Circulating Tumor DNA (ctDNA) Dynamics Predict Early Response to Treatment in Metastasized Gastroesophageal Cancer (mGEC) After 2 Weeks of Systemic Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
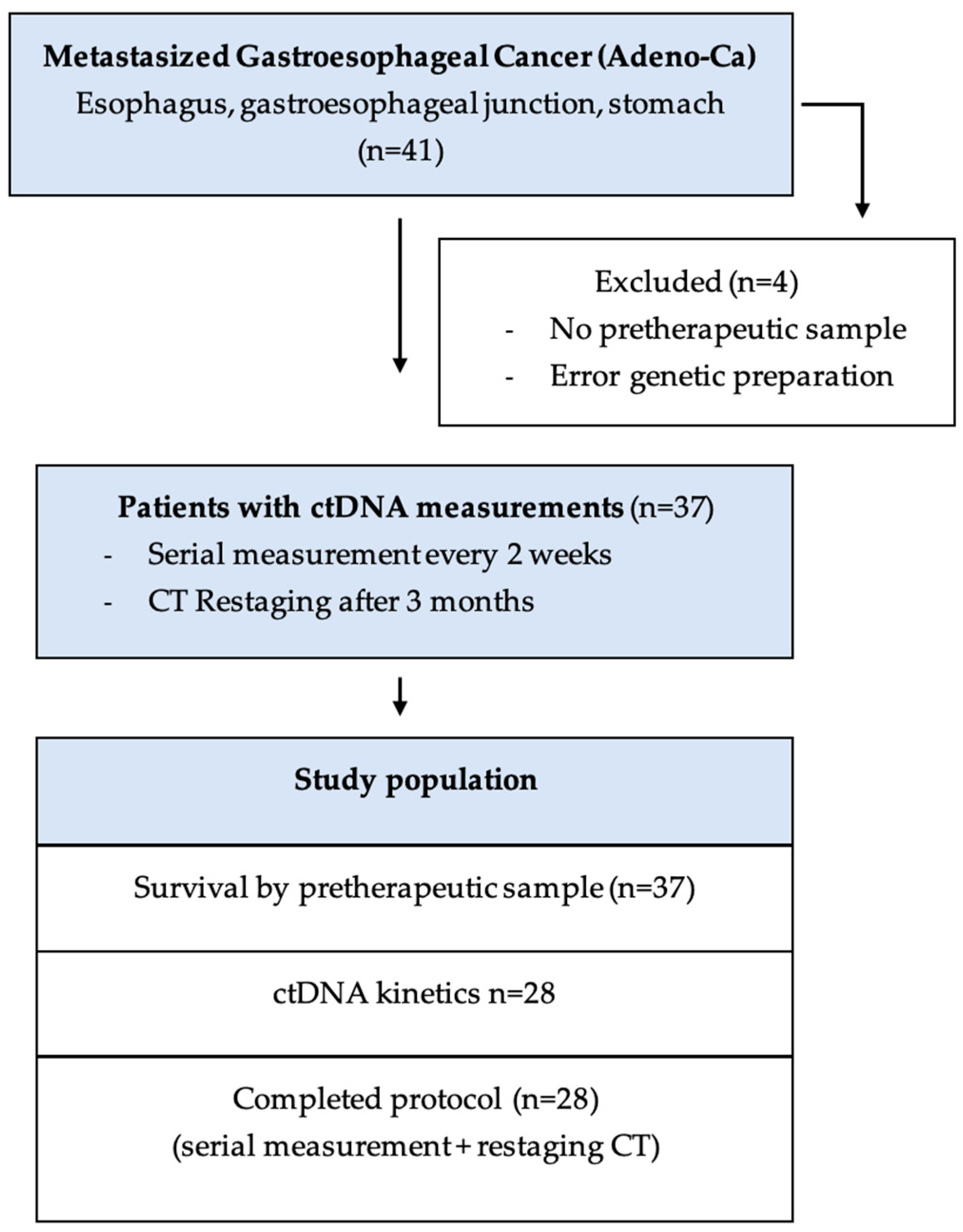
2.1. Patient Cohort
2.2. Sample Preparation
2.3. ctDNA Extraction
2.4. Individual Assay Design and Droplet Digital PCR (ddPCR)
2.5. Statistics
3. Results
3.1. Patient Characteristic
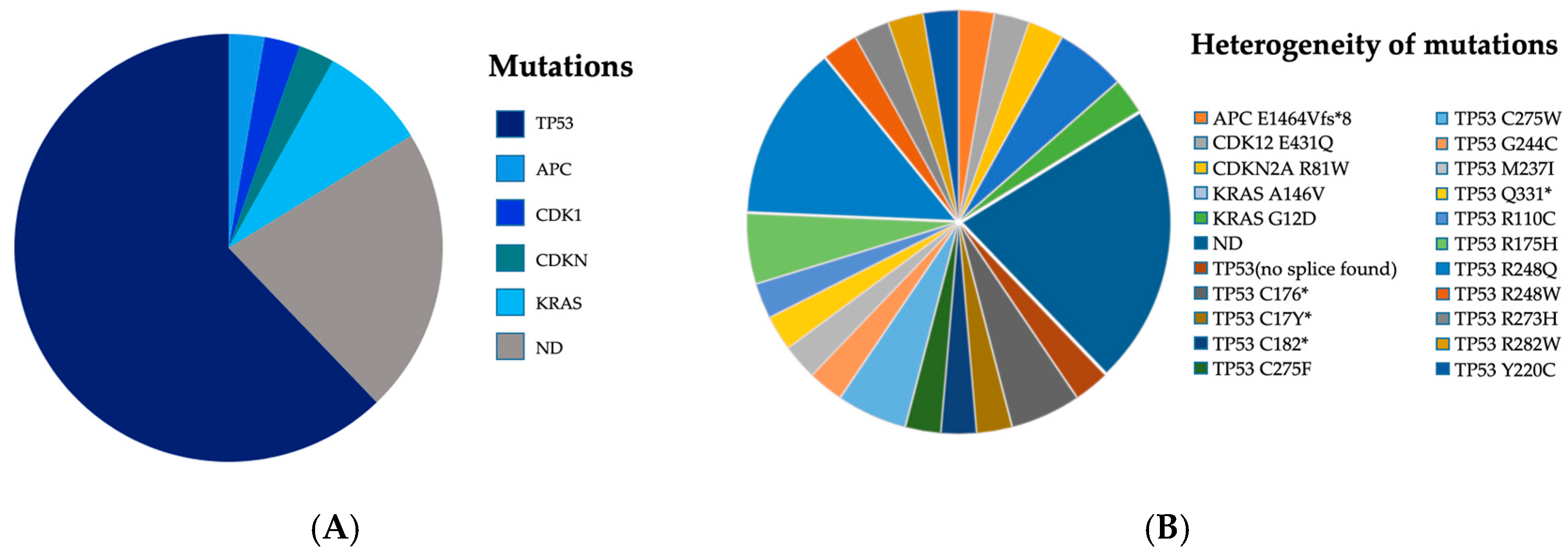
3.2. Mutational Pattern and Detection Rates
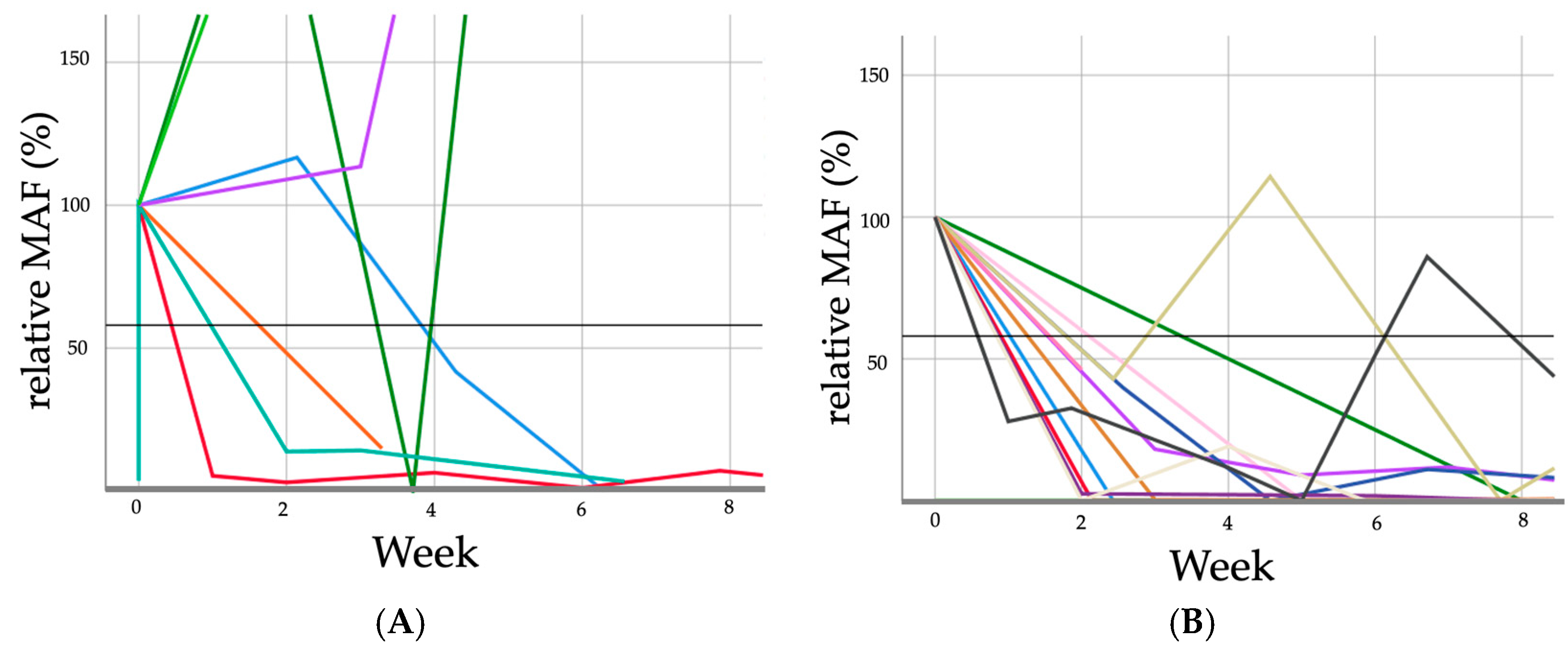
3.3. Response to Treatment Evaluation
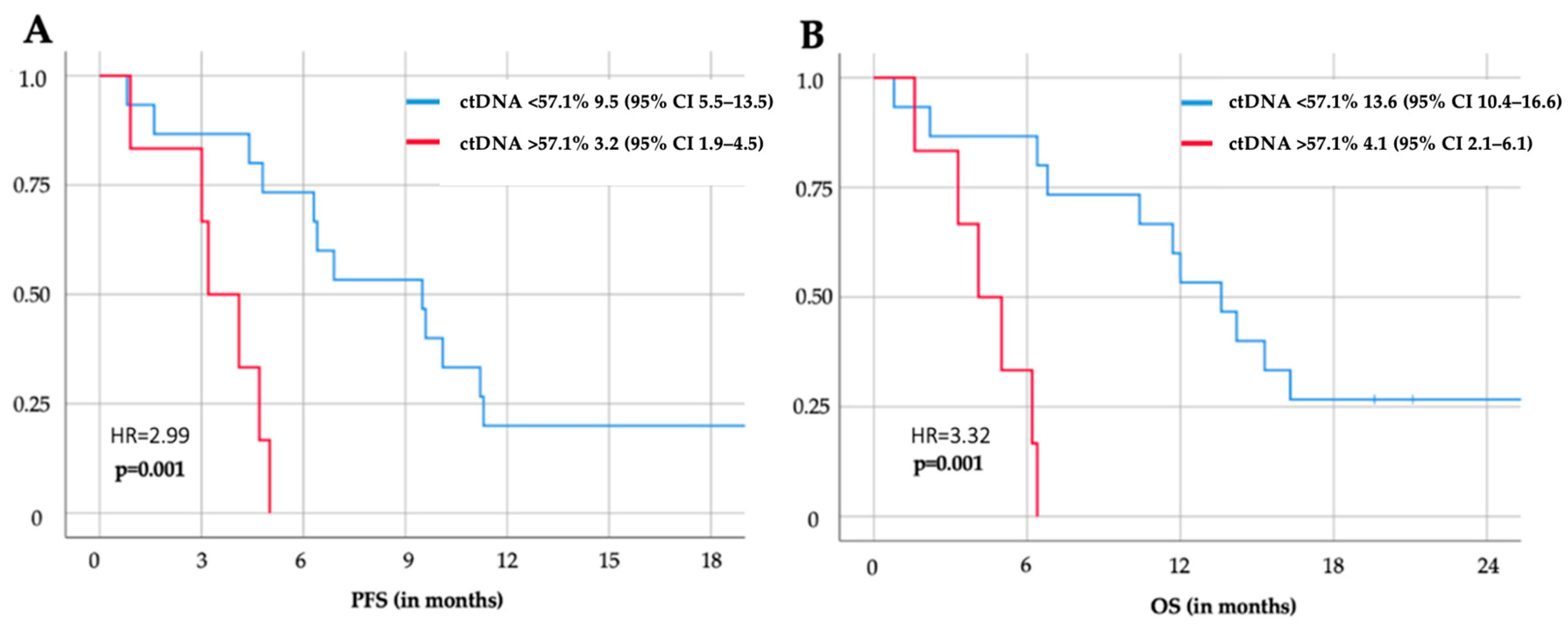
3.4. Survival Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal Cancer. Nat. Rev. Dis. Primer 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef]
- Hoeppner, J.; Kulemann, B. Circulating Tumor Cells in Esophageal Cancer. Oncol. Res. Treat. 2017, 40, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kroese, T.E.; Bronzwaer, S.; van Rossum, P.S.N.; Schoppman, S.F.; Deseyne, P.R.A.J.; van Cutsem, E.; Haustermans, K.; Nafteux, P.; Thomas, M.; Obermannova, R.; et al. European Clinical Practice Guidelines for the Definition, Diagnosis, and Treatment of Oligometastatic Esophagogastric Cancer (OMEC-4). Eur. J. Cancer 2024, 204, 114062. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Pauligk, C.; Homann, N.; Hartmann, J.T.; Moehler, M.; Probst, S.; Rethwisch, V.; Stoehlmacher-Williams, J.; Prasnikar, N.; Hollerbach, S.; et al. The Feasibility of Triple-Drug Chemotherapy Combination in Older Adult Patients with Oesophagogastric Cancer: A Randomised Trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur. J. Cancer 2013, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Relton, A.; Collins, A.; Guttery, D.S.; Gorsia, D.N.; McDermott, H.J.; Moss, E.L. Patient Acceptability of Circulating Tumour DNA Testing in Endometrial Cancer Follow-Up. Eur. J. Cancer Care 2021, 30, e13429. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a Cancer Biomarker: A Broad Overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Yuan, Z. Circulating Tumor Cells and Circulating Tumor DNA Provide New Insights into Pancreatic Cancer. Int. J. Med. Sci. 2016, 13, 902–913. [Google Scholar] [CrossRef]
- Tellez-Gabriel, M.; Knutsen, E.; Perander, M. Current Status of Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes in Breast Cancer Liquid Biopsies. Int. J. Mol. Sci. 2020, 21, 9457. [Google Scholar] [CrossRef]
- Payne, K.; Spruce, R.; Beggs, A.; Sharma, N.; Kong, A.; Martin, T.; Parmar, S.; Praveen, P.; Nankivell, P.; Mehanna, H. Circulating Tumor DNA as a Biomarker and Liquid Biopsy in Head and Neck Squamous Cell Carcinoma. Head Neck 2018, 40, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Jones, G.; Severgnini, M.; Alessi, J.V.; Recondo, G.; Lawrence, M.; Forshew, T.; Lydon, C.; Nishino, M.; Cheng, M.; et al. Early Plasma Circulating Tumor DNA (ctDNA) Changes Predict Response to First-Line Pembrolizumab-Based Therapy in Non-Small Cell Lung Cancer (NSCLC). J. Immunother. Cancer 2021, 9, e001504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Li, B.; Adashek, J.J.; Cha, S.W.; Bianchi-Frias, D.; Qian, D.; Kim, L.; So, T.W.; Mitchell, M.; Kamei, N.; et al. Serial Changes in Liquid Biopsy-Derived Variant Allele Frequency Predict Immune Checkpoint Inhibitor Responsiveness in the Pan-Cancer Setting. Oncoimmunology 2022, 11, 2052410. [Google Scholar] [CrossRef] [PubMed]
- Kirchweger, P.; Kupferthaler, A.; Burghofer, J.; Webersinke, G.; Jukic, E.; Schwendinger, S.; Weitzendorfer, M.; Petzer, A.; Függer, R.; Rumpold, H.; et al. Circulating Tumor DNA Correlates with Tumor Burden and Predicts Outcome in Pancreatic Cancer Irrespective of Tumor Stage. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2022, 48, 1046–1053. [Google Scholar] [CrossRef]
- Rodríguez-Casanova, A.; Bao-Caamano, A.; Lago-Lestón, R.M.; Brozos-Vázquez, E.; Costa-Fraga, N.; Ferreirós-Vidal, I.; Abdulkader, I.; Vidal-Insua, Y.; Rivera, F.V.; Candamio Folgar, S.; et al. Evaluation of a Targeted Next-Generation Sequencing Panel for the Non-Invasive Detection of Variants in Circulating DNA of Colorectal Cancer. J. Clin. Med. 2021, 10, 4487. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.; Kim, H.-S.; Na, K.; Lee, J.-Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Integrating a Next Generation Sequencing Panel into Clinical Practice in Ovarian Cancer. Yonsei Med. J. 2019, 60, 914–923. [Google Scholar] [CrossRef]
- Na, K.; Kim, H.-S.; Shim, H.S.; Chang, J.H.; Kang, S.-G.; Kim, S.H. Targeted Next-Generation Sequencing Panel (TruSight Tumor 170) in Diffuse Glioma: A Single Institutional Experience of 135 Cases. J. Neurooncol. 2019, 142, 445–454. [Google Scholar] [CrossRef]
- Hasenleithner, S.O.; Speicher, M.R. A Clinician’s Handbook for Using ctDNA throughout the Patient Journey. Mol. Cancer 2022, 21, 81. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; George, S.; Gelderblom, H.; Schöffski, P.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.-K.; Razak, A.A.; Trent, J.; et al. Ripretinib versus Sunitinib in Gastrointestinal Stromal Tumor: ctDNA Biomarker Analysis of the Phase 3 INTRIGUE Trial. Nat. Med. 2024, 30, 498–506. [Google Scholar] [CrossRef]
- Roberto, T.-M.; Jorge, M.-A.; Francisco, G.-V.; Noelia, T.; Pilar, R.-G.; Andrés, C. Strategies for Improving Detection of Circulating Tumor DNA Using next Generation Sequencing. Cancer Treat. Rev. 2023, 119, 102595. [Google Scholar] [CrossRef]
- Ococks, E.; Frankell, A.M.; Masque Soler, N.; Grehan, N.; Northrop, A.; Coles, H.; Redmond, A.M.; Devonshire, G.; Weaver, J.M.J.; Hughes, C.; et al. Longitudinal Tracking of 97 Esophageal Adenocarcinomas Using Liquid Biopsy Sampling. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.D.; Chaudhuri, A.A.; Fang, P.; Qiao, Y.; Esfahani, M.S.; Chabon, J.J.; Hamilton, E.G.; Yang, Y.D.; Lovejoy, A.; Newman, A.M.; et al. Circulating Tumor DNA Analysis for Detection of Minimal Residual Disease After Chemoradiotherapy for Localized Esophageal Cancer. Gastroenterology 2020, 158, 494–505.e6. [Google Scholar] [CrossRef] [PubMed]
- Postel, M.; Roosen, A.; Laurent-Puig, P.; Taly, V.; Wang-Renault, S.-F. Droplet-Based Digital PCR and next Generation Sequencing for Monitoring Circulating Tumor DNA: A Cancer Diagnostic Perspective. Expert Rev. Mol. Diagn. 2018, 18, 7–17. [Google Scholar] [CrossRef]
- Martin-Alonso, C.; Tabrizi, S.; Xiong, K.; Blewett, T.; Sridhar, S.; Crnjac, A.; Patel, S.; An, Z.; Bekdemir, A.; Shea, D.; et al. Priming Agents Transiently Reduce the Clearance of Cell-Free DNA to Improve Liquid Biopsies. Science 2024, 383, eadf2341. [Google Scholar] [CrossRef]
- Seelen, L.W.F.; Doppenberg, D.; Stoop, T.F.; Nagelhout, A.; Brada, L.J.H.; Bosscha, K.; Busch, O.R.; Cirkel, G.A.; den Dulk, M.; Daams, F.; et al. Minimum and Optimal CA19-9 Response After Two Months Induction Chemotherapy in Patients With Locally Advanced Pancreatic Cancer: A Nationwide Multicenter Study. Ann. Surg. 2024, 279, 832–841. [Google Scholar] [CrossRef]
- Moehler, M.; Maderer, A.; Thuss-Patience, P.C.; Brenner, B.; Meiler, J.; Ettrich, T.J.; Hofheinz, R.-D.; Al-Batran, S.E.; Vogel, A.; Mueller, L.; et al. Cisplatin and 5-Fluorouracil with or without Epidermal Growth Factor Receptor Inhibition Panitumumab for Patients with Non-Resectable, Advanced or Metastatic Oesophageal Squamous Cell Cancer: A Prospective, Open-Label, Randomised Phase III AIO/EORTC Trial (POWER). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 228–235. [Google Scholar] [CrossRef]
- Chau, I.; Ayers, D.; Goring, S.; Cope, S.; Korytowsky, B.; Abraham, P. Comparative Effectiveness of Nivolumab versus Clinical Practice for Advanced Gastric or Gastroesophageal Junction Cancer. J. Comp. Eff. Res. 2020, 9, 103–114. [Google Scholar] [CrossRef]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B.; et al. Pembrolizumab plus Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus Trastuzumab and Chemotherapy for HER2-Positive Gastric or Gastro-Oesophageal Junction Adenocarcinoma: Interim Analyses from the Phase 3 KEYNOTE-811 Randomised Placebo-Controlled Trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef]
- Topf, V.; Kheifetz, Y.; Daum, S.; Ballhausen, A.; Schwarzer, A.; Trung, K.V.; Stocker, G.; Aigner, A.; Lordick, F.; Scholz, M.; et al. Individual Hematotoxicity Prediction of Further Chemotherapy Cycles by Dynamic Mathematical Models in Patients with Gastrointestinal Tumors. J. Cancer Res. Clin. Oncol. 2023, 149, 6989–6998. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.-H.; Doi, T.; Moriwaki, T.; Kim, S.-B.; Lee, S.-H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Kato, K.; Kim, S.-B.; Ajani, J.A.; Zhao, K.; He, Z.; Yu, X.; Shu, Y.; Luo, Q.; Wang, J.; et al. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3065–3076. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.-Y.; Chin, K.; Kadowaki, S.; Ahn, M.-J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus Chemotherapy in Patients with Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Previous Chemotherapy (ATTRACTION-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Lee, B.; Lipton, L.; Cohen, J.; Tie, J.; Javed, A.A.; Li, L.; Goldstein, D.; Burge, M.; Cooray, P.; Nagrial, A.; et al. Circulating Tumor DNA as a Potential Marker of Adjuvant Chemotherapy Benefit Following Surgery for Localized Pancreatic Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1472–1478. [Google Scholar] [CrossRef]

| mGEC | Overall | ctDNA pos. | ctDNA neg. | p | |||
|---|---|---|---|---|---|---|---|
| n = 37 | n = 21 | n = 16 | |||||
| Age | 61 | (47–81) | 62 | (51–78) | 61 | (51–80) | 0.380 |
| Sex | 0.430 | ||||||
| Male | 32 | (78.05%) | 6 | (100) | 11 | (68.75) | |
| Female | 5 | (21.95%) | 0 | 4 | (31.25) | ||
| ECOG PS | 0.510 | ||||||
| 0 | 29 | (77.8%) | 16 | (76.2) | 13 | (81.3) | |
| 1 | 7 | (18.9) | 4 | (19.0) | 3 | (18.7) | |
| ≥2 | 1 | (3.3) | 1 | (4.8) | |||
| CTX Line | 0.869 | ||||||
| 1st Line | 23 | (62.2%) | 13 | (61.9) | 10 | (62.5) | |
| 2nd Line | 9 | (24.3%) | 6 | (28.6) | 3 | (18.8) | |
| ≥3rd Line | 5 | (13.5%) | 2 | (9.6) | 3 | (18.8) | |
| T-stadium | 0.497 | ||||||
| cTx | 14 | (37.8%) | 9 | (42.9) | 5 | (31.3) | |
| cT1 | |||||||
| cT2 | 3 | (8.1%) | 2 | (9.5) | 1 | (6.3) | |
| cT3 | 17 | (45.9%) | 8 | (38.1) | 9 | (56.3) | |
| cT4 | 3 | (8.1%) | 2 | (9.5) | 1 | (6.3) | |
| N-stadium | 0.871 | ||||||
| 0 | 5 | (13.5%) | 2 | (40) | 3 | (60) | |
| 1 | 15 | (40.5%) | 2 | (15.9) | 13 | (84.1) | |
| 2 | 4 | (10.8%) | 1 | (25) | 3 | (75) | |
| 3 | 2 | (5.4%) | 0 | (0) | 2 | (100) | |
| + | 2 | (24.4%) | 0 | (0) | 2 | (100) | |
| x | 9 | (5.4) | 2 | (22.2) | 7 | (77.8) | |
| Synchronous | 32 | (83.8%) | 18 | (85.7) | 13 | (81.3) | 0.724 |
| Metachronous | 6 | (16.2%) | 3 | (14.3) | 3 | (18.3) | 0.258 |
| (A) | |||
| Initial Relative MAF in % | Relative MAF After 2 Weeks in % | Relative MAF After 4 Weeks in % | Relative MAF After 6 Weeks in % |
| 100 | 113.0 | 346.9 | 1034.3 |
| 100 | 116.7 | 51.7 | 7.0 |
| 100 | 240 | 59.7 | 470 |
| 100 | 45 | 0.0 | 0 |
| 100 | 200 | 17 | 0 |
| 100 | 5.3 | 11.7 | 0.3 |
| 100 | 0 | 0 | 0 |
| 100 | 18.1 | 11.8 | 9.0 |
| (B) | |||
| Initial relative MAF in % | Relative MAF after 2 Weeks in % | Relative MAF after 4 Weeks in % | Relative MAF after 6 Weeks in % |
| 100 | 2.4 | 2.1 | 1.7 |
| 100 | 0 | 19.1 | 0 |
| 100 | 0 | 0 | 0.4 |
| 100 | 5.3 | 3.0 | 6.4 |
| 100 | 0 | 0 | 0 |
| 100 | 18.1 | 9.0 | 11.8 |
| 100 | 14.9 | 0 | 0 |
| 100 | 39.7 | 0 | 11.0 |
| 100 | 46.2 | 12.4 | 13.0 |
| 100 | 42.9 | 114.3 | 0 |
| 100 | 13.8 | 14.2 | 3.3 |
| 100 | 42.9 | 35.7 | 0 |
| 100 | 0.3 | 0.2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatalovic, S.; Doleschal, B.; Kupferthaler, A.; Grundner, S.; Burghofer, J.; Webersinke, G.; Schwendinger, S.; Jukic, E.; Zschocke, J.; Danhel, L.; et al. Circulating Tumor DNA (ctDNA) Dynamics Predict Early Response to Treatment in Metastasized Gastroesophageal Cancer (mGEC) After 2 Weeks of Systemic Treatment. Cancers 2024, 16, 3960. https://doi.org/10.3390/cancers16233960
Tatalovic S, Doleschal B, Kupferthaler A, Grundner S, Burghofer J, Webersinke G, Schwendinger S, Jukic E, Zschocke J, Danhel L, et al. Circulating Tumor DNA (ctDNA) Dynamics Predict Early Response to Treatment in Metastasized Gastroesophageal Cancer (mGEC) After 2 Weeks of Systemic Treatment. Cancers. 2024; 16(23):3960. https://doi.org/10.3390/cancers16233960
Chicago/Turabian StyleTatalovic, Stefan, Bernhard Doleschal, Alexander Kupferthaler, Stephan Grundner, Jonathan Burghofer, Gerald Webersinke, Simon Schwendinger, Emina Jukic, Johannes Zschocke, Lorenz Danhel, and et al. 2024. "Circulating Tumor DNA (ctDNA) Dynamics Predict Early Response to Treatment in Metastasized Gastroesophageal Cancer (mGEC) After 2 Weeks of Systemic Treatment" Cancers 16, no. 23: 3960. https://doi.org/10.3390/cancers16233960
APA StyleTatalovic, S., Doleschal, B., Kupferthaler, A., Grundner, S., Burghofer, J., Webersinke, G., Schwendinger, S., Jukic, E., Zschocke, J., Danhel, L., Kirchweger, A., Havranek, L., Shalamberidze, D., Rezaie, D., Biebl, M., Rumpold, H., & Kirchweger, P. (2024). Circulating Tumor DNA (ctDNA) Dynamics Predict Early Response to Treatment in Metastasized Gastroesophageal Cancer (mGEC) After 2 Weeks of Systemic Treatment. Cancers, 16(23), 3960. https://doi.org/10.3390/cancers16233960








