Long-Term Effects on Gonadal Function After Treatment of Colorectal Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration of Protocols
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
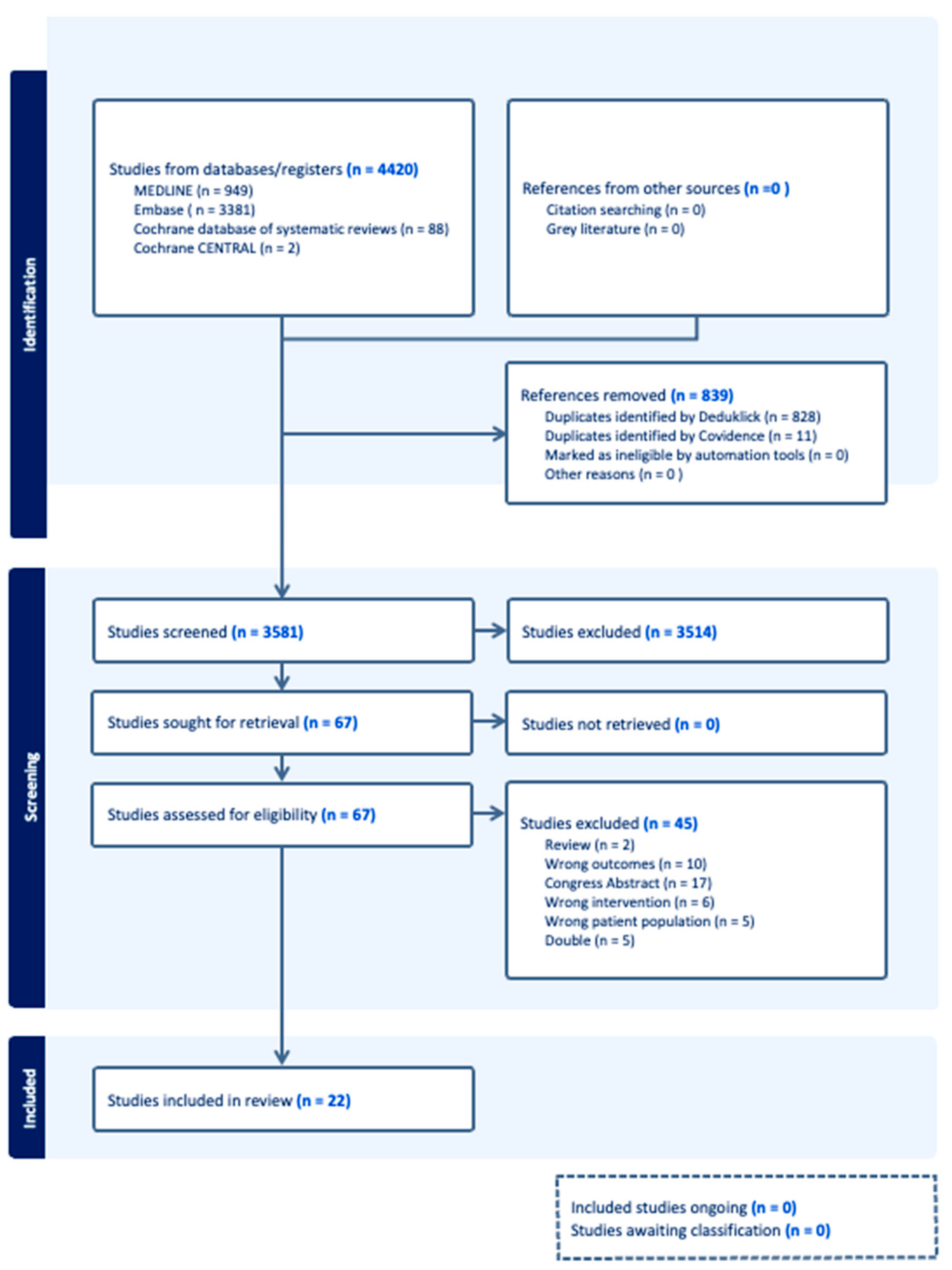
3. Results
3.1. Results of the Systematic Review
3.2. Study Characteristics
3.3. Prevalence of Clinically Relevant Gonadotoxicity
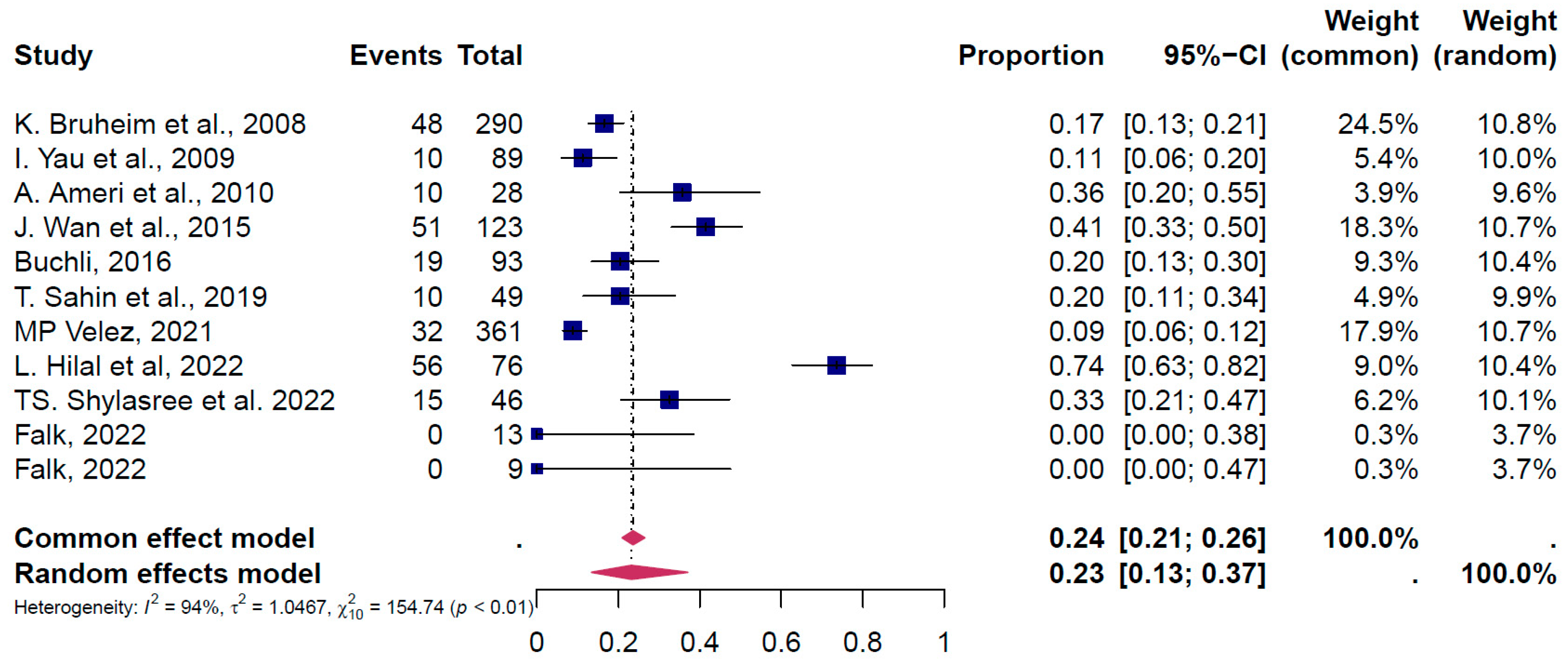
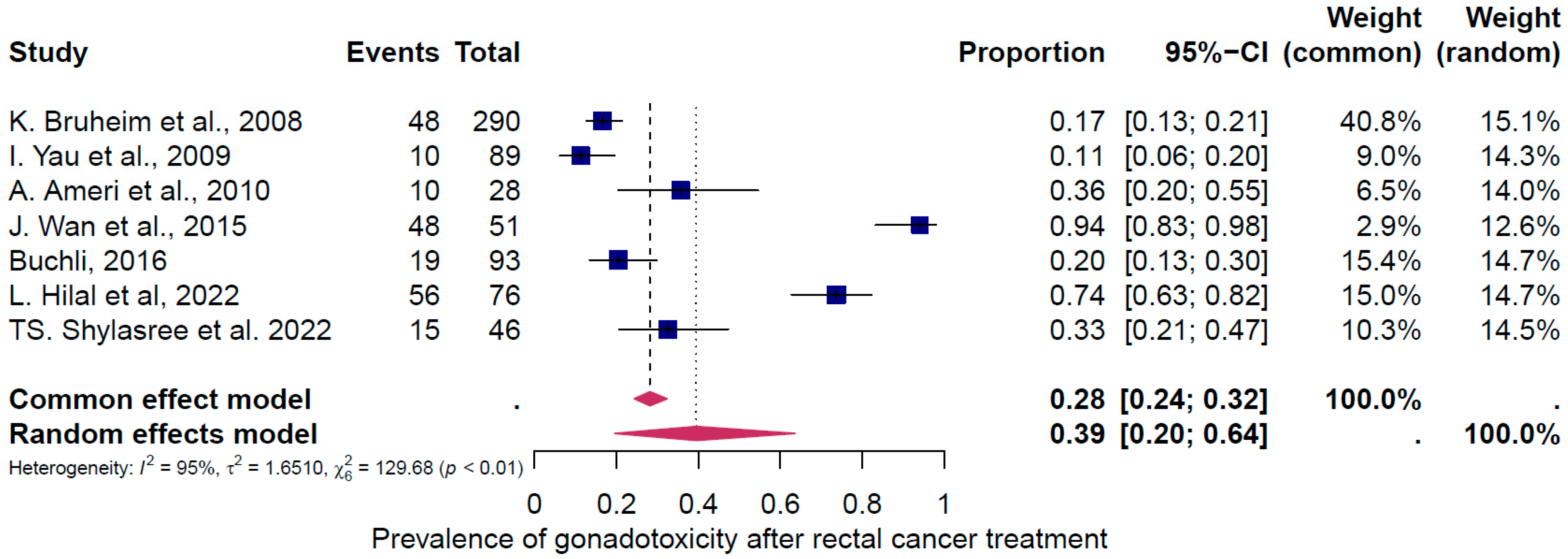
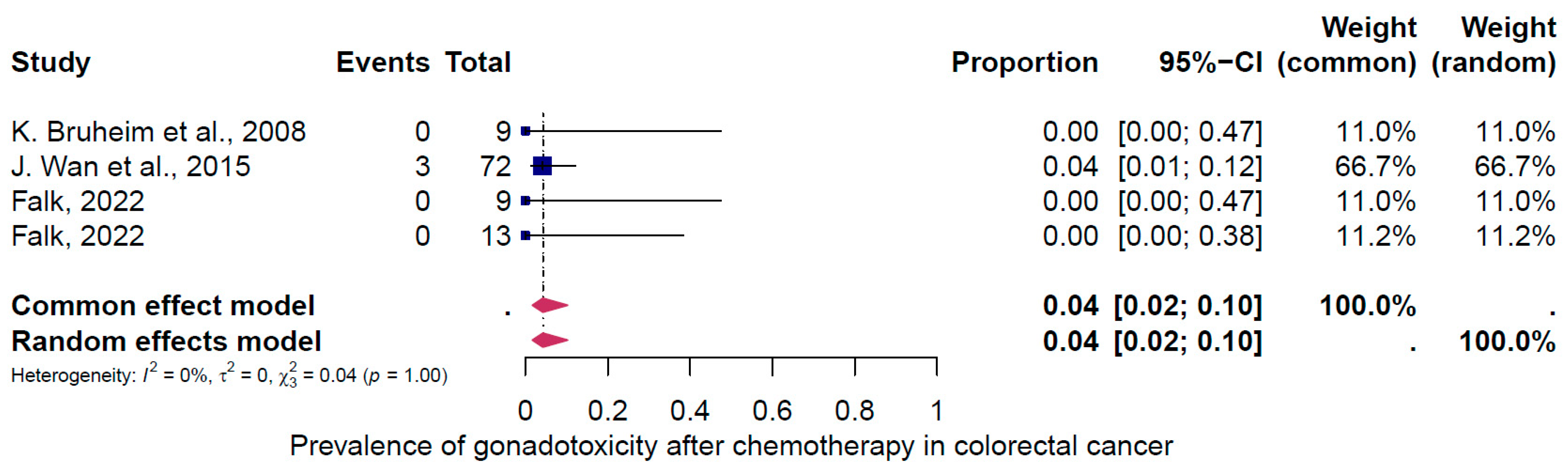
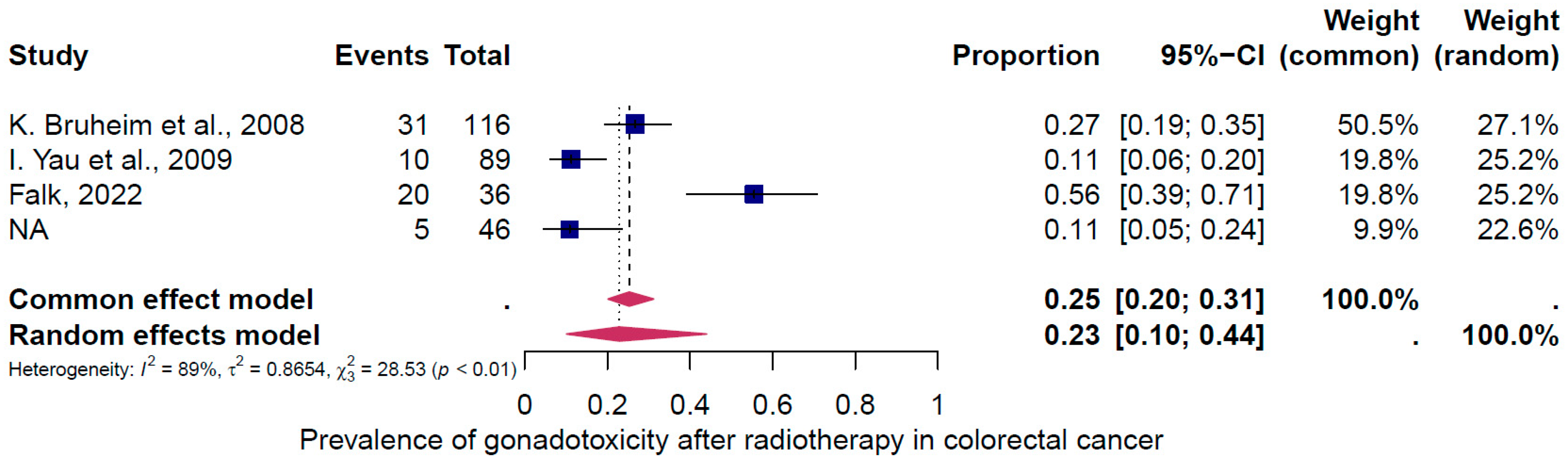
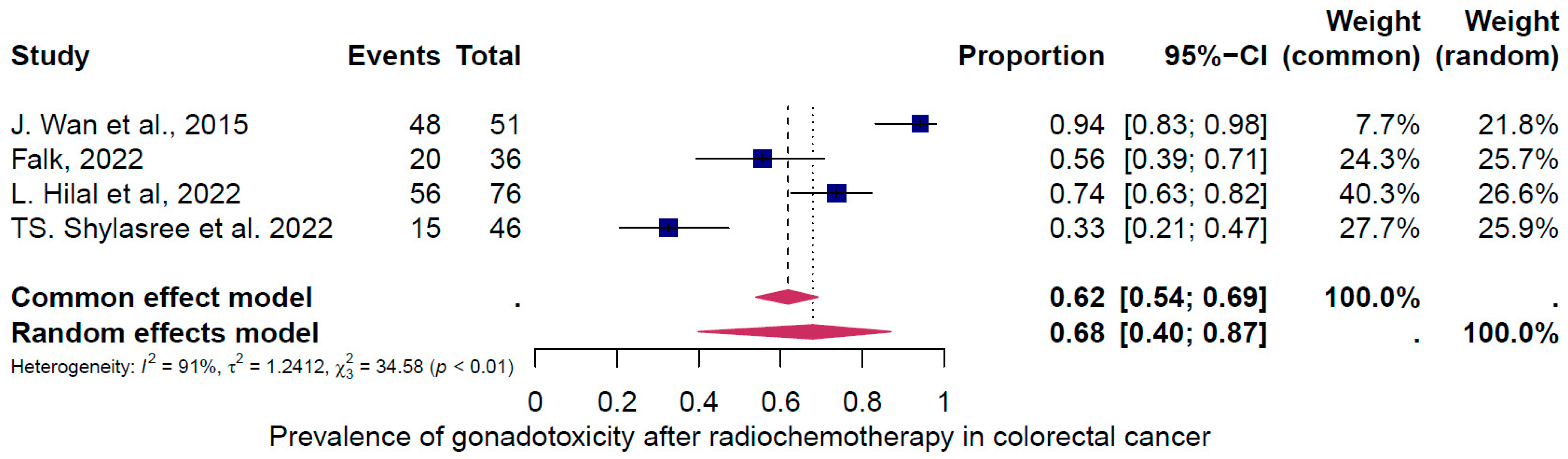
4. Results of the Meta-Analysis
4.1. Pooled Overall Prevalence of Gonadotoxicity After All Types of Treatment
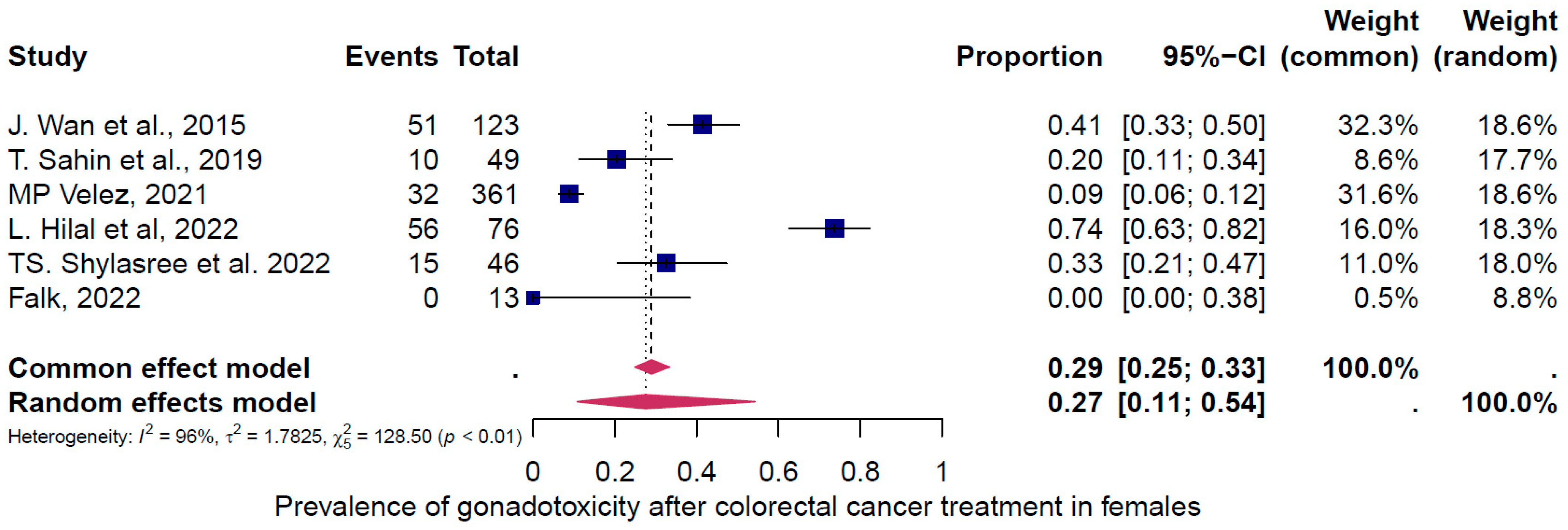
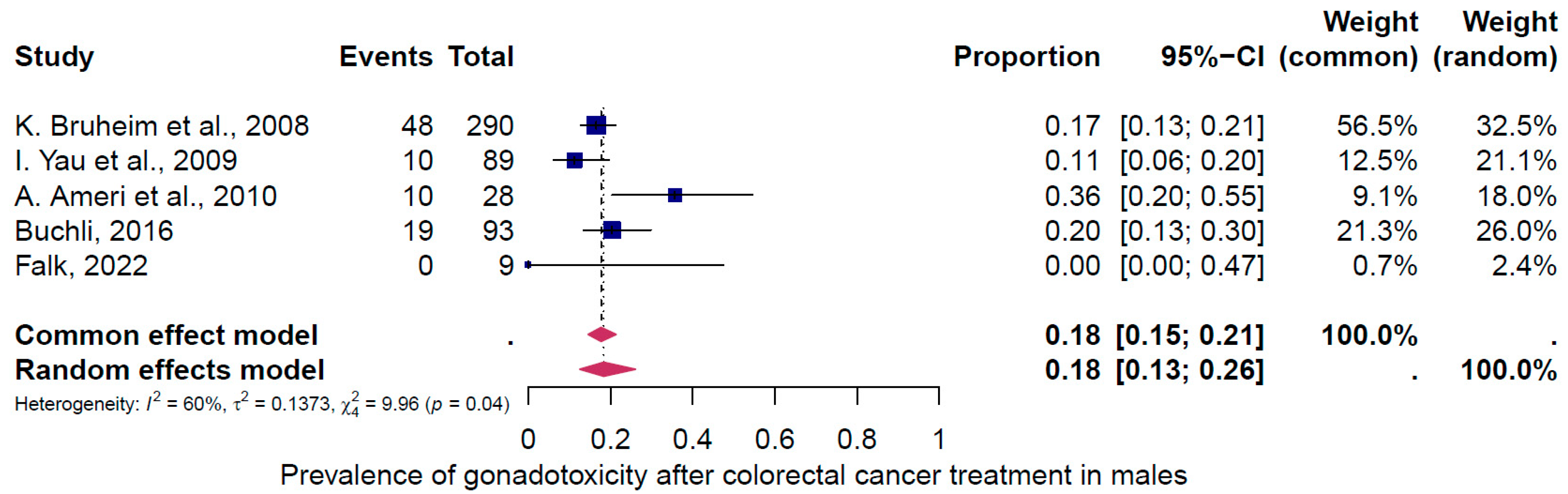
4.2. Subgroup Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Paschke, S.; Jafarov, S.; Staib, L.; Kreuser, E.-D.; Maulbecker-Armstrong, C.; Roitman, M.; Holm, T.; Harris, C.C.; Link, K.-H.; Kornmann, M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 2577. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Giovannucci, E.L.; Colditz, G.A.; Hunter, D.J.; Speizer, F.E.; Willett, W.C. A prospective study of family history and the risk of colorectal cancer. N. Engl. J. Med. 1994, 331, 1669–1674. [Google Scholar] [CrossRef]
- Edelstein, D.L.; Axilbund, J.; Baxter, M.; Hylind, L.M.; Romans, K.; Griffin, C.A.; Cruz-Correa, M.; Giardiello, F.M. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin. Gastroenterol. Hepatol. 2011, 9, 340–343. [Google Scholar] [CrossRef]
- Church, J.M.; McGannon, E.; Burke, C.; Clark, B. Teenagers with familial adenomatous polyposis: What is their risk for colorectal cancer? Dis. Colon. Rectum 2002, 45, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, M.W.M.D.; Vleggaar, F.P.; Schipper, M.E.I.; Stokkers, P.C.F.; van der Woude, C.J.; Hommes, D.W.; de Jong, D.J.; Dijkstra, G.; van Bodegraven, A.A.; Oldenburg, B.; et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut 2008, 57, 1246–1251. [Google Scholar] [CrossRef]
- Tanaka, L.F.; Hechenbichler Figueroa, S.; Popova, V.; Klug, S.J.; Buttmann-Schweiger, N. The rising incidence of early-onset colorectal cancer. Dtsch. Ärzteblatt Int. 2023, 120, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [PubMed]
- Spanos, C.P.; Mamopoulos, A.; Tsapas, A.; Syrakos, T.; Kiskinis, D. Female fertility and colorectal cancer. Int. J. Color. Dis. 2008, 23, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Anselmi, M.C.; Schneider, G.A.; Rodrigues Furtado, J.P.; Mohamed Abau Shwareb, M.G.; Linhares, J.C. First live birth after uterine transposition. Fertil. Steril. 2023, 120, 188–193. [Google Scholar] [CrossRef]
- Sipaviciute, A.; Sileika, E.; Burneckis, A.; Dulskas, A. Late gastrointestinal toxicity after radiotherapy for rectal cancer: A systematic review. Int. J. Color. Dis. 2020, 35, 977–983. [Google Scholar] [CrossRef]
- Von Wolff, M. Fertility Preservation in Oncological and Non-Oncological Diseases: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 3-030-47570-0. [Google Scholar]
- von Wolff, M.; Germeyer, A.; Böttcher, B.; Magaton, I.M.; Marcu, I.; Pape, J.; Sänger, N.; Nordhoff, V.; Roumet, M.; Weidlinger, S. Evaluation of the Gonadotoxicity of Cancer Therapies to Improve Counseling of Patients About Fertility and Fertility Preservation Measures: Protocol for a Retrospective Systematic Data Analysis and a Prospective Cohort Study. JMIR Res. Protoc. 2024, 13, e51145. [Google Scholar] [CrossRef]
- Weidlinger, S.; Graber, S.; Bratschi, I.; Pape, J.; Kollár, A.; Karrer, T.; von Wolff, M. A Systematic Review of the Gonadotoxicity of Osteosarcoma and Ewing’s Sarcoma Chemotherapies in Postpubertal Females and Males. J. Adolesc. Young Adult Oncol. 2024, 13, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, M.; Rietschin, A.; Pagano, F.; Karrer, T.; Kollár, A.; Weidlinger, S.; von Wolff, M. Systematic Review of the Gonadotoxicity and Risk of Infertility of Soft Tissue Sarcoma Chemotherapies in Pre- and Postpubertal Females and Males. J. Adolesc. Young Adult Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Fernando, J.; Megaritis, D.; Weidlinger, S.; Vidal, A.; Birkhäuser, F.D.; Karrer, T.; Von Wolff, M. Oncological treatments have limited effects on the fertility prognosis in testicular cancer: A systematic review and meta-analysis. Andrology 2024. [Google Scholar] [CrossRef]
- Pape, J.; Gudzheva, T.; Danijela, B.; Weidlinger, S.; Vidal, A.; Furtwängler, R.; Karrer, T.; Von Wolff, M. Long-term effects on fertility after central nervous system cancer: A systematic review and meta-analysis. Neuro-Oncol. Pract. 2024, 11, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Borissov, N.; Haas, Q.; Minder, B.; Kopp-Heim, D.; von Gernler, M.; Janka, H.; Teodoro, D.; Amini, P. Reducing systematic review burden using Deduklick: A novel, automated, reliable, and explainable deduplication algorithm to foster medical research. Syst. Rev. 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Van der Mierden, S.; Tsaioun, K.; Bleich, A.; Leenaars, C.H.C. Software tools for literature screening in systematic reviews in biomedical research. ALTEX 2019, 36, 508–517. [Google Scholar] [CrossRef]
- Al-Badawi, I.A.; Al-Aker, M.; AlSubhi, J.; Salem, H.; Abduljabbar, A.; Balaraj, K.; Munkarah, A. Laparoscopic Ovarian Transposition Before Pelvic Irradiation: A Saudi Tertiary Center Experience. Int. J. Gynecol. Cancer 2010, 20, 1082–1086. [Google Scholar] [CrossRef]
- Cercek, A.; Siegel, C.L.; Capanu, M.; Reidy-Lagunes, D.; Saltz, L.B. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin. Color. Cancer 2013, 12, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Barahmeh, S.; Al Masri, M.; Badran, O.; Masarweh, M.; El-Ghanem, M.; Jaradat, I.; Lataifeh, I. Ovarian Transposition before Pelvic Irradiation: Indications and Functional Outcome. J. Obs. Gynaecol. 2013, 39, 1533–1537. [Google Scholar] [CrossRef]
- Wan, J.; Gai, Y.; Li, G.; Tao, Z.; Zhang, Z. Incidence of chemotherapy- and chemoradiotherapy-induced amenorrhea in premenopausal women with stage II/III colorectal cancer. Clin. Color. Cancer 2015, 14, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Shalgi, R.; Brenner, B.; Perl, G.; Purim, O.; Amit, L.; Stemmer, S.M.; Ben-Aharon, I. The impact of oxaliplatin on the gonads: From bedside to the bench. Mol. Hum. Reprod. 2015, 21, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Sioulas, V.D.; Jorge, S.; Chern, J.-Y.; Schiavone, M.B.; Weiser, M.R.; Kelvin, J.F.; Gardner, G.J.; Sonoda, Y.; Abu-Rustum, N.R.; Goodman, K.A.; et al. Robotically Assisted Laparoscopic Ovarian Transposition in Women with Lower Gastrointestinal Cancer Undergoing Pelvic Radiotherapy. Ann. Surg. Oncol. 2017, 24, 251–256. [Google Scholar] [CrossRef]
- Sahin, T.; Dizdar, O.; Ozdemir, N.; Zengin, N.; Ates, O.; Oksuzoglu, B.; Sendur, M.A.N.; Bilgin, B.; Demir, M.; Bozbulut, U.B.; et al. The frequency and predictors of persistent amenorrhea in premenopausal women with colorectal cancer who received adjuvant chemotherapy. Anti-Cancer Drugs 2019, 30, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Svanström Röjvall, A.; Buchli, C.; Bottai, M.; Ahlberg, M.; Flöter-Rådestad, A.; Martling, A.; Segelman, J. Effect of radiotherapy for rectal cancer on female sexual function: A prospective cohort study. Br. J. Surg. 2020, 107, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Velez, M.P.; Richardson, H.; Baxter, N.N.; McClintock, C.; Greenblatt, E.; Barr, R.; Green, M. Risk of infertility in female adolescents and young adults with cancer: A population-based cohort study. Human. Reprod. 2021, 36, 1981–1988. [Google Scholar] [CrossRef]
- Hilal, L.; Cercek, A.; Navilio, J.; Hsu, M.; Zhang, Z.; Brady, P.; Wu, A.J.; Reyngold, M.; Cuaron, J.J.; Romesser, P.B.; et al. Factors Associated With Premature Ovarian Insufficiency in Young Women With Locally Advanced Rectal Cancer Treated With Pelvic Radiation Therapy. Adv. Radiat. Oncol. 2022, 7, 100801. [Google Scholar] [CrossRef]
- Shylasree, T.S.; Singh, P.; Kazi, M.; Gupta, S.; Engineer, R.; Patil, P.S.; DeSouza, A.; Saklani, A. Laparoscopic ovarian transposition in teenage and young women with locally advanced rectal cancer: Respite amidst cancer chaos. Color. Dis. 2022, 24, 697–705. [Google Scholar] [CrossRef]
- Falk, P.; Severin, M.; Berglund, Å.; Guren, M.G.; Hofsli, E.; Österlund, P.; Tandberg, A.; Eberhard, J.; Sorbye, H. Sex hormones and sperm parameters after adjuvant oxaliplatin-based treatment for colorectal cancer. Cancer Treat. Res. Commun. 2022, 31, 100517. [Google Scholar] [CrossRef]
- Piroth, M.D.; Hensley, F.; Wannenmacher, M.; Zierhut, D. Radiogene Hodenbelastung durch Streustrahlung bei adjuvanter 3-D-Beckenbestrahlung nach anteriorer Resektion beim Rektumkarzinom: Einfluss auf die Fertilität. Strahlenther Onkol 2003, 179, 754–759. [Google Scholar] [CrossRef]
- Bruheim, K.; Svartberg, J.; Carlsen, E.; Dueland, S.; Haug, E.; Skovlund, E.; Tveit, K.M.; Guren, M.G. Radiotherapy for Rectal Cancer Is Associated With Reduced Serum Testosterone and Increased FSH and LH. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Yau, I.; Vuong, T.; Garant, A.; Ducruet, T.; Doran, P.; Faria, S.; Liberman, S.; Richard, C.; Letellier, F.; Charlebois, P.; et al. Risk of Hypogonadism From Scatter Radiation During Pelvic Radiation in Male Patients With Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Yoon, F.H.; Perera, F.; Fisher, B.; Stitt, L. Alterations in Hormone Levels After Adjuvant Chemoradiation in Male Rectal Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1186–1190. [Google Scholar] [CrossRef]
- Ameri, A.; Sobhani, M.; Alidoosti, A.; Sharafi, A.A.; Arbabi, A.; Taslimi, F.; Fazlalizadeh, H. Different irradiation machines and their effects on testes’ exposure levels and sex hormones profile in patients with rectal cancer. J. Radiother. Pract. 2010, 9, 99–105. [Google Scholar] [CrossRef]
- Hennies, S.; Wolff, H.A.; Jung, K.; Rave-Fränk, M.; Gaedcke, J.; Ghadimi, M.; Hess, C.F.; Becker, H.; Hermann, R.M.; Christiansen, H. Testicular Radiation Dose after Multimodal Curative Therapy for Locally Advanced Rectal Cancer: Influence on Hormone Levels, Quality of Life, and Sexual Functioning. Strahlenther Onkol 2012, 188, 926–932. [Google Scholar] [CrossRef]
- Buchli, C.; Tapper, J.; Bottai, M.; Holm, T.; Arver, S.; Blomqvist, L.; Martling, A. Testosterone and Body Composition in Men after Treatment for Rectal Cancer. J. Sex. Med. 2015, 12, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Buchli, C.; Martling, A.; Abani, M.A.; Frödin, J.-E.; Bottai, M.; Lax, I.; Arver, S.; Holm, T. Risk of Acute Testicular Failure After Preoperative Radiotherapy for Rectal Cancer: A Prospective Cohort Study. Ann. Surg. 2018, 267, 326–331. [Google Scholar] [CrossRef]
- De La Motte, L.; Custovic, S.; Tapper, J.; Arver, S.; Martling, A.; Buchli, C. Effect of Preoperative Radiotherapy for Rectal Cancer on Spermatogenesis. Br. J. Surg. 2021, 108, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.; Fernandes, D.; Athiyamaan, M.S.; Shankar, S.; Rao, S.; Hasib, A.G. Assessment of Variations in Serum Testosterone, Follicle Stimulating Hormone, and Luteinizing Hormone Levels in Patients Receiving Radiotherapy for Rectal Cancer. Middle East J. Cancer 2022, 13. [Google Scholar] [CrossRef]
- Ullah, F.; Pillai, A.B.; Omar, N.; Dima, D.; Harichand, S. Early-Onset Colorectal Cancer: Current Insights. Cancers 2023, 15, 3202. [Google Scholar] [CrossRef]
- Dolmans, M.-M. Recent advances in fertility preservation and counseling for female cancer patients. Expert. Rev. Anticancer. Ther. 2018, 18, 115–120. [Google Scholar] [CrossRef]
- Lambertini, M.; Del Mastro, L.; Pescio, M.C.; Andersen, C.Y.; Azim, H.A.; Peccatori, F.A.; Costa, M.; Revelli, A.; Salvagno, F.; Gennari, A.; et al. Cancer and fertility preservation: International recommendations from an expert meeting. BMC Med. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Schüring, A.N.; Fehm, T.; Behringer, K.; Goeckenjan, M.; Wimberger, P.; Henes, M.; Henes, J.; Fey, M.F.; von Wolff, M. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part I: Indications for fertility preservation. Arch. Gynecol. Obstet. 2018, 297, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.B.; Shalet, S.M.; Hendry, J.H.; Morris-Jones, P.H.; Gattamaneni, H.R. Ovarian failure following abdominal irradiation in childhood: The radiosensitivity of the human oocyte. Br. J. Radiol. 1989, 62, 995–998. [Google Scholar] [CrossRef]
- Smith, K.L.; Gracia, C.; Sokalska, A.; Moore, H. Advances in Fertility Preservation for Young Women With Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 27–37. [Google Scholar] [CrossRef]
- Kye, B.-H.; Cho, H.-M. Overview of radiation therapy for treating rectal cancer. Ann. Coloproctol. 2014, 30, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Lohynska, R.; Jirkovska, M.; Novakova-Jiresova, A.; Mazana, E.; Vambersky, K.; Veselsky, T.; Kindlova, A.; Stankusova, H.; Malinova, B. Radiotherapy dose limit for uterus fertility sparing in curative chemoradiotherapy for rectal cancer. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2021, 165, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Grønlie Guren, M.; Rune Olsen, D.; Poulsen, J.P.; Magne Tveit, K. Radiation therapy induced changes in male sex hormone levels in rectal cancer patients. Radiother. Oncol. 2003, 68, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Pogue-Geile, K.; Yothers, G.; Taniyama, Y.; Tanaka, N.; Gavin, P.; Colangelo, L.; Blackmon, N.; Lipchik, C.; Kim, S.R.; Sharif, S.; et al. Defective Mismatch Repair and Benefit from Bevacizumab for Colon Cancer: Findings from NSABP C-08. JNCI J. Natl. Cancer Inst. 2013, 105, 989–992. [Google Scholar] [CrossRef]
- Morse, M.A. Adjuvant therapy of colon cancer: Current status and future developments. Clin. Colon. Rectal Surg. 2005, 18, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tsukada, Y.; Watanabe, J.; Fukunaga, Y.; Hirano, Y.; Sakamoto, K.; Hamamoto, H.; Yoshimitsu, M.; Horie, H.; Matsuhashi, N.; et al. Long-term survival and functional outcomes of laparoscopic surgery for clinical stage I ultra-low rectal cancers located within 5 cm of the anal verge: A prospective phase II trial (Ultimate trial). Ann. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- REACCT Collaborative. Post-Operative Functional Outcomes in Early Age Onset Rectal Cancer. Front. Oncol. 2022, 12, 868359. [Google Scholar]
- Thyø, A.; Elfeki, H.; Laurberg, S.; Emmertsen, K.J. Female sexual problems after treatment for colorectal cancer—A population-based study. Color. Dis. 2019, 21, 1130–1139. [Google Scholar] [CrossRef]
- Holowatyj, A.N.; Eng, C.; Lewis, M.A. Incorporating Reproductive Health in the Clinical Management of Early-Onset Colorectal Cancer. JCO Oncol. Pract. 2022, 18, 169–172. [Google Scholar] [CrossRef]
- Chodoff, A.; Smith, K.C.; Shukla, A.; Blackford, A.L.; Ahuja, N.; Johnston, F.M.; Peairs, K.S.; Ngaiza, J.R.; Warczynski, T.; Nettles, B.; et al. Colorectal cancer survivorship care plans: Variations in documentation and posttreatment surveillance recommendations. J. Surg. Oncol. 2022, 125, 678–691. [Google Scholar] [CrossRef] [PubMed]
| Females | Males |
|---|---|
| Menstrual cycle disorders Amenorrhea/oligomenorrhea Hormonal treatment: puberty induction/hormonal replacement therapy | Disorders of sperm quality Azoospermia Oligozoospermia |
| Hormone levels above the normal range Follicle-stimulating hormone (FSH) Luteinizing hormone (LH) | Hormone levels above the normal range Follicle-stimulating hormone (FSH) Luteinizing hormone (LH) |
| Premature ovarian insufficiency (POI) Oligo-/amenorrhea for at least 4 months and an elevated FSH level > 25 IU/L on two occasions at 4 weeks apart before the age of 40. (ESHRE Definition) | Gonadal dysfunction Low testosterone levels Hormonal treatment: testosterone therapy |
| Low ovarian reserve parameters Anti-Müllerian hormone (AMH) not detectable | Hormone levels below the normal range Inhibin B |
| First Author, Year of Publication | Country | Study Design | Number of Participants of Interest (Females) | Age of Participants of Interest at Time of Diagnosis/Therapy (Years, Range) | Age (Years, Mean ± SD) at Outcome/Evaluation | Follow-Up After Diagnosis/Treatment, Length in Years (Range) | Tumor Type Number (%) | Chemotherapy, Details | Radiotherapy, Details | Suspected Infertility | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Badawi et al., 2010 [24] | Saudi Arabia | Retrospective | 4 | 23 (18–36) | Not specified | 2.67 (0.83–5) | RC | Not specified | Yes, without specifications | 2/4 (50%) | Calculated in women with persistent amenorrhea. Laparoscopic ovarian transposition to paracolic gutters with uterine conservation. |
| Cercek et al., 2013 [25] | USA | Retrospective | 49 | 31–35 (21–50) | Not specified | >0.5 (range not specified) | CRC | FOLFOX standard modified mFOLFOX | No | 8/49 (16%) | Calculated in women with persistent amenorrhea (>1 year). |
| Barahmeh et al., 2013 [26] | Jordan | Retrospective | 4 | Not specified | Not specified | 3.5 (2.83–4.17) | RC | 5-FU concomitantly with radiotherapy | Estimated irradiation dose to both ovaries after pelvic radiotherapy: 2.1 Gy for three patients and 18 Gy for one patient. External pelvic irradiation (45–60 Gy) | 1/4 (25%) | Calculated in women with hypergonadotropic hypogonadism. Bilateral ovarian transposition to the paracolic gutter. |
| Wan et al., 2015 [27] | China | Retrospective | 123 | CC: 36 (17–40) RC: 35 (24–40) | Not specified | CC: 3.16 (1.52–6.32) RC: 3.35 (1.21–6.36) | CC 58.6 RC 41.4 | FOLFOX XELOX Capecitabine only | CC: no RC: intensity-modulated radiotherapy to pelvis (total dose 45–55 Gy in 25–30 fractions) | colon cancer 3/72 (4.2%) rectal cancer 48/51 (94.1%) | Calculated in women with persistent amenorrhea > 1 year. |
| Levi et al., 2015 [28] | Israel | Prospective | 11 | 36 | 36.5 | 0.5 | CRC | FOLFOX or XELOX | In 1 patient | 0/11 (0%) | Calculated in women with hypergonadotropic hypogonadism. |
| Sioulas et al., 2017 [29] | USA | Retrospective | 22 | 39 (26–45) | Not specified | 2.42 (0.09–6) | RC (90.9) AC (9.1) | FOLFOX CAPOX FOLFOX/bevacizumab FOLFOX/FOLFIRINOX Capecitabine 5-FU Mitomycin C | RC: 5000 to 5400 cGy to the rectal tumor 4500 cGy to the pelvic nodes AC: 5600 cGy to the primary tumor 4500 cGy to the pelvic nodes | 6/18 (33.3%) | Calculated in women with hypergonadotropic hypogonadism. Only 18 patients were evaluable for ovarian function. Nineteen patients underwent OT. |
| Sahin et al., 2019 [30] | Turkey | Retrospective | 60 | 40 (19–50) | Not specified | Min. 1 | CC | 5-FU alone 5-FU + oxaliplatin FOLFOX CAPOX | No | 10/49 (20.4%) | Calculated in women with persistent amenorrhea >1 year. |
| Svanström Röjvall, 2020 [31] | Sweden | Prospective | 6 | Not specified | Not specified | 2 | RC | Yes | Short course (5 Gy × 5) Long course (2 Gy × 25 or 1· 8 Gy × 28) + 3 fractions of boost | 5/6 (83.3%) | Calculated in women with undetectable AMH. |
| Velez, 2021 [32] | Canada | Retrospective | 361 | Not specified | Not specified | Not specified | CRC | Not specified | Not specified | 32/361 (8.9%) | Calculated in women with infertility diagnosis using the health administrative database. |
| Hilal et al., 2022 [33] | USA | Retrospective | 76 | 43 (20–49) | Not specified | 4.48 (0.48–15.44) | RC | FOLFOX/XELOX 5FU/LV Xeloda Cisplatin–Etoposide | Median dose: 50 Gy (25–56) 25 (5–28) fractions 3D-CRT IMRT | 56/76 (75%) | Twenty-six (34%) underwent OT. Calculated in women with hypergonadotropic hypogonadism. |
| Shylasree, 2022 [34] | India | Retrospective | 46 | 25.2 | Not specified | 3.5 (0.42–6.75) | RC | Capecitabine 5-FU + oxaliplatin | Neoadjuvant chemoradiation: 50.4 Gy in 28 fractions (1.8 Gy) with concurrent capecitabine. Short-course RT: 25 Gy in five fractions (5 Gy). | 15/43 (34.9%) | Calculated in women with hypergonadotropic hypogonadism and a need for puberty induction. |
| Falk, 2022 [35] | Norway, Sweden, Finland | Prospective | 16 | 35 (range 20–40) | Not specified | 1–5 | CC RC AA CRC | FOLFOX CAPOX Nordic FLOX | No | 0/13 (0%) | Calculated in women with hypergonadotropic hypogonadism, amenorrhea, and undetectable AMH. |
| First Author, Year of Publication | Country | Study Design | Number of Participants of Interest (Males) | Age of Participants of Interest at Time of Diagnosis/Therapy | Age, yrs (Mean ± SD) at Outcome/ Evaluation | Follow-Up After Diagnosis/ Treatment, Length in Years (Range) | Tumor Type | Chemotherapy, Details | Radiotherapy, Details | Suspected Infertility (…/…/%) MALES | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Piroth et al., 2003 [36] | Germany | Prospective | 18 | not specified | Not specified | Not specified | RC | 5-FU | Total dose: 50.4 Gy Single dose: 1.8 Gy per day 5 × per week TD: Mean: 0.057 Gy (0.035–0.114) Cumulative: 1.60 Gy (0.98–3.19) | n/a | |
| Bruheim et al., 2008 [37] | Norway | Retrospective | 290 | irradiated 66.0 (45.1–86.0) non-irradiated 71.4 (40.2–94.8) | Not specified | 2–12 | RC | 5-FU + leucovorin | Mean dose: 50.07 Gy (25 fractions of 2 Gy given in 5 weeks) Treatment time: 35 days (7–106) Preoperative: 74 (63.8%) Postoperative: 42 (36.2%) | 48/290 (16.6%) | Calculated in men with testosterone values under the normal limit |
| Yau et al., 2009 [38] | Canada | Prospective | 89 | EBRT 62.25 (32–87) HDRBT 61.03 (37–84) | Not specified | 1.42 - 1.17 EBRT - 1.67 HDRBT | RC | 5-FU | EBRT (38 patients) 45.0–50.4 Gy in 1.8 Gy per day (5 days per week over 5–5.5 weeks) HDRBT (51 patients) 26 Gy (4 times per day; 6.5 Gy daily) TD: - EBRT: 1.24 Gy (0.06–7.80) - HDRBT: 0.27 Gy (0.14–0.65) | EBRT 9/51 (17.6%) HDRBT 1/38 (2.6%) total 10/89 (11.2%) | 2-year hypogonadism rates |
| Yoon et al., 2009 [39] | England | Prospective | 43 | 56.5 (35–72) | Not specified | 6,1 (1.3–9.4) | RC | Adjuvant: 5-FU (bolus) Concurrent: - CIVI (36; 84%) - bolus (7; 16%) 2 additional 5-day cycles of 5-FU (450 mg/m2/d) | Median dose: 54.0 Gy in 30 fractions TD: 4 Gy (1.5–8.9 Gy) Three-field pelvic technique (36, 84%) Four-field technique (5, 11.6%) | Only mean values | |
| Ameri et al., 2010 [40] | Iran | Prospective | 28 | 52.72 ± 13 | Not specified | 0.13 | RC | Adjuvant 18 (Co60: 10 LINAC: 8) Neo-adjuvant 6 (Co60: 2 LINAC: 4) Palliative 1 (Co60) 5-FU (CIVI) (Co60: 4 LINAC: 1) 5-FU + oxaliplatin (Co60: 3 LINAC: 1) Capecitabine (Co60: 5 LINAC: 8) | Co60 (14 patients) 47.88 Gy ± 2.77 LINAC (14 patients) 47.55 Gy ± 3.24 TD: Co60 (4) 55 mGy (±24.7) (29–80) Mean cumulative: 3.27 Gy (2.4–3.8) 6.6% (4.7–7.5%) of total target dose LINAC (5): 120 mGy (±20.3) (85–135) Mean cumulative: 1.4 Gy (0.73–2) 3% (1.6–4.45) of total target dose | (10/28) 35.71% | Of patients with a decrease in testosterone post-radiotherapy |
| Hennies et al., 2012 [41] | Germany | Prospective | 83 | 65 (39–83) | Not specified | 1 | RC | Concomitant: 5-FU (53, 64%) 5-FU + oxaliplatin (30, 36%) Adjuvant: 5-FU (68, 88%) 5-FU + oxaliplatin (9, 12%) | Isocentric three-field posterior–anterior/lateral technique Total dose: 50.4 Gy (1.8 Gy daily, 5 days/week) TD: 3.9 Gy | Only mean values | |
| Buchli et al., 2015 [42] | Sweden | Prospective | 40 | 59.9 ± 12.8 | Not specified | 1 | RC | Postoperative chemotherapy (12/40 patients) | Preoperative radiotherapy: short-course (5 × 5 Gy) (30/40 patients) 28 × 1.8 Gy (10/40 patients) | 6/40 (15%) | Calculated in men with testosterone values under the normal limit |
| Levi et al., 2015 [28] | Israel | Prospective | 8 | 38 (33–41) | 38.5 | 0.5 | CRC | FOLFOX XELOX | n/a | none | |
| Buchli et al., 2016 [43] | Sweden | Prospective | 105 | 60.3 (±11.3) | 60.3 (±11.3) | 0.1 (0.01–0.53) | RC | Concomitant chemotherapy (23/25) with long-course RT Full-dose preoperative chemotherapy (11/68) with short-course RT | Preoperative RT: 25 Gy (short-course RT, 5 Gy × 5) or 50.4 Gy (long-course RT, 1.8 Gy × 28) Full-dose preoperative chemotherapy: after short-course RT according to the protocol of the RAPIDO trial | n/a | |
| Motte et al., 2021 [44] | Sweden | Prospective | 115 | Group A: 52 Group B: 63 | Not specified | 2 | RC | Capecitabine, 5-FU, oxaliplatin, leucovorin, irinotecan | TD: Group A: 2.6% Group B: 1.8% | (5/8) 62.5% | Patients with oligospermia 2 years after therapy Group A = semen sample Group B = no semen sample |
| Falk et al., 2022 [35] | Norway Sweden Finland | Prospective | 20 | 35 (20–40) | Not specified | 1–5 | CC (90%) RC (10%) | CAPOX Nordic FLOX (17, 85%) FOLFOX/FLOX CAPOX | No radiotherapy | 0/9 (0%) | Calculated in men with normal FSH/LH |
| Krishna et al., 2022 [45] | India | Prospective | 20 | 59.5 | Not specified | 0.1 | RC | Concurrent: capecitabine 825 mg/m2 (2x per day, five days a week, along with radiation) | 3DCRT (6, 30%) IMRT (14, 70%) neoadjuvant (5, 33%) adjuvant (15, 67%) 50.4 Gy for 5 weeks delivered in 28 fractions TD: 2.65 Gy (1.96 Gy to 4.96 Gy) 5.25% of the total dose | 5/20 (25%) | Calculated in men with testosterone values under the normal limit |
| Selection | Comparability | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year of Publication | Representativeness of Exposed Cohort | Selection of Non-exposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Study Start | Comparability of Cohorts on the Basis of the Design or Analysis Controlled for Confounders | Assessment of Outcome | Sufficient Length of Follow-Up for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | Total | Quality Assessment | Comments |
| Piroth et al., 2003 [36] | ★ | - | ★ | ★ | - | - | - | ★ | 4/8 | poor | no non-exposed cohort group |
| Bruheim et al., 2008 [37] | ★ | ★ | ★ | - | ★ | ★ | ★ | - | 6/8 | good | |
| Yau et al., 2009 [38] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Yoon et al., 2009 [39] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Al-Badawi et al., 2010 [24] | ★ | - | ★ | - | - | ★ | ★ | ★ | 5/8 | poor | no non-exposed cohort group |
| Ameri et al., 2010 [40] | ★ | - | ★ | ★ | - | ★ | - | ★ | 5/8 | poor | no non-exposed cohort group |
| Hennies et al., 2012 [41] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Barahmeh et al., 2013 [26] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Cercek et al., 2013 [25] | ★ | - | ★ | - | - | - | ★ | ★ | 4/8 | poor | no non-exposed cohort group |
| Buchli et al., 2015 [42] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Levi et al., 2015 [28] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Wan et al., 2015 [27] | ★ | - | ★ | - | - | ★ | ★ | - | 4/8 | poor | no non-exposed cohort group |
| Buchli et al., 2016 [43] | ★ | ★ | ★ | ★ | ★ | ★ | - | ★ | 7/8 | good | |
| Sioulas et al., 2017 [29] | ★ | - | ★ | - | - | - | ★ | ★ | 4/8 | poor | no non-exposed cohort group |
| Sahin et al., 2019 [30] | ★ | - | ★ | ★ | - | - | ★ | ★ | 5/8 | poor | no non-exposed cohort group |
| Svanström Röjvall et al., 2020 [31] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/8 | good | |
| Motte et al., 2021 [44] | ★ | - | ★ | ★ | - | ★ | ★ | - | 5/8 | poor | no non-exposed cohort group |
| Velez et al., 2021 [32] | ★ | ★ | ★ | - | ★ | ★ | ★ | ★ | 7/8 | good | |
| Falk et al., 2022 [35] | ★ | - | ★ | ★ | - | ★ | ★ | ★ | 6/8 | poor | no non-exposed cohort group |
| Hilal et al., 2022 [33] | ★ | ★ | ★ | - | ★ | ★ | ★ | ★ | 7/8 | good | |
| Krishna et al., 2022 [45] | ★ | - | ★ | ★ | - | ★ | - | ★ | 5/8 | poor | no non-exposed cohort group |
| Shylasree et al., 2022 [34] | ★ | - | ★ | ★ | - | - | ★ | ★ | 5/8 | poor | no non-exposed cohort group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anthon, C.; Vidal, A.; Recker, H.; Piccand, E.; Pape, J.; Weidlinger, S.; Kornmann, M.; Karrer, T.; von Wolff, M. Long-Term Effects on Gonadal Function After Treatment of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 4005. https://doi.org/10.3390/cancers16234005
Anthon C, Vidal A, Recker H, Piccand E, Pape J, Weidlinger S, Kornmann M, Karrer T, von Wolff M. Long-Term Effects on Gonadal Function After Treatment of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(23):4005. https://doi.org/10.3390/cancers16234005
Chicago/Turabian StyleAnthon, Christiane, Angela Vidal, Hanna Recker, Eva Piccand, Janna Pape, Susanna Weidlinger, Marko Kornmann, Tanya Karrer, and Michael von Wolff. 2024. "Long-Term Effects on Gonadal Function After Treatment of Colorectal Cancer: A Systematic Review and Meta-Analysis" Cancers 16, no. 23: 4005. https://doi.org/10.3390/cancers16234005
APA StyleAnthon, C., Vidal, A., Recker, H., Piccand, E., Pape, J., Weidlinger, S., Kornmann, M., Karrer, T., & von Wolff, M. (2024). Long-Term Effects on Gonadal Function After Treatment of Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers, 16(23), 4005. https://doi.org/10.3390/cancers16234005







