Machine Perfusion as a Strategy to Decrease Ischemia-Reperfusion Injury and Lower Cancer Recurrence Following Liver Transplantation
Simple Summary
Abstract
1. Introduction
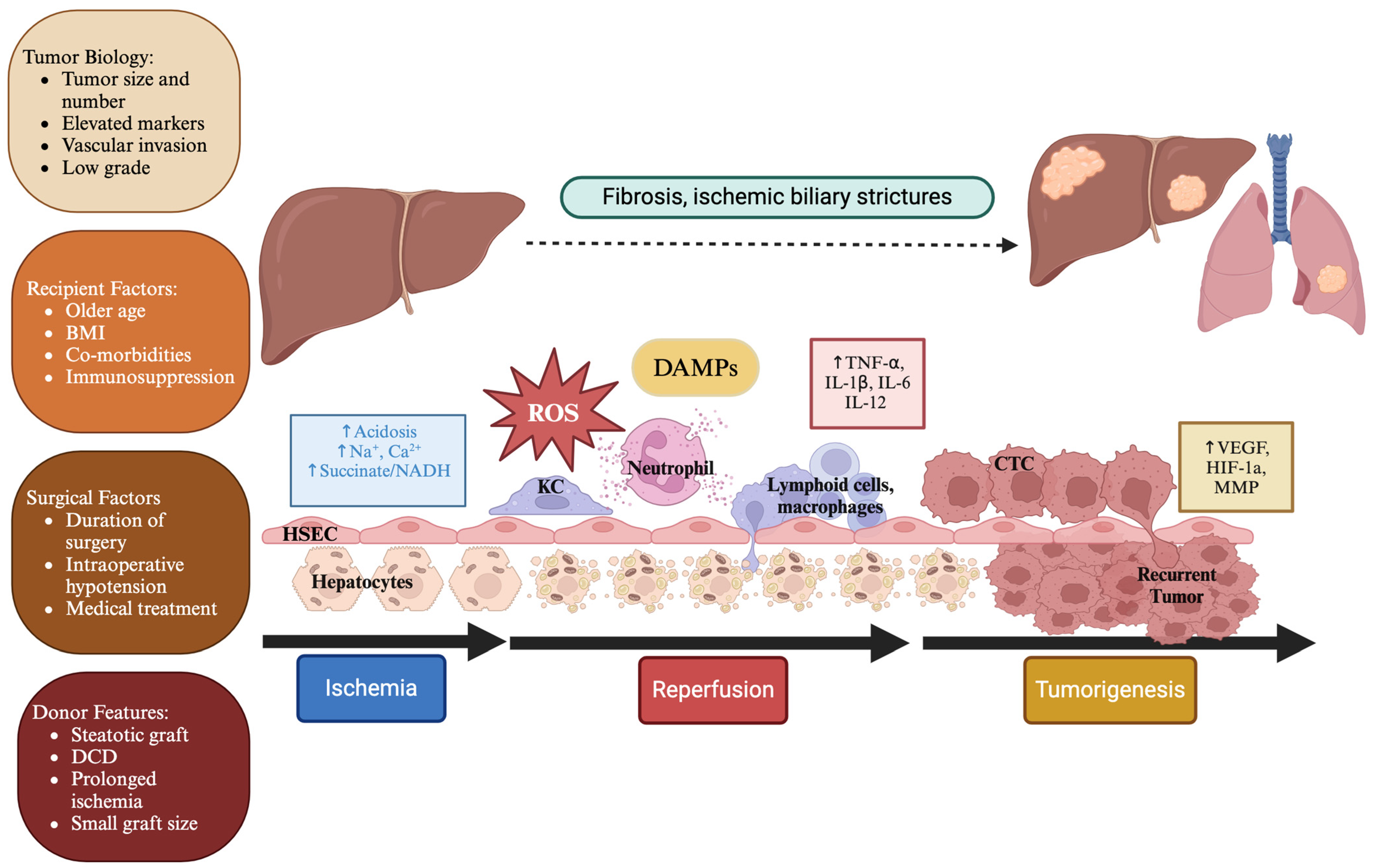
2. Mechanism of Hepatic Ischemia-Reperfusion Injury
3. The Link Between Ischemia-Reperfusion Injury and Cancer Recurrence
4. Risk Factors for Elevated Ischemia-Reperfusion Injury and Cancer Recurrence Following Liver Transplantation
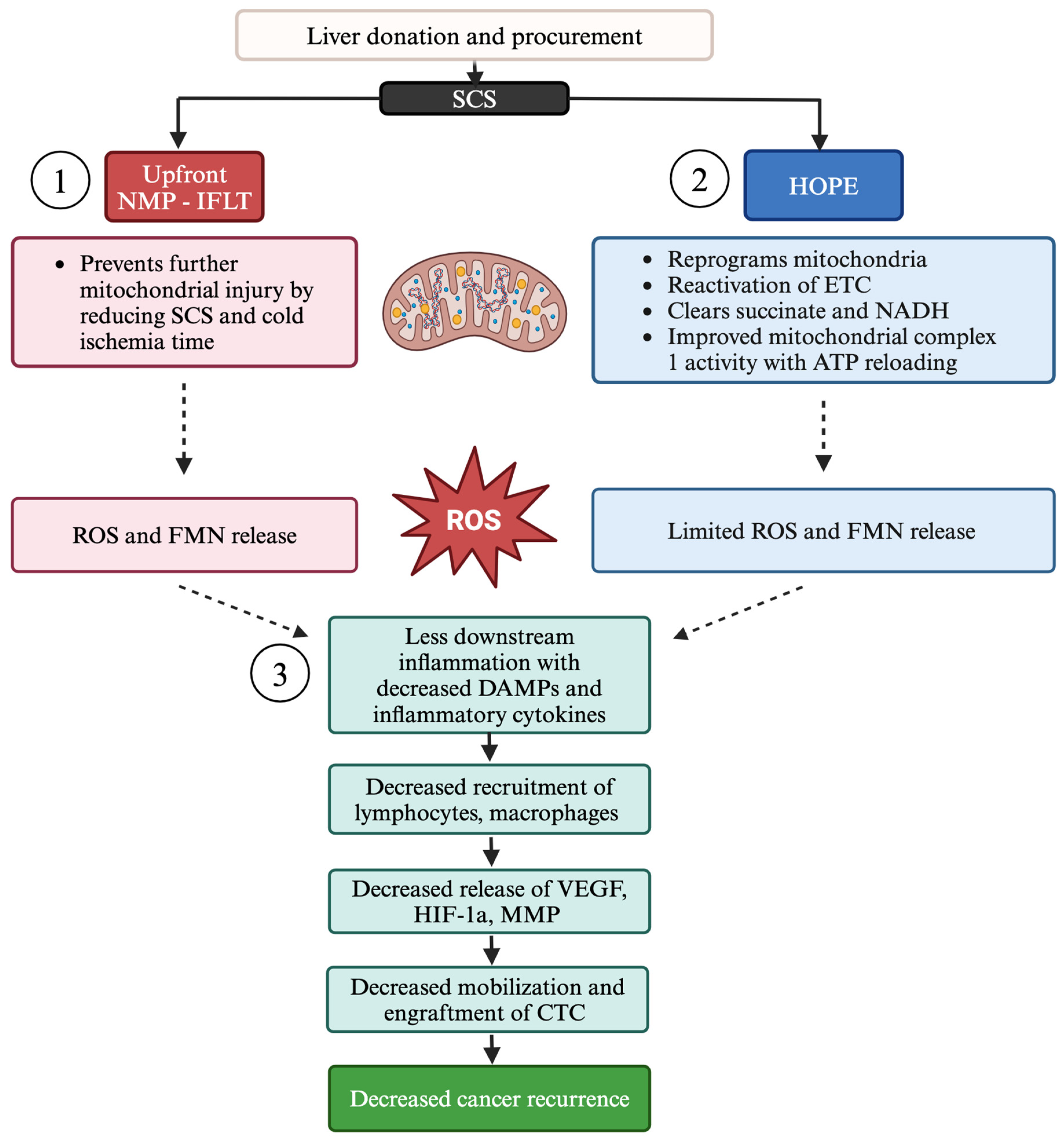
5. Mitigation of Tumor Recurrence Through Machine Perfusion
5.1. Hypothermic Oxygenated Perfusion
5.2. Normothermic Machine Perfusion Techniques
6. Other Strategies to Reduce IRI and Mitigate Cancer Recurrence
6.1. Pharmacological Agents
6.1.1. Anti-Inflammatory Agents
6.1.2. Adjuvant Systemic Therapy
6.1.3. Immunosuppression
6.1.4. Immunotherapy
6.2. Other Surgical Interventions
7. Summary and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzaferro, V.; Toso, C.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Maspero, M.; Satish, S.; Eghtesad, B.; Pita, A.; Kim, J.; Khalil, M.; Calderon, E.; Orabi, D.; et al. Risk assessment in liver transplantation for hepatocellular carcinoma: Long-term follow-up of a two-centre experience. Int. J. Surg. 2024, 110, 2818–2831. [Google Scholar] [CrossRef]
- Facciuto, M.E.; Singh, M.K.; Katta, U.; Samaniego, S.; Sharma, J.; Rodriguez-Davalos, M.; Sheiner, P.; Kim-Schluger, L.; Wolf, D.C. Liver transplantation for hepatocellular carcinoma: Defining the impact of using extended criteria liver allografts. Transplantation 2011, 92, 446–452. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Gorgen, A.; Roayaie, S.; Droz Dit Busset, M.; Sapisochin, G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 364–377. [Google Scholar] [CrossRef]
- Rossi, R.E.; Burroughs, A.K.; Caplin, M.E. Liver transplantation for unresectable neuroendocrine tumor liver metastases. Ann. Surg. Oncol. 2014, 21, 2398–2405. [Google Scholar] [CrossRef]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjornbeth, B.A.; Hagness, M.; Line, P.D. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Solheim, J.M.; Dueland, S.; Line, P.D.; Hagness, M. Transplantation for Nonresectable Colorectal Liver Metastases: Long-Term Follow-Up of the First Prospective Pilot Study. Ann. Surg. 2023, 278, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Burra, P.; Ghobrial, M.; Hibi, T.; Metselaar, H.; Sapisochin, G.; Bhoori, S.; Kwan Man, N.; Mas, V.; Ohira, M.; et al. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1143–1149. [Google Scholar] [CrossRef]
- Aggarwal, A.; Te, H.S.; Verna, E.C.; Desai, A.P. A National Survey of Hepatocellular Carcinoma Surveillance Practices Following Liver Transplantation. Transplant. Direct 2021, 7, e638. [Google Scholar] [CrossRef]
- Najjar, M.; Agrawal, S.; Emond, J.C.; Halazun, K.J. Pretreatment neutrophil-lymphocyte ratio: Useful prognostic biomarker in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2018, 5, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, N.A. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transpl. 2018, 24, 1470–1475. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Kusakabe, J.; Akabane, M.; Maspero, M.; Zervos, B.; Modaresi Esfeh, J.; Whitsett Linganna, M.; Imaoka, Y.; Khalil, M.; Pita, A.; et al. Expanding Selection Criteria in Deceased Donor Liver Transplantation for Hepatocellular Carcinoma: Long-term Follow-up of a National Registry and 2 Transplant Centers. Transplantation 2024, 108, 10-1097. [Google Scholar] [CrossRef]
- Mathur, A.; Franco, E.S.; Leone, J.P.; Osman-Mohamed, H.; Rojas, H.; Kemmer, N.; Neff, G.W.; Rosemurgy, A.S.; Alsina, A.E. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB 2013, 15, 504–510. [Google Scholar] [CrossRef]
- Robinson, S.M.; Wilson, C.H.; Burt, A.D.; Manas, D.M.; White, S.A. Chemotherapy-associated liver injury in patients with colorectal liver metastases: A systematic review and meta-analysis. Ann. Surg. Oncol. 2012, 19, 4287–4299. [Google Scholar] [CrossRef]
- Vivarelli, M.; Cucchetti, A.; Piscaglia, F.; La Barba, G.; Bolondi, L.; Cavallari, A.; Pinna, A.D. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: Key role of immunosuppression. Liver Transpl. 2005, 11, 497–503. [Google Scholar] [CrossRef]
- Maspero, M.; Yilmaz, S.; Cazzaniga, B.; Raj, R.; Ali, K.; Mazzaferro, V.; Schlegel, A. The role of ischaemia-reperfusion injury and liver regeneration in hepatic tumour recurrence. JHEP Rep. 2023, 5, 100846. [Google Scholar] [CrossRef]
- Man, K.; Ng, K.T.; Lo, C.M.; Ho, J.W.; Sun, B.S.; Sun, C.K.; Lee, T.K.; Poon, R.T.; Fan, S.T. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases--activation of cell invasion and migration pathways. Liver Transpl. 2007, 13, 1669–1677. [Google Scholar] [CrossRef]
- Ogawa, T.; Tashiro, H.; Miyata, Y.; Ushitora, Y.; Fudaba, Y.; Kobayashi, T.; Arihiro, K.; Okajima, M.; Asahara, T. Rho-associated kinase inhibitor reduces tumor recurrence after liver transplantation in a rat hepatoma model. Am. J. Transplant. 2007, 7, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; De Stefano, N.; Patrono, D.; De Donato, V.; Campi, L.; Turturica, D.; Doria, T.; Sciannameo, V.; Berchialla, P.; Tandoi, F.; et al. Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation. J. Pers. Med. 2023, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Roggio, D.; Mazzeo, A.T.; Catalano, G.; Mazza, E.; Rizza, G.; Gambella, A.; Rigo, F.; Leone, N.; Elia, V.; et al. Clinical assessment of liver metabolism during hypothermic oxygenated machine perfusion using microdialysis. Artif. Organs 2022, 46, 281–295. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- Patrono, D.; Colli, F.; Colangelo, M.; De Stefano, N.; Apostu, A.L.; Mazza, E.; Catalano, S.; Rizza, G.; Mirabella, S.; Romagnoli, R. How Can Machine Perfusion Change the Paradigm of Liver Transplantation for Patients with Perihilar Cholangiocarcinoma? J. Clin. Med. 2023, 12, 2026. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Hong, H.; Gross, A.; Liu, Q.; Ali, K.; Cazzaniga, B.; Miyazaki, Y.; Tuul, M.; Modaresi Esfeh, J.; Khalil, M.; et al. The impact of normothermic machine perfusion and acuity circles on waitlist time, mortality, and cost in liver transplantation: A multicenter experience. Liver Transpl. 2024, 30, 10-1097. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Zhang, M.; Khalil, M.; Pita, A.; Modaresi Esfeh, J.; Diago-Uso, T.; Kim, J.; Aucejo, F.; Kwon, D.C.H.; Ali, K.; et al. Impact of Back-to-Base Normothermic Machine Perfusion on Complications and Costs: A Multicenter, Real-World Risk-Matched Analysis. Ann. Surg. 2024, 280, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.; Bruggenwirth, I.M.A.; Berlakovich, G.; Buchholz, B.M.; Botea, F.; Camagni, S.; Cescon, M.; Cillo, U.; Colli, F.; Compagnon, P.; et al. Long-term outcomes after hypothermic oxygenated machine perfusion and transplantation of 1,202 donor livers in a real-world setting (HOPE-REAL study). J. Hepatol. 2024, in press. [CrossRef]
- Parente, A.; Tirotta, F.; Pini, A.; Eden, J.; Dondossola, D.; Manzia, T.M.; Dutkowski, P.; Schlegel, A. Machine perfusion techniques for liver transplantation—A meta-analysis of the first seven randomized-controlled trials. J. Hepatol. 2023, 79, 1201–1213. [Google Scholar] [CrossRef]
- Wehrle, C.J.; de Goeij, F.H.C.; Zhang, M.; Abbassi, F.; Satish, S.; Jiao, C.; Sun, K.; Pinna, A.D.; Hashimoto, K.; Miller, C.; et al. Core outcome sets and benchmarking complications: Defining best practices for standardized outcome reporting in liver transplantation. Liver Transpl. 2024, 30, 10-1097. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Miller, C.; Hashimoto, K.; Schlegel, A. Standardization is needed in reporting risk and outcomes of machine perfusion in liver transplantation. Hepatobiliary Surg. Nutr. 2024, 13, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Otarashvili, G.; Meszaros, A.; Ebner, S.; Weissenbacher, A.; Cardini, B.; Oberhuber, R.; Resch, T.; Ofner, D.; Schneeberger, S.; et al. Restoring Mitochondrial Function While Avoiding Redox Stress: The Key to Preventing Ischemia/Reperfusion Injury in Machine Perfused Liver Grafts? Int. J. Mol. Sci. 2020, 21, 3132. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, T.; Ju, W.; Li, F.; Zhang, Q.; Chen, Z.; Gong, J.; Zhao, Q.; Wang, D.; Chen, M.; et al. Ischemic-Free Liver Transplantation Reduces the Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Front. Oncol. 2021, 11, 773535. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramirez, A.; Mosbah, I.B.; Ramalho, F.; Rosello-Catafau, J.; Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006, 79, 1881–1894. [Google Scholar] [CrossRef]
- Fan, C.; Zwacka, R.M.; Engelhardt, J.F. Therapeutic approaches for ischemia/reperfusion injury in the liver. J. Mol. Med. 1999, 77, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Zwacka, R.M.; Zhou, W.; Zhang, Y.; Darby, C.J.; Dudus, L.; Halldorson, J.; Oberley, L.; Engelhardt, J.F. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kappaB activation. Nat. Med. 1998, 4, 698–704. [Google Scholar] [CrossRef]
- Selzner, M.; Selzner, N.; Jochum, W.; Graf, R.; Clavien, P.A. Increased ischemic injury in old mouse liver: An ATP-dependent mechanism. Liver Transpl. 2007, 13, 382–390. [Google Scholar] [CrossRef]
- Wang, D.; Dou, K.; Song, Z.; Liu, Z. The Na+/H+ exchange inhibitor: A new therapeutic approach for hepatic ischemia injury in rats. Transplant. Proc. 2003, 35, 3134–3135. [Google Scholar] [CrossRef]
- Carini, R.; De Cesaris, M.G.; Splendore, R.; Bagnati, M.; Bellomo, G.; Albano, E. Alterations of Na(+) homeostasis in hepatocyte reoxygenation injury. Biochim. Biophys. Acta 2000, 1500, 297–305. [Google Scholar] [CrossRef]
- Gores, G.J.; Nieminen, A.L.; Wray, B.E.; Herman, B.; Lemasters, J.J. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J. Clin. Invest. 1989, 83, 386–396. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Z.M.; Liu, L.; Zhang, C.; Zhang, Y.L.; Zhang, Z.C. Effects of Ca2+ channel blockers on store-operated Ca2+ channel currents of Kupffer cells after hepatic ischemia/reperfusion injury in rats. World J. Gastroenterol. 2006, 12, 4694–4698. [Google Scholar] [CrossRef]
- Barritt, G.J.; Chen, J.; Rychkov, G.Y. Ca(2+) -permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology. Biochim. Biophys. Acta 2008, 1783, 651–672. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Panconesi, R.; Widmer, J.; Carvalho, M.F.; Eden, J.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Mitochondria and ischemia reperfusion injury. Curr. Opin. Organ. Transplant. 2022, 27, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, G.M.; Wu, D.; Soong, Y.; Birk, A.V.; Schiller, P.W.; Szeto, H.H. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 2004, 279, 34682–34690. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Serviddio, G.; Pereda, J.; Minana, J.B.; Arduini, A.; Vendemiale, G.; Poli, G.; Pallardo, F.V.; Vina, J. Mitochondrial function in liver disease. Front. Biosci. 2007, 12, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; Murphy, M.P.; Frezza, C.; Prag, H.A.; Chouchani, E.T.; O’Neill, L.A.; Mills, E.L. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 2019, 1, 16–33. [Google Scholar] [CrossRef]
- Vairetti, M.; Richelmi, P.; Berte, F.; Currin, R.T.; Lemasters, J.J.; Imberti, R. Role of pH in protection by low sodium against hypoxic injury in isolated perfused rat livers. J. Hepatol. 2006, 44, 894–901. [Google Scholar] [CrossRef]
- Wang, H.G.; Pathan, N.; Ethell, I.M.; Krajewski, S.; Yamaguchi, Y.; Shibasaki, F.; McKeon, F.; Bobo, T.; Franke, T.F.; Reed, J.C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999, 284, 339–343. [Google Scholar] [CrossRef]
- Anderson, C.D.; Pierce, J.; Nicoud, I.; Belous, A.; Knox, C.D.; Chari, R.S. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury. Liver Transpl. 2005, 11, 663–668. [Google Scholar] [CrossRef]
- Vogel, T.; Brockmann, J.G.; Quaglia, A.; Morovat, A.; Jassem, W.; Heaton, N.D.; Coussios, C.C.; Friend, P.J. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transpl. 2017, 23, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Bautista, A.P.; Spolarics, Z.; Spitzer, J.J. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J. Leukoc. Biol. 1992, 52, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Moldawer, L.L.; Shires, G.T.; Lowry, S.F. The biologic characteristics of cytokines and their implication in surgical injury. Surg. Gynecol. Obstet. 1990, 170, 363–378. [Google Scholar] [PubMed]
- Leifeld, L.; Cheng, S.; Ramakers, J.; Dumoulin, F.L.; Trautwein, C.; Sauerbruch, T.; Spengler, U. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology 2002, 36, 1001–1008. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Yoshidome, H.; Kato, A.; Warner, R.L.; Cheadle, W.G.; Ward, P.A.; Edwards, M.J. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology 1999, 30, 1448–1453. [Google Scholar] [CrossRef]
- Husted, T.L.; Blanchard, J.; Schuster, R.; Shen, H.; Lentsch, A.B. Potential role for IL-23 in hepatic ischemia/reperfusion injury. Inflamm. Res. 2006, 55, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Flores Carvalho, M.; Schlegel, A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 10091. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), 173–179. [Google Scholar] [CrossRef]
- Kawada, N.; Tran-Thi, T.A.; Klein, H.; Decker, K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur. J. Biochem. 1993, 213, 815–823. [Google Scholar] [CrossRef]
- Kawamura, E.; Yamanaka, N.; Okamoto, E.; Tomoda, F.; Furukawa, K. Response of plasma and tissue endothelin-1 to liver ischemia and its implication in ischemia-reperfusion injury. Hepatology 1995, 21, 1138–1143. [Google Scholar] [CrossRef]
- Lefer, A.M.; Lefer, D.J. Nitric oxide. II. Nitric oxide protects in intestinal inflammation. Am. J. Physiol. 1999, 276, G572–G575. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, Y.; Kiriyama, H.; Fukushi, Y.; Nagura, T.; Takada, H.; Hai, K.; Kamii, K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig. Dis. Sci. 1994, 39, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Wahid, B.; Ali, A.; Rafique, S.; Saleem, K.; Waqar, M.; Wasim, M.; Idrees, M. Role of altered immune cells in liver diseases: A review. Gastroenterol. Hepatol. 2018, 41, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, V.; Vetrugno, L.; Zanini, V.; Pravisani, R.; Ventin, M.; Lorenzin, D.; Adani, G.L.; Clocchiatti, L.; Boscolo, E.; Vit, A.; et al. Association between indocyanine green clearance test and ischemic type biliary lesions within one year after orthotopic liver transplantation. Gastroenterol. Hepatol. 2021, 44, 687–695. [Google Scholar] [CrossRef]
- Guo, Z.; Luo, T.; Mo, R.; Zhao, Q.; He, X. Ischemia-free organ transplantation—A review. Curr. Opin. Organ. Transplant. 2022, 27, 300–304. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, X.D.; Gao, F.; Zhao, A.; Freitas, M.C.; Lassman, C.; Luster, A.D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology 2008, 47, 207–214. [Google Scholar] [CrossRef]
- Orci, L.A.; Berney, T.; Majno, P.E.; Lacotte, S.; Oldani, G.; Morel, P.; Mentha, G.; Toso, C. Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation. Br. J. Surg. 2015, 102, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Shih, K.C.; Ng, K.T.; Xiao, J.W.; Guo, D.Y.; Sun, C.K.; Lim, Z.X.; Cheng, Q.; Liu, Y.; Fan, S.T.; et al. Molecular signature linked to acute phase injury and tumor invasiveness in small-for-size liver grafts. Ann. Surg. 2010, 251, 1154–1161. [Google Scholar] [CrossRef]
- Lim, C.; Broqueres-You, D.; Brouland, J.P.; Merkulova-Rainon, T.; Faussat, A.M.; Hilal, R.; Rouquie, D.; Eveno, C.; Audollent, R.; Levy, B.I.; et al. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J. Surg. Res. 2013, 184, 888–897. [Google Scholar] [CrossRef]
- Ling, C.C.; Ng, K.T.; Shao, Y.; Geng, W.; Xiao, J.W.; Liu, H.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Yeung, W.H.; et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J. Hepatol. 2014, 60, 103–109. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Gu, X.; Zheng, Q.; Zhu, H. Regulatory mechanism of HIF-1alpha and its role in liver diseases: A narrative review. Ann. Transl. Med. 2022, 10, 109. [Google Scholar] [CrossRef]
- Graeber, T.G.; Osmanian, C.; Jacks, T.; Housman, D.E.; Koch, C.J.; Lowe, S.W.; Giaccia, A.J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996, 379, 88–91. [Google Scholar] [CrossRef]
- Zheng, S.S.; Chen, X.H.; Yin, X.; Zhang, B.H. Prognostic significance of HIF-1alpha expression in hepatocellular carcinoma: A meta-analysis. PLoS ONE 2013, 8, e65753. [Google Scholar] [CrossRef]
- Shimomura, M.; Hinoi, T.; Kuroda, S.; Adachi, T.; Kawaguchi, Y.; Sasada, T.; Takakura, Y.; Egi, H.; Okajima, M.; Tashiro, H.; et al. Overexpression of hypoxia inducible factor-1 alpha is an independent risk factor for recurrence after curative resection of colorectal liver metastases. Ann. Surg. Oncol. 2013, 20 (Suppl. 3), S527–S536. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Tejado, M.; Naranjo-Suarez, S.; Jimenez, C.; Carrera, A.C.; Landazuri, M.O.; del Peso, L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: Protective role in apoptosis. J. Biol. Chem. 2001, 276, 22368–22374. [Google Scholar] [CrossRef] [PubMed]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Demaria, M.; Giorgi, C.; Lebiedzinska, M.; Esposito, G.; D’Angeli, L.; Bartoli, A.; Gough, D.J.; Turkson, J.; Levy, D.E.; Watson, C.J.; et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging 2010, 2, 823–842. [Google Scholar] [CrossRef]
- Panconesi, R.; Carvalho, M.F.; Muiesan, P.; Dutkowski, P.; Schlegel, A. Liver perfusion strategies: What is best and do ischemia times still matter? Curr. Opin. Organ. Transplant. 2022, 27, 285–299. [Google Scholar] [CrossRef]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig. Dis. Sci. 2015, 60, 2832–2839. [Google Scholar] [CrossRef]
- Nagai, S.; Yoshida, A.; Facciuto, M.; Moonka, D.; Abouljoud, M.S.; Schwartz, M.E.; Florman, S.S. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology 2015, 61, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.A.; Saeb-Parsy, K.; Pettigrew, G. Liver Transplantation from Donation After Circulatory Death Donors in Patients with Hepatocellular Carcinoma Results in Good Outcomes. Int. J. Surg. 2013, 11, 706–707. [Google Scholar] [CrossRef][Green Version]
- Teng, D.H.; Zhu, Z.J.; Zheng, H.; Deng, Y.L.; Sun, L.Y.; Pan, C.; Liu, Y.H.; Song, H.L.; Shen, Z.Y. Effect of steatosis donor liver transplantation on hepatocellular carcinoma recurrence: Experience at a single institution. Hepatogastroenterology 2012, 59, 858–862. [Google Scholar] [PubMed]
- Zhou, J.; Huang, Z.; Chen, Z.; Xu, F.; Tong, R.; Zheng, S. Impact of donor age on liver transplant outcomes in patients with hepatocellular carcinoma: Analysis of the SRTR database. BMC Gastroenterol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Cusumano, C.; De Carlis, L.; Centonze, L.; Lesourd, R.; Levi Sandri, G.B.; Lauterio, A.; De Carlis, R.; Ferla, F.; Di Sandro, S.; Camus, C.; et al. Advanced donor age does not increase risk of hepatocellular carcinoma recurrence after liver transplantation: A retrospective two-centre analysis using competing risk analysis. Transpl. Int. 2021, 34, 1948–1958. [Google Scholar] [CrossRef]
- Lee, E.C.; Kim, S.H.; Shim, J.R.; Park, S.J. Small-for-size grafts increase recurrence of hepatocellular carcinoma in liver transplantation beyond milan criteria. Liver Transpl. 2018, 24, 35–43. [Google Scholar] [CrossRef]
- Kim, D.G.; Hwang, S.; Lee, K.W.; Kim, J.M.; You, Y.K.; Choi, D.; Ryu, J.H.; Kim, B.W.; Kim, D.S.; Cho, J.Y.; et al. Small graft size and hepatocellular carcinoma outcomes in living donor liver transplantation: A retrospective multicentric cohort study. Int. J. Surg. 2024, 110, 10-1097. [Google Scholar] [CrossRef]
- Adeniji, N.; Arjunan, V.; Prabhakar, V.; Mannalithara, A.; Ghaziani, T.; Ahmed, A.; Kwo, P.; Nguyen, M.; Melcher, M.L.; Busuttil, R.W.; et al. Posttransplant Outcomes in Older Patients With Hepatocellular Carcinoma Are Driven by Non-Hepatocellular Carcinoma Factors. Liver Transpl. 2021, 27, 684–698. [Google Scholar] [CrossRef]
- Siegel, A.B.; Lim, E.A.; Wang, S.; Brubaker, W.; Rodriguez, R.D.; Goyal, A.; Jacobson, J.S.; Hershman, D.L.; Verna, E.C.; Zaretsky, J.; et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation 2012, 94, 539–543. [Google Scholar] [CrossRef]
- Wu, J.C.; Huang, Y.H.; Chau, G.Y.; Su, C.W.; Lai, C.R.; Lee, P.C.; Huo, T.I.; Sheen, I.J.; Lee, S.D.; Lui, W.Y. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J. Hepatol. 2009, 51, 890–897. [Google Scholar] [CrossRef]
- Nutu, A.; Justo, I.; Marcacuzco, A.; Caso, O.; Manrique, A.; Calvo, J.; Garcia-Sesma, A.; Garcia-Conde, M.; Gallego, M.S.; Jimenez-Romero, C. Liver transplantation for hepatocellular carcinoma using grafts from uncontrolled circulatory death donation. Sci. Rep. 2021, 11, 13520. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Mergental, H.; Fondevila, C.; Porte, R.J.; Friend, P.J.; Dutkowski, P. Machine perfusion of the liver and bioengineering. J. Hepatol. 2023, 78, 1181–1198. [Google Scholar] [CrossRef]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am. J. Transplant. 2022, 22, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Mueller, M.; Muller, X.; Eden, J.; Panconesi, R.; von Felten, S.; Steigmiller, K.; Sousa Da Silva, R.X.; de Rougemont, O.; Mabrut, J.Y.; et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J. Hepatol. 2023, 78, 783–793. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Kalisvaart, M.; O’Rourke, J.; Shetty, S.; Parente, A.; Muller, X.; Isaac, J.; Muellhaupt, B.; Muiesan, P.; Shah, T.; et al. Hypothermic Oxygenated Liver Perfusion (HOPE) Prevents Tumor Recurrence in Liver Transplantation From Donation After Circulatory Death. Ann. Surg. 2020, 272, 759–765. [Google Scholar] [CrossRef]
- Dajti, G.; Germinario, G.; Prosperi, E.; Siniscalchi, A.; Vasuri, F.; Valente, S.; Odaldi, F.; Maroni, L.; Serenari, M.; Bertuzzo, V.; et al. The role of cold ischemia time and hypothermic perfusion in predicting early hepatocellular carcinoma recurrences after liver transplantation. Artif. Organs 2024, 48, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Xie, H.Y.; Li, J.H.; He, Y.; Jiang, L.; He, N.; Zhou, L.; Wang, W.; Zheng, S.S. Graft protection of the liver by hypothermic machine perfusion involves recovery of graft regeneration in rats. J. Int. Med. Res. 2019, 47, 427–437. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef]
- Rossignol, G.; Muller, X.; Ruiz, M.; Collardeau-Frachon, S.; Boulanger, N.; Depaulis, C.; Antonini, T.; Dubois, R.; Mohkam, K.; Mabrut, J.Y. HOPE Mitigates Ischemia-Reperfusion Injury in Ex-Situ Split Grafts: A Comparative Study With Living Donation in Pediatric Liver Transplantation. Transpl. Int. 2024, 37, 12686. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, Q.; Jia, Z.; Huang, C.; Wang, D.; Ju, W.; Zhang, J.; Yang, L.; Huang, S.; Chen, M.; et al. A randomized-controlled trial of ischemia-free liver transplantation for end-stage liver disease. J. Hepatol. 2023, 79, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; Garcia-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef]
- Chapman, W.C.; Barbas, A.S.; D’Alessandro, A.M.; Vianna, R.; Kubal, C.A.; Abt, P.; Sonnenday, C.; Barth, R.; Alvarez-Casas, J.; Yersiz, H.; et al. Normothermic Machine Perfusion of Donor Livers for Transplantation in the United States: A Randomized Controlled Trial. Ann. Surg. 2023, 278, e912–e921. [Google Scholar] [CrossRef]
- Meszaros, A.T.; Hofmann, J.; Buch, M.L.; Cardini, B.; Dunzendorfer-Matt, T.; Nardin, F.; Blumer, M.J.; Fodor, M.; Hermann, M.; Zelger, B.; et al. Mitochondrial respiration during normothermic liver machine perfusion predicts clinical outcome. EBioMedicine 2022, 85, 104311. [Google Scholar] [CrossRef]
- Jassem, W.; Xystrakis, E.; Ghnewa, Y.G.; Yuksel, M.; Pop, O.; Martinez-Llordella, M.; Jabri, Y.; Huang, X.; Lozano, J.J.; Quaglia, A.; et al. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatology 2019, 70, 682–695. [Google Scholar] [CrossRef]
- Jia, D.; Guo, S.; Jia, Z.; Gao, Z.; You, K.; Gong, J.; Li, S. N-acetylcysteine in the Donor, Recipient, or Both Donor and Recipient in Liver Transplantation: A Systematic Review With Meta-analysis and Trial Sequential Analysis. Transplantation 2023, 107, 1976–1990. [Google Scholar] [CrossRef]
- Santiago, F.M.; Bueno, P.; Olmedo, C.; Muffak-Granero, K.; Comino, A.; Serradilla, M.; Mansilla, A.; Villar, J.M.; Garrote, D.; Ferron, J.A. Effect of N-acetylcysteine administration on intraoperative plasma levels of interleukin-4 and interleukin-10 in liver transplant recipients. Transplant. Proc. 2008, 40, 2978–2980. [Google Scholar] [CrossRef]
- Peng, C.; Li, X.; Ao, F.; Li, T.; Guo, J.; Liu, J.; Zhang, X.; Gu, J.; Mao, J.; Zhou, B. Mitochondrial ROS driven by NOX4 upregulation promotes hepatocellular carcinoma cell survival after incomplete radiofrequency ablation by inducing of mitophagy via Nrf2/PINK1. J. Transl. Med. 2023, 21, 218. [Google Scholar] [CrossRef]
- Kretzmann, N.A.; Chiela, E.; Matte, U.; Marroni, N.; Marroni, C.A. N-acetylcysteine improves antitumoural response of Interferon alpha by NF-kB downregulation in liver cancer cells. Comp. Hepatol. 2012, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Natori, S.; Fujii, Y.; Kurosawa, H.; Nakano, A.; Shimada, H. Prostaglandin E1 protects against ischemia-reperfusion injury of the liver by inhibition of neutrophil adherence to endothelial cells. Transplantation 1997, 64, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Song, S.H.; Kim, J.M.; Kim, S.J.; Joh, J.W.; Lee, S.K.; Kwon, C.H. Effectiveness of intraportal prostaglandin E1 administration after liver transplantation. Transplant. Proc. 2012, 44, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Treating ischaemia-reperfusion injury with prostaglandin E1 reduces the risk of early hepatocellular carcinoma recurrence following liver transplantation. Aliment. Pharmacol. Ther. 2015, 42, 1101–1110. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, H.; Li, Q.; Zang, Y.; Chen, X.; Zou, W.; Wang, L.; Shen, Z.Y. Combination adjuvant chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin after liver transplantation for hepatocellular carcinoma: A preliminary open-label study. Investig. New Drugs 2011, 29, 1360–1369. [Google Scholar] [CrossRef]
- Shetty, K.; Dash, C.; Laurin, J. Use of adjuvant sorafenib in liver transplant recipients with high-risk hepatocellular carcinoma. J. Transplant. 2014, 2014, 913634. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Das, K.; Kocak, M.; Helmick, R.A.; Eason, J.D.; Nair, S.P.; Vanatta, J.M. No apparent benefit of preemptive sorafenib therapy in liver transplant recipients with advanced hepatocellular carcinoma on explant. Clin. Transplant. 2018, 32, e13246. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Galle, P.R.; Zhu, A.X.; Ducreux, M.; Cheng, A.L.; Ikeda, M.; Tsuchiya, K.; Aoki, K.I.; Jia, J.; et al. IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study. Liver Cancer 2023, 12, 238–250. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Pelizzaro, F.; Gambato, M.; Gringeri, E.; Vitale, A.; Cillo, U.; Farinati, F.; Burra, P.; Russo, F.P. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2021, 13, 4882. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, I.A.; Evangeliou, A.P.; Giannis, D.; Hayat, M.H.; Mylonas, K.S.; Tohme, S.; Geller, D.A.; Elias, N.; Goyal, L.; Tsoulfas, G. The Role of Immunotherapy in Hepatocellular Carcinoma: A Systematic Review and Pooled Analysis of 2,402 Patients. Oncologist 2021, 26, e1036–e1049. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Y.; Ling, Q.; Xu, L.; Wang, T.; Zhu, J.; Lin, Y.; Lu, X.; Qu, W.; Zhang, F.; et al. Pretransplant use of immune checkpoint inhibitors for hepatocellular carcinoma: A multicenter, retrospective cohort study. Am. J. Transplant. 2024, 24, 1837–1856. [Google Scholar] [CrossRef]
- Rodriguez-Peralvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; Garcia-Caparros, C.; O’Beirne, J.; Poyato-Gonzalez, A.; Ferrin-Sanchez, G.; Montero-Alvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Vivarelli, M.; Cucchetti, A.; La Barba, G.; Ravaioli, M.; Del Gaudio, M.; Lauro, A.; Grazi, G.L.; Pinna, A.D. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: Reassessment of risk factors for tumor recurrence. Ann. Surg. 2008, 248, 857–862. [Google Scholar] [CrossRef]
- Bhat, M.; Sonenberg, N.; Gores, G.J. The mTOR pathway in hepatic malignancies. Hepatology 2013, 58, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Toso, C.; Merani, S.; Bigam, D.L.; Shapiro, A.M.; Kneteman, N.M. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010, 51, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Mamou, C.; Rodriguez-Castro, K.I.; Burra, P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: A systematic review. Transpl. Int. 2014, 27, 1039–1049. [Google Scholar] [CrossRef]
- De Simone, P.; Precisi, A.; Lai, Q.; Ducci, J.; Campani, D.; Marchetti, P.; Gitto, S. Everolimus Mitigates the Risk of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2024, 16, 1243. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schnitzbauer, A.A.; Zulke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; De Carlis, L.; Del Gaudio, M.; De Simone, P.; Fagiuoli, S.; Lupo, F.; Tisone, G.; Volpes, R. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: Consensus recommendations from an Italian Working Group. Hepatol. Int. 2020, 14, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Ohira, M.; Hotta, R.; Tanaka, Y.; Matsuura, T.; Tekin, A.; Selvaggi, G.; Vianna, R.; Ricordi, C.; Ruiz, P.; Nishida, S.; et al. Pilot study to determine the safety and feasibility of deceased donor liver natural killer cell infusion to liver transplant recipients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2022, 71, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Ohira, M.; Ishiyama, K.; Tanaka, Y.; Doskali, M.; Igarashi, Y.; Tashiro, H.; Hiraga, N.; Imamura, M.; Sakamoto, N.; Asahara, T.; et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J. Clin. Invest. 2009, 119, 3226–3235. [Google Scholar] [CrossRef]
- Jakubauskiene, L.; Jakubauskas, M.; Stiegler, P.; Leber, B.; Schemmer, P.; Strupas, K. Ischemic Preconditioning for Liver Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Visc. Med. 2021, 37, 329–337. [Google Scholar] [CrossRef]
- Qi, B.; Wang, X.Q.; Pan, S.T.; Li, P.Y.; Chen, L.K.; Xia, Q.; Yang, L.Q.; Yu, W.F. Effect of remote ischemic preconditioning among donors and recipients following pediatric liver transplantation: A randomized clinical trial. World J. Gastroenterol. 2021, 27, 345–357. [Google Scholar] [CrossRef]
- Orci, L.A.; Lacotte, S.; Oldani, G.; Slits, F.; De Vito, C.; Crowe, L.A.; Rubbia-Brandt, L.; Vallee, J.P.; Morel, P.; Toso, C. Effect of ischaemic preconditioning on recurrence of hepatocellular carcinoma in an experimental model of liver steatosis. Br. J. Surg. 2016, 103, 417–426. [Google Scholar] [CrossRef]
| Study | Study Design and Country | Perfusion Techniques (Device) and Duration | Donor Type | Donor Risk Factors | Outside HCC Transplant Criteria | Follow-Up | HCC Recurrence Rate | Key Findings |
|---|---|---|---|---|---|---|---|---|
| Clinical studies with hypothermic oxygenated perfusion | ||||||||
| Mueller et al., 2020 [97] | Retrospective multicenter - Center A: Switzerland - Center B: United Kingdom | HOPE (XVIVO Liver Assist) (n = 70) with 2 h (IQR:1.7–2.5) SCS (n = 210) | DCD treated with HOPE: n = 70 (Center A) DBD control: n = 70 (Center A) DCD control: n = 70 (Center B) DBD control: n = 70 (Center B) | Donor age was significantly higher in HOPE group (p = 0.007) 59.5 years (IQR: 48.75–72.0) FDWIT was significantly higher in HOPE group (p < 0.0001) 30.5 min (IQR: 26–35) | Milan: HOPE: 25/70 (35.7%) DCD control: 13/70 (18.6%) DBD control (A): 26/70 (37.1%) DBD control (B): 14/70 (20%) UCSF: HOPE: 20/70 (28.6%) DCD control: 6/70 (8.6%) DBD control (A): 21/70 (30%) DBD control (B): 4/70 (5.7%) Metroticket 2.0: HOPE: 13/70 (18.6%) DCD control: 1/70 (1.4%) DBD control (A): 12/70 (17.1%) DBD control (B): 1/70 (1.4%) | HOPE: 32 mo (15.8–55.6) DCD control: 54 mo (31.5–68.8) DBD control (A): 54.3 mo (24.6–87.8) DBD control (B): 44.9 mo (27.4–67.5) | DCD-HOPE: 4/70 (5.7%) DCD control: 10/70 (14.3%) DBD control (Center A): 18/70 (25.7%) DBD control (Center B): 12/70 (17.1%) | Fourfold higher tumor recurrence in unperfused DBD livers (25.7%, 18/70), compared to only 5.7% recipients (4/70) with tumor recurrence in the HOPE-treated DCD cohort (p = 0.002) in Center A and twofold higher tumor recurrence rate in unperfused DBD and DCD from Center B. Five-year RFS: 92% in HOPE group, compared to 73%, 82.7%, and 81.2% in patients receiving unperfused DBD or DCD livers. |
| Rigo et al., 2023 [22] | Retrospective single center; Italy | D-HOPE (XVIVO Liver Assist) (n = 80) with 2.4 h (1.95–3) SCS (n = 246) | D-HOPE: DCD—14/80 (18%); DBD—66/80 (82%) SCS: DCD: 0%; DBD: 246/246 (100%) | Donor age was significantly higher in HOPE group 71.8 years (IQR: 60.7–82.4, p = 0.003), FDWIT not reported | Metroticket 2.0, <80% estimated survival at 5 years D-HOPE: 8/80 (10%) SCS: 20/246 (8%) Downstaging proportion comparable (p = 0.673) | D-HOPE: 40 mo (32–52) SCS: 59 mo (36–72) | D-HOPE: 8/80 (10%) SCS: 22/246 (9%) | Similar 1-year RFS: 96% SCS, 95% D-HOPE. Adjusted analysis for donor age, donor BMI, macrovesicular steatosis, CIT, recipient age, HCC grading, and MVI showed no significant difference in RFS. |
| Dajti et al., 2024 [98] | Retrospective single center; Italy | HOPE (instituitional device) (n = 60) SCS (n = 177) | HOPE: DCD—16/60 (27%); DBD—44/60 (73%) SCS: DCD: 0%; DBD: 177/177 (100%) | Donor age was comparable (p = 0.2) FDWIT not reported | Milan: HOPE: 1/60 (7%), 17/60 (28.3%) outside Milan, 10/60 downstaged SCS: 22/177 (10%), 51/177 (28.8%) outside Milan, 29/177 downstaged | HOPE: 32 mo (24–48) SCS: 45 mo (27–64) | At 2 years: HOPE: 1/60 (2%) SCS: 14/177 (8%) | HOPE associated with lower risk of HCC recurrence (OR 0.126, p = 0.049) and higher RFS (HR 0.132, p = 0.050) after balancing recipient age, sex, MELD, DRI, and Milan criteria status at listing. MVI (OR 3.737, p = 0.019) and longer CIT (OR 1.155, p = 0.049) were associated with higher risk of recurrence. |
| Clinical studies with ischemia-free liver transplantation | ||||||||
| Tang et al., 2021 [33] | Retrospective single center; China | IFLT (XVIVO Liver Assist) (n = 30) CLT (n = 85) | All donors were DBD | Donor age was comparable (p = 0.117) | Milan: IFLT: 13/30 (43.3%) CLT: 44/30 (51.8%) AFP is lower in IFLT group (p = 0.016) | IFLT: 22.9 ± 11.2 mo CLT: 22.6 ± 11.4 mo | At 1 year, RFS IFLT: 92.2% SCS: 88.1% | RFS at 1 and 3 year was significantly improved with IFLT: 92.2% and 86.7% vs. 88.1% and 53.6% for CLT (p = 0.048). Difference between OS at 1 and 3 years not statistically significant: IFLT 96.7% and 90.6%, vs. CLT 94.1% and 70.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, K.B.; Hussein, A.; Satish, S.; Wehrle, C.J.; Karakaya, O.; Panconesi, R.; Sun, K.; Jiao, C.; Fernandes, E.; Pinna, A.; et al. Machine Perfusion as a Strategy to Decrease Ischemia-Reperfusion Injury and Lower Cancer Recurrence Following Liver Transplantation. Cancers 2024, 16, 3959. https://doi.org/10.3390/cancers16233959
Garcia KB, Hussein A, Satish S, Wehrle CJ, Karakaya O, Panconesi R, Sun K, Jiao C, Fernandes E, Pinna A, et al. Machine Perfusion as a Strategy to Decrease Ischemia-Reperfusion Injury and Lower Cancer Recurrence Following Liver Transplantation. Cancers. 2024; 16(23):3959. https://doi.org/10.3390/cancers16233959
Chicago/Turabian StyleGarcia, Karla Bracho, Ahmed Hussein, Sangeeta Satish, Chase J. Wehrle, Omer Karakaya, Rebecca Panconesi, Keyue Sun, Chunbao Jiao, Eduardo Fernandes, Antonio Pinna, and et al. 2024. "Machine Perfusion as a Strategy to Decrease Ischemia-Reperfusion Injury and Lower Cancer Recurrence Following Liver Transplantation" Cancers 16, no. 23: 3959. https://doi.org/10.3390/cancers16233959
APA StyleGarcia, K. B., Hussein, A., Satish, S., Wehrle, C. J., Karakaya, O., Panconesi, R., Sun, K., Jiao, C., Fernandes, E., Pinna, A., Hashimoto, K., Miller, C., Aucejo, F., & Schlegel, A. (2024). Machine Perfusion as a Strategy to Decrease Ischemia-Reperfusion Injury and Lower Cancer Recurrence Following Liver Transplantation. Cancers, 16(23), 3959. https://doi.org/10.3390/cancers16233959






