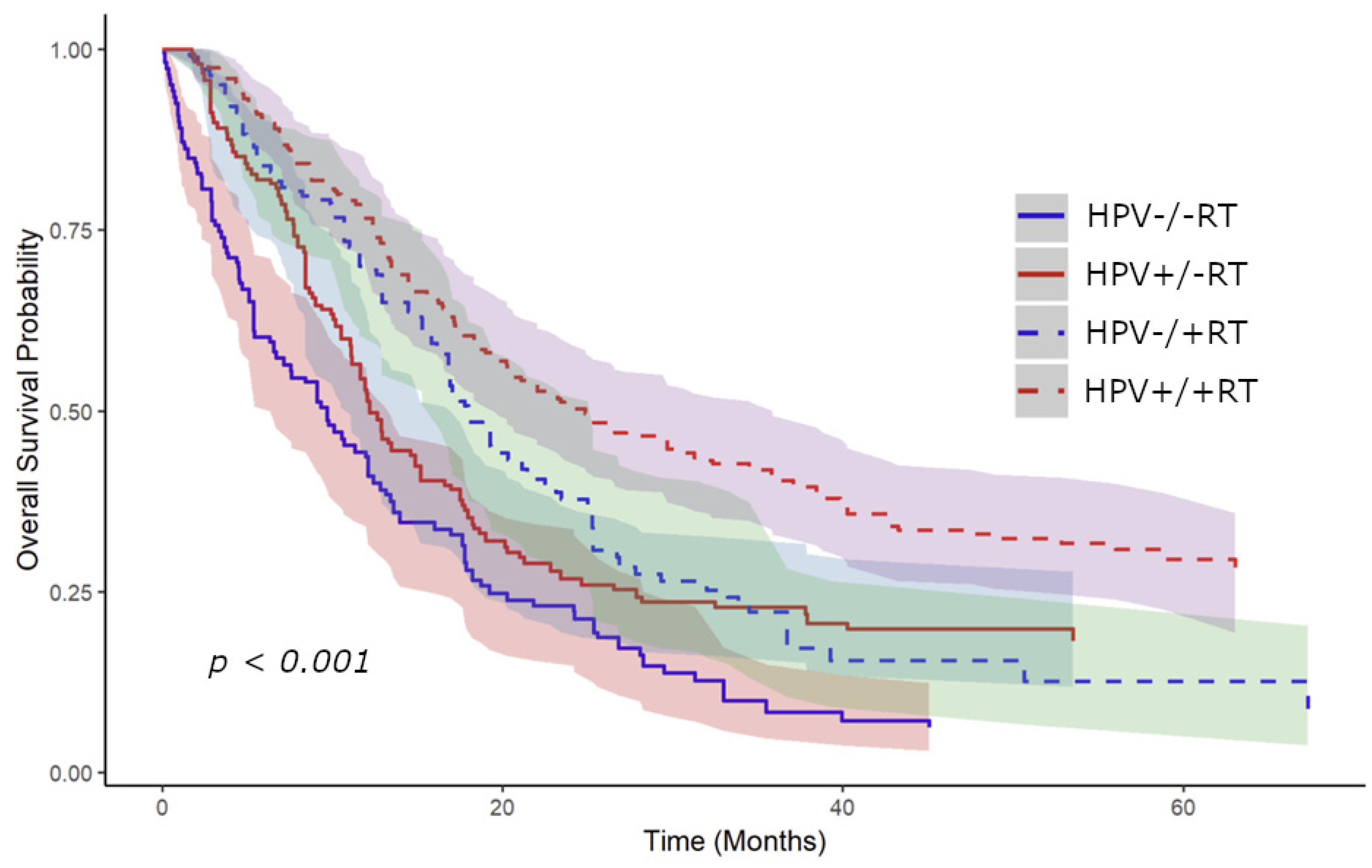
3.3. Kaplan–Meier Survival Analysis Stratified by RT to the Primary Site and HPV Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Duprez, F.; Berwouts, D.; De Neve, W.; Bonte, K.; Boterberg, T.; Deron, P.; Huvenne, W.; Rottey, S.; Mareel, M. Distant Metastases in Head and Neck Cancer. Head Neck 2017, 39, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.I.; Auethavekiat, V.; Adkins, D.R.; Nussenbaum, B.; Collins, S.; Boonchalermvichian, C.; Trinkaus, K.; Chen, L.; Morgensztern, D. Squamous Cell Cancer of the Head and Neck with Distant Metastasis at Presentation. Head Neck 2011, 33, 714–718. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Wise-Draper, T.M.; Bahig, H.; Tonneau, M.; Karivedu, V.; Burtness, B. Current Therapy for Metastatic Head and Neck Cancer: Evidence, Opportunities, and Challenges. In American Society of Clinical Oncology Educational Book; ASCO Publications: Alexandria, VA, USA, 2022; Volume 42. [Google Scholar] [CrossRef]
- Ordoñez, R.; Otero, A.; Jerez, I.; Medina, J.A.; Lupiañez-Pérez, Y.; Gomez-Millan, J. Role of Radiotherapy in the Treatment of Metastatic Head and Neck Cancer. Onco. Targets. Ther. 2019, 12, 677–683. [Google Scholar] [CrossRef] [PubMed]
- McBride, S.M.; Busse, P.M.; Clark, J.R.; Wirth, L.J.; Ancukiewicz, M.; Chan, A.W. Long-Term Survival after Distant Metastasis in Patients with Oropharyngeal Cancer. Oral Oncol. 2014, 50, 208–212. [Google Scholar] [CrossRef]
- Bahig, H.; Huang, S.H.; O’Sullivan, B. Oligometastatic Head and Neck Cancer: Challenges and Perspectives. Cancers 2022, 14, 3894. [Google Scholar] [CrossRef]
- Tang, É.; Nguyen, T.-V.-F.; Clatot, F.; Rambeau, A.; Johnson, A.; Sun, X.S.; Tao, Y.; Thariat, J. Radiation Therapy on Primary Tumour of Synchronous Metastatic Head and Neck Squamous Cell Carcinomas. Cancer/Radiothérapie 2020, 24, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P.; Carvalho, A.L. Natural History of Untreated Head and Neck Cancer. Eur. J. Cancer 2000, 36, 1032–1037. [Google Scholar] [CrossRef]
- Coatesworth, A.P.; Tsikoudas, A.; MacLennan, K. The Cause of Death in Patients with Head and Neck Squamous Cell Carcinoma. J. Laryngol. Otol. 2002, 116, 269–271. [Google Scholar] [CrossRef]
- Slootweg, P.J.; Hordijk, G.J.; Koole, R. Autopsy Findings in Patients with Head and Neck Squamous Cell Cancer and Their Therapeutic Relevance. Eur. J. Cancer Part B Oral Oncol. 1996, 32, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Danna, E.A.; Sinha, P.; Gilbert, M.; Clements, V.K.; Pulaski, B.A.; Ostrand-Rosenberg, S. Surgical Removal of Primary Tumor Reverses Tumor-Induced Immunosuppression Despite the Presence of Metastatic Disease. Cancer Res. 2004, 64, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Watanabe, A.; Aburatani, H.; Maru, Y. Tumour-Mediated Upregulation of Chemoattractants and Recruitment of Myeloid Cells Predetermines Lung Metastasis. Nat. Cell Biol. 2006, 8, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Bol, V.; Grégoire, V. Biological Basis for Increased Sensitivity to Radiation Therapy in HPV-Positive Head and Neck Cancers. BioMed Res. Int. 2014, 2014, 696028. [Google Scholar] [CrossRef]
- Cohan, D.M.; Popat, S.; Kaplan, S.E.; Rigual, N.; Loree, T.; Hicks, W.L. Oropharyngeal Cancer: Current Understanding and Management. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 88–94. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Glastonbury, C.M. Critical Changes in the Staging of Head and Neck Cancer. Radiol. Imaging Cancer 2020, 2, e190022. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef]
- Slotman, B.J.; van Tinteren, H.; Praag, J.O.; Knegjens, J.L.; El Sharouni, S.Y.; Hatton, M.; Keijser, A.; Faivre-Finn, C.; Senan, S. Use of Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Phase 3 Randomised Controlled Trial. Lancet 2015, 385, 36–42. [Google Scholar] [CrossRef]
- Rusthoven, C.G.; Jones, B.L.; Flaig, T.W.; Crawford, E.D.; Koshy, M.; Sher, D.J.; Mahmood, U.; Chen, R.C.; Chapin, B.F.; Kavanagh, B.D.; et al. Improved Survival with Prostate Radiation in Addition to Androgen Deprivation Therapy for Men with Newly Diagnosed Metastatic Prostate Cancer. J. Clin. Oncol. 2016, 34, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Sun, M.; Leow, J.J.; Preston, M.A.; Cole, A.P.; Gelpi-Hammerschmidt, F.; Hanna, N.; Meyer, C.P.; Kibel, A.S.; Lipsitz, S.R.; et al. Efficacy of High-Intensity Local Treatment for Metastatic Urothelial Carcinoma of the Bladder: A Propensity Score–Weighted Analysis from the National Cancer Data Base. J. Clin. Oncol. 2016, 34, 3529–3536. [Google Scholar] [CrossRef]
- Mayadev, J.; Daly, M.; Chen, A.; Bold, R.; Chew, H. The Potential Role of Radiation Therapy to the Primary Site of Disease in Stage IV Breast Cancer Presenting with Synchronous Metastasis. Clin. Breast Cancer 2014, 14, 10–12. [Google Scholar] [CrossRef][Green Version]
- Rambeau, A.; Bastit, V.; Thureau, S.; Thariat, J.; Moldovan, C.; Roge, M.; Babin, E.; Gery, B.; Di Fiore, F.; Florescu, C.; et al. Impact of Locoregional Irradiation in Patients with Upfront Metastatic Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2019, 93, 46–51. [Google Scholar] [CrossRef]
- Nguy, S.; Oh, C.; Karp, J.M.; Wu, S.P.; Li, Z.; Persky, M.J.; Hu, K.S.; Givi, B.; Tam, M.M. Radiotherapy in Metastatic Oropharyngeal Cancer. Laryngoscope 2021, 131, E1847–E1853. [Google Scholar] [CrossRef] [PubMed]
- Kabarriti, R.; Baliga, S.; Ohri, N.; Guha, C.; Kalnicki, S.; Garg, M.K. Radiation Therapy for Patients with Newly Diagnosed Metastatic Head and Neck Squamous Cell Carcinoma. Head Neck 2019, 41, 130–138. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Luu, M.; Yoshida, E.J.; Kim, S.; Tighiouart, M.; David, J.M.; Shiao, S.L.; Mita, A.C.; Scher, K.S.; Sherman, E.J.; et al. Combined High-intensity Local Treatment and Systemic Therapy in Metastatic Head and Neck Squamous Cell Carcinoma: An Analysis of the National Cancer Data Base. Cancer 2017, 123, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- Ampil, F.L.; Kim, D.D.; Ghali, G.E.; Baluna, R.G. How Intensive Should Radiotherapy for Head and Neck Cancer with Synchronous Distant Metastases Be? Review of Cases. J. Oral Maxillofac. Surg. 2012, 70, 730–733. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.-P.; Huang, P.-Y.; Zou, X.; Sun, R.; He, Y.-X.; Wu, Y.-S.; Shen, G.-P.; Zhang, H.-D.; Duan, C.-Y.; et al. Efficacy and Safety of Locoregional Radiotherapy with Chemotherapy vs Chemotherapy Alone in de Novo Metastatic Nasopharyngeal Carcinoma. JAMA Oncol. 2020, 6, 1345. [Google Scholar] [CrossRef]
- Maxwell, J.H.; Grandis, J.R.; Ferris, R.L. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annu. Rev. Med. 2016, 67, 91–101. [Google Scholar] [CrossRef]
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef]
- Mirghani, H.; Amen, F.; Tao, Y.; Deutsch, E.; Levy, A. Increased Radiosensitivity of HPV-Positive Head and Neck Cancers: Molecular Basis and Therapeutic Perspectives. Cancer Treat. Rev. 2015, 41, 844–852. [Google Scholar] [CrossRef]
- Gibson, M.K.; Forastiere, A.A. Multidisciplinary Approaches in the Management of Advanced Head and Neck Tumors: State of the Art. Curr. Opin. Oncol. 2004, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Babu, S. Combining Radiotherapy and Systemic Therapies in Oropharyngeal Cancer: A Comprehensive Review of Recent Developments. Oral Oncol. Reports 2024, 12, 100659. [Google Scholar] [CrossRef]
- Ravi, P.; Babu, S. Emerging Immune Checkpoint Inhibitors for the Treatment of Oropharyngeal Squamous Cell Carcinoma. Oral Oncol. Reports 2024, 12, 100650. [Google Scholar] [CrossRef]
- Borson, S.; Shuai, Y.; Branstetter, B.F.; Nilsen, M.L.; Hughes, M.A.; Fenton, M.; Kubik, M.; Sridharan, S.; Clump, D.A.; Skinner, H.D.; et al. Definitive Local Therapy to Head and Neck Squamous Cell Carcinoma with Distant Metastasis. Laryngoscope Investig. Otolaryngol. 2022, 7, 757–765. [Google Scholar] [CrossRef]
- Weissmann, T.; Höfler, D.; Hecht, M.; Semrau, S.; Haderlein, M.; Filimonova, I.; Frey, B.; Bert, C.; Lettmaier, S.; Mantsopoulos, K.; et al. Oligometastatic Head and Neck Cancer: Which Patients Benefit from Radical Local Treatment of All Tumour Sites? Radiat. Oncol. 2021, 16, 62. [Google Scholar] [CrossRef]
- Trotti, A. Toxicity in Head and Neck Cancer: A Review of Trends and Issues. Int. J. Radiat. Oncol. 2000, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B. To Treat or Not to Treat: Balancing Therapeutic Outcomes, Toxicity and Quality of Life in Patients with Recurrent and/or Metastatic Head and Neck Cancer. J. Support. Oncol. 2013, 11, 149–159. [Google Scholar] [CrossRef][Green Version]
- Bates, J.E.; De Leo, A.N.; Morris, C.G.; Amdur, R.J.; Dagan, R. Oligometastatic Squamous Cell Carcinoma of the Head and Neck Treated with Stereotactic Body Ablative Radiotherapy: Single-institution Outcomes. Head Neck 2019, 41, 2309–2314. [Google Scholar] [CrossRef]
- Boffa, D.J.; Rosen, J.E.; Mallin, K.; Loomis, A.; Gay, G.; Palis, B.; Thoburn, K.; Gress, D.; McKellar, D.P.; Shulman, L.N.; et al. Using the National Cancer Database for Outcomes Research. JAMA Oncol. 2017, 3, 1722. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for Head and Neck Cancer: Recent Advances and Future Directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef] [PubMed]

| Patient Variable | Total Patients, n (%) (n = 1056) |
|---|---|
| Median Age (range) | 61 (30–90) |
| Sex | |
| Male | 877 (83.0%) |
| Female | 179 (17.0%) |
| Race | |
| White | 855 (81.0%) |
| Black | 163 (15.4%) |
| Other/Unknown | 38 (3.6%) |
| Patient Variable | Total Patients, n (%) (n = 1056) |
|---|---|
| Charlson–Deyo Comorbidity Score | |
| 0 | 797 (75.5%) |
| 1 | 177 (16.8%) |
| 2 | 57 (5.4%) |
| 3 | 25 (2.4%) |
| Tumor Subsite | |
| Tonsil | 427 (40.4%) |
| Base of Tongue | 435 (41.2%) |
| Other Oropharynx | 194 (18.4%) |
| Tumor Grade | |
| 1 | 36 (3.4%) |
| 2 | 257 (24.3%) |
| 3 | 326 (30.9%) |
| 4 | 11 (1.0%) |
| Unknown | 426 (40.3%) |
| HPV Status | |
| Positive | 217 (20.5%) |
| Negative | 280 (26.5%) |
| Unknown | 559 (52.9%) |
| Clinical T Stage | |
| cT1 | 117 (11.1%) |
| cT2 | 295 (27.9%) |
| cT3 | 255 (24.1%) |
| cT4 | 389 (36.8%) |
| Clinical N Stage | |
| cN0 | 61 (5.8%) |
| cN1 | 105 (9.9%) |
| cN2 | 741 (70.2%) |
| cN3 | 149 (14.1%) |
| Site of Metastasis | |
| Bone | 201 (19.0%) |
| Brain | 8 (0.8%) |
| Lung | 559 (52.9%) |
| Liver | 107 (10.1%) |
| Distant Lymph Node | 215 (20.4%) |
| Presence of Lymphovascular Invasion | |
| Yes | 168 (15.9%) |
| No | 66 (6.3%) |
| Unknown | 822 (77.8%) |
| Radiotherapy | |
| Yes | 577 (54.6%) |
| No | 479 (45.4%) |
| Systemic Therapy | |
| Yes | 738 (69.9%) |
| No | 318 (30.1%) |
| Immunotherapy | |
| Yes | 89 (8.5%) |
| No | 967 (91.5%) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |
| Any RT | 0.72 | <0.001 | 0.7 | <0.001 |
| HPV Status | 1.03 | 0.0017 | 1.02 | 0.049 |
| Age | 1.02 | <0.001 | 1.01 | 0.0024 |
| CDCC | 1.21 | 0.0011 | 1.16 | 0.0021 |
| T Category | ||||
| 1 | - | - | - | - |
| 2 | 1.27 | 0.06 | 1.13 | 0.36 |
| 3 | 1.38 | 0.027 | 1.35 | 0.025 |
| 4 | 1.8 | <0.001 | 1.64 | <0.001 |
| N Category | ||||
| 0 | - | - | - | - |
| 1 | 1.16 | 0.44 | 1.34 | 0.11 |
| 2 | 1 | 0.99 | 1.28 | 0.11 |
| 3 | 1.24 | 0.24 | 1.53 | 0.014 |
| Tumor Size | 1 | 0.84 | - | - |
| Bone Mets | 1.34 | 0.0018 | 1.81 | <0.001 |
| Liver Mets | 1.11 | 0.44 | 1.41 | 0.0045 |
| Lung Mets | 1.35 | <0.001 | 1.58 | <0.001 |
| Brain Mets | 1.74 | 0.087 | 2.13 | 0.069 |
| LN Mets | 0.54 | <0.001 | - | - |
| LVSI | 1.02 | 0.056 | 1.02 | 0.04 |
| Any Chemo | 0.5 | <0.001 | 0.55 | <0.001 |
| HPV Positive | HPV Negative | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |
| Any RT | 0.67 | 0.013 | 0.64 | 0.0026 | 0.69 | 0.018 | 0.65 | 0.0047 |
| Age | 1.01 | 0.089 | 1.01 | 0.093 | 0.99 | 0.27 | 0.99 | 0.14 |
| CDCC | 1.16 | 0.288 | 1.22 | 0.052 | 1.11 | 0.28 | 1.28 | 0.017 |
| T Category | ||||||||
| 1 | - | - | - | - | - | - | - | - |
| 2 | 1.76 | 0.038 | - | - | 1.11 | 0.68 | 1.12 | 0.7 |
| 3 | 2.04 | 0.0088 | - | - | 1.35 | 0.26 | 1.27 | 0.42 |
| 4 | 1.7 | 0.062 | - | - | 2.13 | 0.0025 | 1.99 | 0.012 |
| N Category | ||||||||
| 0 | - | - | - | - | - | - | - | - |
| 1 | 0.73 | 0.56 | - | - | 1.44 | 0.37 | - | - |
| 2 | 0.73 | 0.49 | - | - | 1.31 | 0.44 | - | - |
| 3 | 0.87 | 0.78 | - | - | 1.65 | 0.23 | - | - |
| Tumor Size | 1 | 0.97 | - | - | 1 | 0.016 | 1 | 0.11 |
| Bone Mets | 1.42 | 0.048 | 1.75 | 0.0041 | 1.89 | 0.0027 | 2.52 | <0.001 |
| Liver Mets | 1.11 | 0.616 | - | - | 0.79 | 0.45 | - | - |
| Lung Mets | 1.35 | 0.044 | 1.39 | 0.041 | 1.14 | 0.401 | 1.49 | 0.035 |
| Brain Mets | 0 | p < 0.001 | - | - | 44.03 | <0.001 | 48.53 | <0.001 |
| LN Mets | 0.6 | 0.0061 | - | - | 0.69 | 0.042 | - | - |
| LVSI | 0.99 | 0.6 | - | - | 1.06 | 0.014 | 1.1 | <0.001 |
| Any Chemo | 0.66 | 0.074 | 0.57 | 0.0057 | 0.59 | 0.0093 | 0.45 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharouta, M.; Lorenz, F.J.; Mahase, S.; Shi, H.; Goyal, N.; Yao, M. The Role of Radiotherapy to the Primary Site in Oropharyngeal Cancer with Limited Metastases—An Analysis of a Hospital-Based Registry. Cancers 2024, 16, 4130. https://doi.org/10.3390/cancers16244130
Kharouta M, Lorenz FJ, Mahase S, Shi H, Goyal N, Yao M. The Role of Radiotherapy to the Primary Site in Oropharyngeal Cancer with Limited Metastases—An Analysis of a Hospital-Based Registry. Cancers. 2024; 16(24):4130. https://doi.org/10.3390/cancers16244130
Chicago/Turabian StyleKharouta, Michael, F. Jeffrey Lorenz, Sean Mahase, Hongyun Shi, Neerav Goyal, and Min Yao. 2024. "The Role of Radiotherapy to the Primary Site in Oropharyngeal Cancer with Limited Metastases—An Analysis of a Hospital-Based Registry" Cancers 16, no. 24: 4130. https://doi.org/10.3390/cancers16244130
APA StyleKharouta, M., Lorenz, F. J., Mahase, S., Shi, H., Goyal, N., & Yao, M. (2024). The Role of Radiotherapy to the Primary Site in Oropharyngeal Cancer with Limited Metastases—An Analysis of a Hospital-Based Registry. Cancers, 16(24), 4130. https://doi.org/10.3390/cancers16244130






