PSMA-Guided Imaging and Therapy of Advanced Adenoid Cystic Carcinomas and Other Salivary Gland Carcinomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
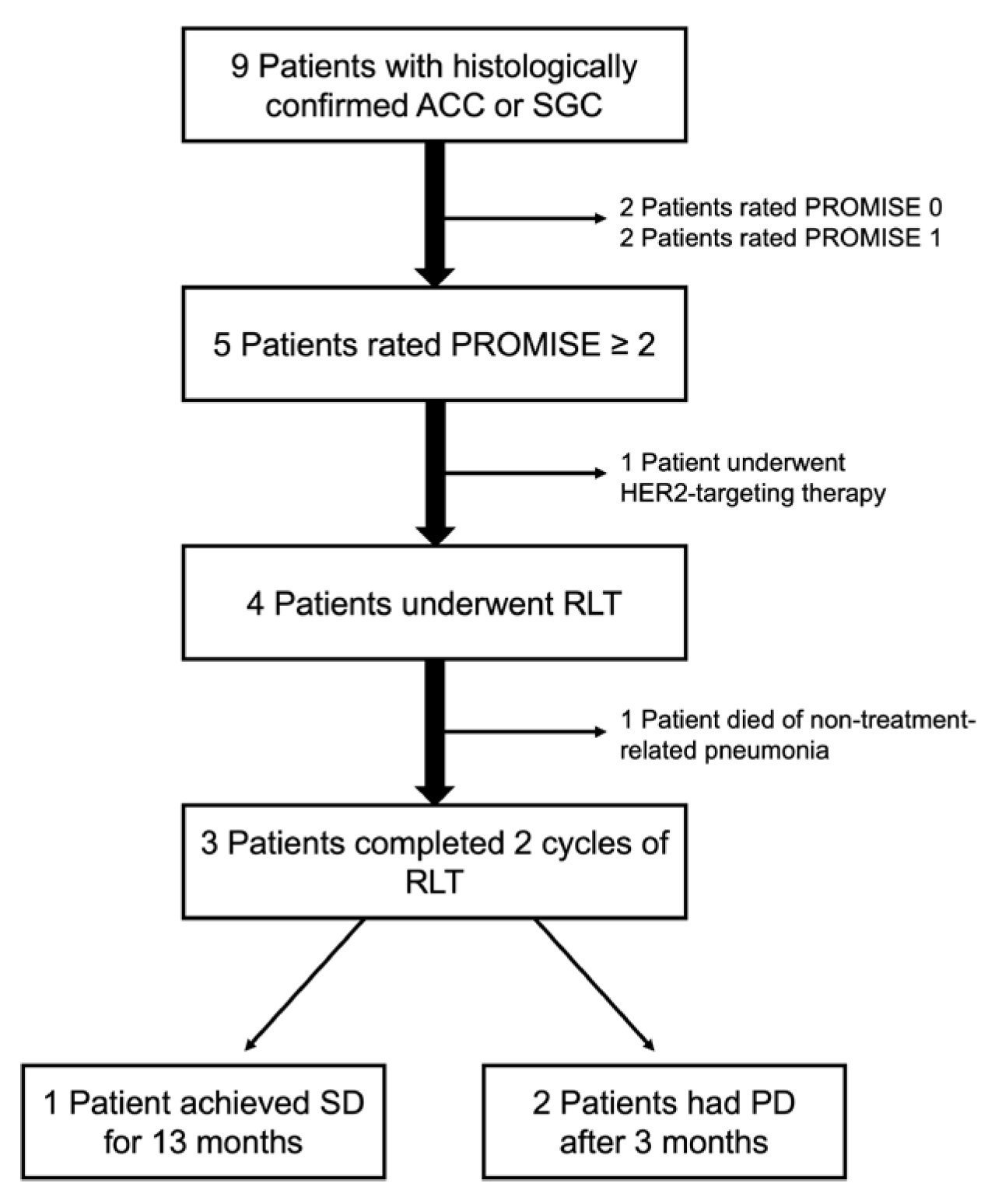
2.1. Study Cohort
2.2. [18F]PSMA-1007 and [177Lu]Lu-PSMA-I&T
2.3. PSMA PET Image Acquisition
2.4. [177Lu]Lu-PSMA-I&T SPECT/CT Acquisition and Reconstruction
2.5. Imaging Analysis
2.6. [177Lu]Lu-PSMA-I&T RLT Patient Selection and Treatment Administration
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
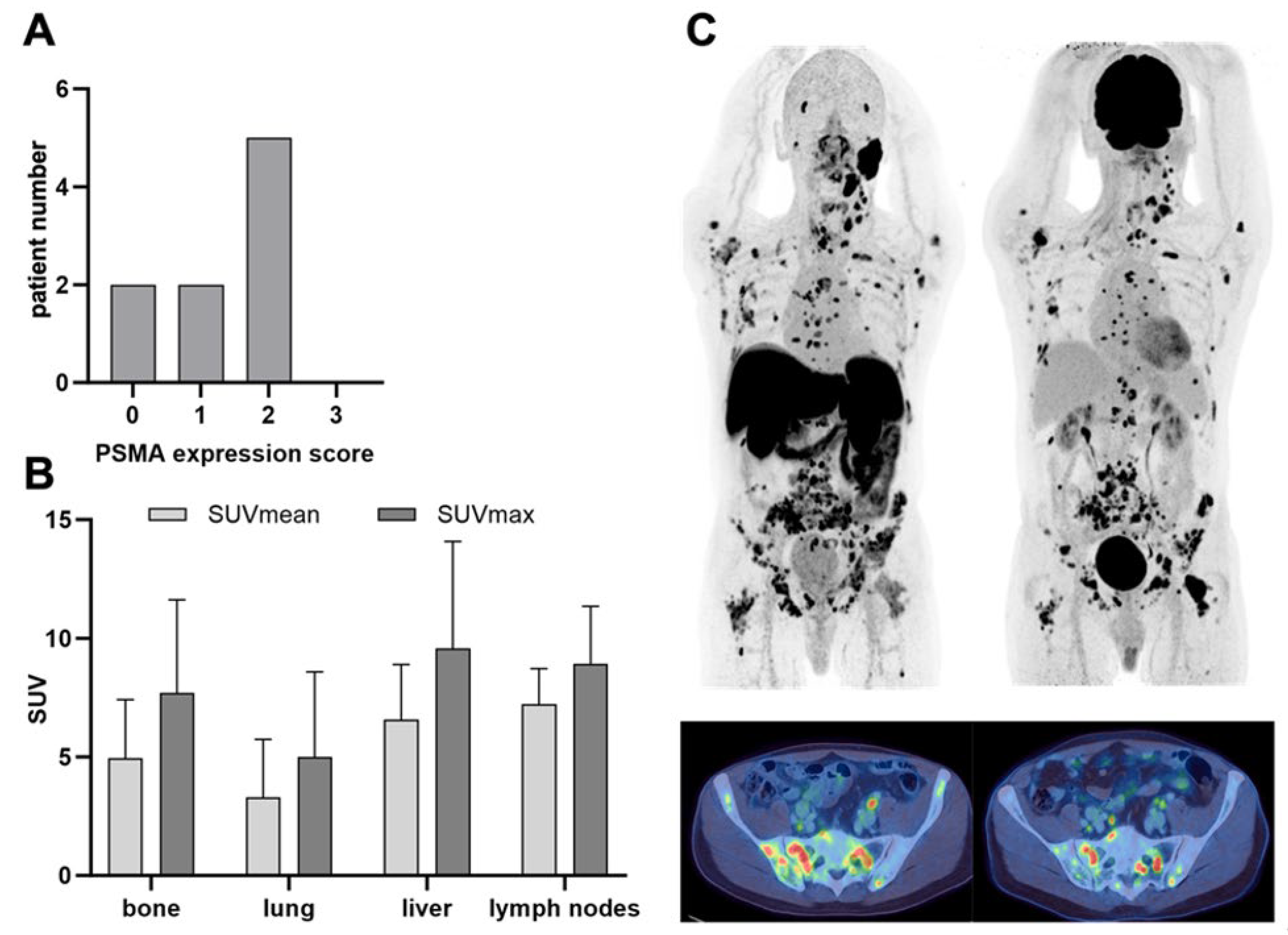
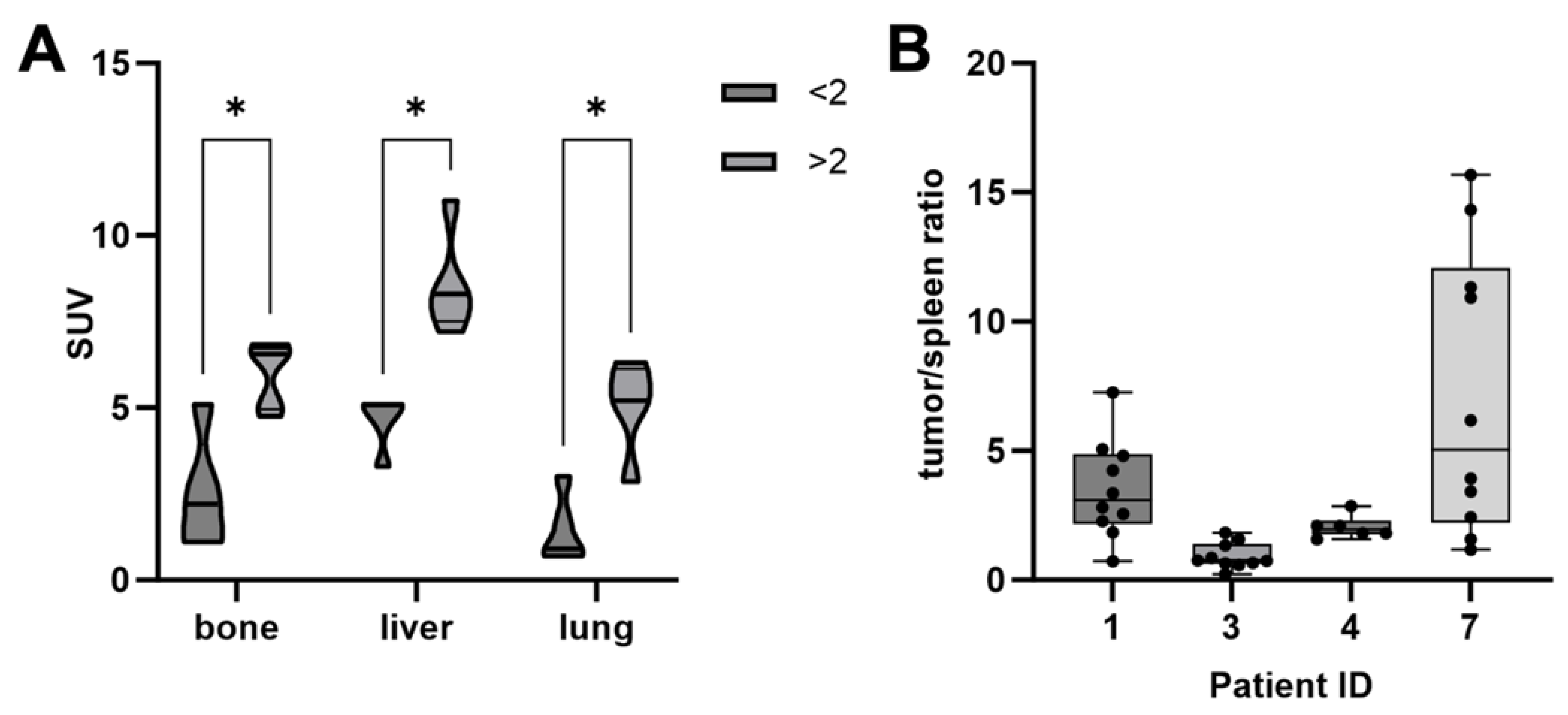
3.2. PSMA PET
3.3. PSMA RLT
3.4. Post Therapeutic SPECT/CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Dijk, B.A.; Gatta, G.; Capocaccia, R.; Pierannunzio, D.; Strojan, P.; Licitra, L.; Group, R.W. Rare cancers of the head and neck area in Europe. Eur. J. Cancer 2012, 48, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Mayor, C.V.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.N.; Asarian, A.; Xiao, P. Adenoid cystic carcinoma of the breast. J. Surg. Case Rep. 2019, 2019, rjy355. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhu, S.; Zhao, Q.; Chang, H.; Li, G.; Shao, Q.; Zhang, C.; Wu, P. Epidemiology of and factors associated with overall survival for patients with head and neck adenoid cystic carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 14071–14080. [Google Scholar] [CrossRef]
- Gao, M.; Hao, Y.; Huang, M.X.; Ma, D.Q.; Luo, H.Y.; Gao, Y.; Peng, X.; Yu, G.Y. Clinicopathological study of distant metastases of salivary adenoid cystic carcinoma. Int. J. Oral. Maxillofac. Surg. 2013, 42, 923–928. [Google Scholar] [CrossRef]
- Saleh, E.; Ukwas, A. Adenoid Cystic Carcinoma of Salivary Glands: A Ten-Year Review and an Assessment of the Current Management, Surgery, Radiotherapy, and Chemotherapy. Int. J. Otolaryngol. 2023, 2023, 7401458. [Google Scholar] [CrossRef]
- Meulemans, J.; Van Lierde, C.; Delaere, P.; Vranckx, J.J.; Vander Poorten, V. New Developments in Surgery for Malignant Salivary Gland Tumors; Springer International Publishing: Cham, Switzerland, 2023; pp. 315–326. [Google Scholar]
- Moratin, J.; Horn, D.; Semmelmayer, K.; Ristow, O.; Engel, M.; Hoffmann, J.; Bleymehl, M.; Held, T.; Zittel, S.; Freudlsperger, C. Surgical Treatment of Carcinomas of the Oral Minor Salivary Glands-Oncological Outcome in Dependence of Tumor Entity and Therapeutic Strategies. Cancers 2023, 15, 3895. [Google Scholar] [CrossRef]
- Thariat, J.; Ferrand, F.R.; Fakhry, N.; Even, C.; Vergez, S.; Chabrillac, E.; Sarradin, V.; Digue, L.; Troussier, I.; Bensadoun, R.-J. Radiotherapy for salivary gland cancer: REFCOR recommendations by the formal consensus method. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2024, 141, 221–226. [Google Scholar] [CrossRef]
- Fang, Y.; Peng, Z.; Wang, Y.; Gao, K.; Liu, Y.; Fan, R.; Zhang, H.; Xie, Z.; Jiang, W. Current opinions on diagnosis and treatment of adenoid cystic carcinoma. Oral. Oncol. 2022, 130, 105945. [Google Scholar] [CrossRef]
- Nakano, K.; Sato, Y.; Sasaki, T.; Shimbashi, W.; Fukushima, H.; Yonekawa, H.; Mitani, H.; Kawabata, K.; Takahashi, S. Combination chemotherapy of carboplatin and paclitaxel for advanced/metastatic salivary gland carcinoma patients: Differences in responses by different pathological diagnoses. Acta Otolaryngol. 2016, 136, 948–951. [Google Scholar] [CrossRef]
- Onaga, R.; Enokida, T.; Ito, K.; Ueda, Y.; Okano, S.; Fujisawa, T.; Wada, A.; Sato, M.; Tanaka, H.; Takeshita, N.; et al. Combination chemotherapy with taxane and platinum in patients with salivary gland carcinoma: A retrospective study of docetaxel plus cisplatin and paclitaxel plus carboplatin. Front. Oncol. 2023, 13, 1185198. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Huang, Z.; Chen, R.; Chen, Y.; Wang, Y.; Huang, Z. RPS3 Promotes the Metastasis and Cisplatin Resistance of Adenoid Cystic Carcinoma. Front. Oncol. 2022, 12, 804439. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Dunn, L.; Sherman, E.J.; Fury, M.G.; Baxi, S.S.; Chandramohan, R.; Dogan, S.; Morris, L.G.; Cullen, G.D.; Haque, S.; et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann. Oncol. 2016, 27, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Tran, C.; Baxi, S.; Katabi, N.; Antonescu, C.R.; Ostrovnaya, I.; Haque, S.S.; Pfister, D.G.; et al. Phase II Study of Lenvatinib in Patients With Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2019, 37, 1529–1537. [Google Scholar] [CrossRef]
- Kang, E.J.; Ahn, M.J.; Ock, C.Y.; Lee, K.W.; Kwon, J.H.; Yang, Y.; Choi, Y.H.; Kim, M.K.; Ji, J.H.; Yun, T.; et al. Randomized Phase II Study of Axitinib versus Observation in Patients with Recurred or Metastatic Adenoid Cystic Carcinoma. Clin. Cancer Res. 2021, 27, 5272–5279. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Singh, A.; Schuchardt, C.; Niepsch, K.; Sayeg, M.; Leshch, Y.; Wester, H.-J.; Baum, R.P. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J. Nucl. Med. 2016, 57, 97S–104S. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Virgolini, I.; Baum, R. Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer. Cancers 2024, 16, 2520. [Google Scholar] [CrossRef]
- Bakht, M.K.; Beltran, H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat. Rev. Urol. 2024, 1–20. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Nishida, H.; Kondo, Y.; Kusaba, T.; Kadowaki, H.; Daa, T. Immunohistochemical Reactivity of Prostate-Specific Membrane Antigen in Salivary Gland Tumors. Head. Neck Pathol. 2022, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; Valstar, M.H.; Smit, L.A.; Smeele, L.E.; Zuithoff, N.P.A.; de Keizer, B.; de Bree, R.; van Es, R.J.J.; Willems, S.M. Prostate-specific membrane antigen (PSMA) expression in adenoid cystic carcinoma of the head and neck. BMC Cancer 2020, 20, 519. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Eppard, E.; Kurpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.D.; Gartner, F.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477–12488. [Google Scholar] [CrossRef]
- van Boxtel, W.; Lutje, S.; van Engen-van Grunsven, I.C.H.; Verhaegh, G.W.; Schalken, J.A.; Jonker, M.A.; Nagarajah, J.; Gotthardt, M.; van Herpen, C.M.L. (68)Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: A phase 2 imaging study. Theranostics 2020, 10, 2273–2283. [Google Scholar] [CrossRef]
- Civan, C.; Kasper, S.; Berliner, C.; Fragoso-Costa, P.; Grunwald, V.; Pogorzelski, M.; Schaarschmidt, B.M.; Lang, S.; Kersting, D.; Nader, M.; et al. PSMA-Directed Imaging and Therapy of Salivary Gland Tumors: A Single-Center Retrospective Study. J. Nucl. Med. 2023, 64, 372–378. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of (177)Lu-labeled PSMA-targeted radioligand-therapy ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Sato, R.; Yamaki, H.; Komatsuda, H.; Wakisaka, R.; Inoue, T.; Kumai, T.; Takahara, M. Exploring Immunological Effects and Novel Immune Adjuvants in Immunotherapy for Salivary Gland Cancers. Cancers 2024, 16, 1205. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Fizazi, K.; Morris, M.J.; Shore, N.D.; Chi, K.N.; Crosby, M.; Bono, J.S.D.; Herrmann, K.; Roubaud, G.; Nagarajah, J.; Fleming, M.T.; et al. Health-related quality of life and pain in a phase 3 study of [177Lu]Lu-PSMA-617 in taxane-naïve patients with metastatic castration-resistant prostate cancer (PSMAfore). J. Clin. Oncol. 2024, 42, 5003. [Google Scholar] [CrossRef]
- Roy, J.; Warner, B.M.; Basuli, F.; Zhang, X.; Wong, K.; Pranzatelli, T.; Ton, A.T.; Chiorini, J.A.; Choyke, P.L.; Lin, F.I.; et al. Comparison of Prostate-Specific Membrane Antigen Expression Levels in Human Salivary Glands to Non-Human Primates and Rodents. Cancer Biother. Radiopharm. 2020, 35, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Czernin, J.; Thin, P.; Gartmann, J.; Nguyen, K.; Armstrong, W.R.; Allen-Auerbach, M.; Quon, A.; Bahri, S.; Gupta, P.; et al. Safety of PSMA-Targeted Molecular Radioligand Therapy with 177Lu-PSMA-617: Results from the Prospective Multicenter Phase 2 Trial RESIST-PC (NCT03042312). J. Nucl. Med. 2021, 62, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Tauber, R.; Knorr, K.; Retz, M.; Rauscher, I.; Grigorascu, S.; Hansen, K.; D’Alessandria, C.; Wester, H.-J.; Gschwend, J.; Weber, W.; et al. Safety and Efficacy of [177Lu]-PSMA-I&T Radioligand Therapy in Octogenarians with Metastatic Castration-Resistant Prostate Cancer: Report on 80 Patients over the Age of 80 Years. J. Nucl. Med. 2023, 64, 1244–1251. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.; Devriese, L.A.; de Bree, R.; de Keizer, B. First experiences with (177)Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef]
- Zhang, J.; Schuchardt, C.; Chen, X.; Baum, R.P. Rapid Tumor Washout of 177Lu-PSMA Radioligand in Renal Cell Carcinoma. Clin. Nucl. Med. 2023, 48, 732–734. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Buteau, J.P.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; et al. Overall survival with [(177)Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024, 25, 99–107. [Google Scholar] [CrossRef]

| Patient ID | Gender | Age | Years from Initial Diagnosis | Histology | Tissue of Origin | Surgery | Radiotherapy | Systemic Therapies |
|---|---|---|---|---|---|---|---|---|
| 1 | f | 60 | 10.3 | Adenocarcinoma | Submandibular gland | Yes | Yes | Doxorubicin + Cisplatin + Cyclophosphamide; Vinorelbine; Paclitaxel; Carboplatin + Gemcitabine; Irinotecan + ATR-Inhibitor |
| 2 | f | 51 | 23 | ACC | Parotid gland | Yes | Yes | Cabozantinib |
| 3 | f | 64 | 3.9 | Acinic cell carcinoma | Parotid gland | Yes | Yes | Cisplatin+ 5FU; Trastuzumab + Docetaxel; Doxycycline + Cisplatin + Cyclophosphamide |
| 4 | f | 34 | 5.1 | ACC | Bronchial gland | No | Yes | Nivolumab + Ipilimumab; Cisplatin+ Paclitaxel; Carboplatin + Paclitaxel; Sorafenib, |
| 5 | f | 35 | 3.5 | ACC | Mammary gland | Yes | Yes | Carboplatin + Paclitaxel; Cisplatin+ Doxorubicin + Cyclophosphamide; Lenvatinib |
| 6 | f | 71 | 16 | ACC | Greater vestibular gland | Yes | Yes | Gemcitabine + ATR-Inhibitor |
| 7 | m | 58 | 9.8 | ACC | Parotid gland | Yes | Yes | Cisplatin + 5FU, Cetuximab; Nivolumab; Docetaxel; Lenvatinib; Cisplatin + Doxorubicin |
| 8 | f | 61 | 13.7 | ACC | Paranasal sinus | No | Yes | Lenvatinib |
| 9 | m | 72 | 3.2 | Adenocarcinoma | Parotid gland | Yes | Yes | Cyclophosphamide + Doxorubicin+ Cisplatin; Docetaxel + Trastuzumab |
| Patient ID | Tumor Sites | Promise Score | Cycles | Total Activity (MBq) | RECIST Response | Time to Progression (mon.) |
|---|---|---|---|---|---|---|
| 1 | bone, lung | 2 | 4 | 30.289 | SD | 13 |
| 3 | lymph nodes, bone | 2 | 1 | 7.753 | NA | 1 |
| 4 | lung, liver | 2 | 2 | 14.911 | PD | 3 |
| 7 | bone, lung | 2 | 2 | 15.072 | PD | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trautwein, N.F.; Brendlin, A.; Reischl, G.; Mattke, M.; Paulsen, F.; Loewenheim, H.; Zender, L.; la Fougère, C.; Dittmann, H. PSMA-Guided Imaging and Therapy of Advanced Adenoid Cystic Carcinomas and Other Salivary Gland Carcinomas. Cancers 2024, 16, 3843. https://doi.org/10.3390/cancers16223843
Trautwein NF, Brendlin A, Reischl G, Mattke M, Paulsen F, Loewenheim H, Zender L, la Fougère C, Dittmann H. PSMA-Guided Imaging and Therapy of Advanced Adenoid Cystic Carcinomas and Other Salivary Gland Carcinomas. Cancers. 2024; 16(22):3843. https://doi.org/10.3390/cancers16223843
Chicago/Turabian StyleTrautwein, Nils F., Andreas Brendlin, Gerald Reischl, Moritz Mattke, Frank Paulsen, Hubert Loewenheim, Lars Zender, Christian la Fougère, and Helmut Dittmann. 2024. "PSMA-Guided Imaging and Therapy of Advanced Adenoid Cystic Carcinomas and Other Salivary Gland Carcinomas" Cancers 16, no. 22: 3843. https://doi.org/10.3390/cancers16223843
APA StyleTrautwein, N. F., Brendlin, A., Reischl, G., Mattke, M., Paulsen, F., Loewenheim, H., Zender, L., la Fougère, C., & Dittmann, H. (2024). PSMA-Guided Imaging and Therapy of Advanced Adenoid Cystic Carcinomas and Other Salivary Gland Carcinomas. Cancers, 16(22), 3843. https://doi.org/10.3390/cancers16223843







