Identification of Molecular Subtypes and Prognostic Traits Based on Chromosomal Instability Phenotype-Related Genes in Lung Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Transcriptome Data Analysis
2.2. Mutation Profiling
2.3. Analysis of Copy Number Variation
2.4. Statistical Analysis
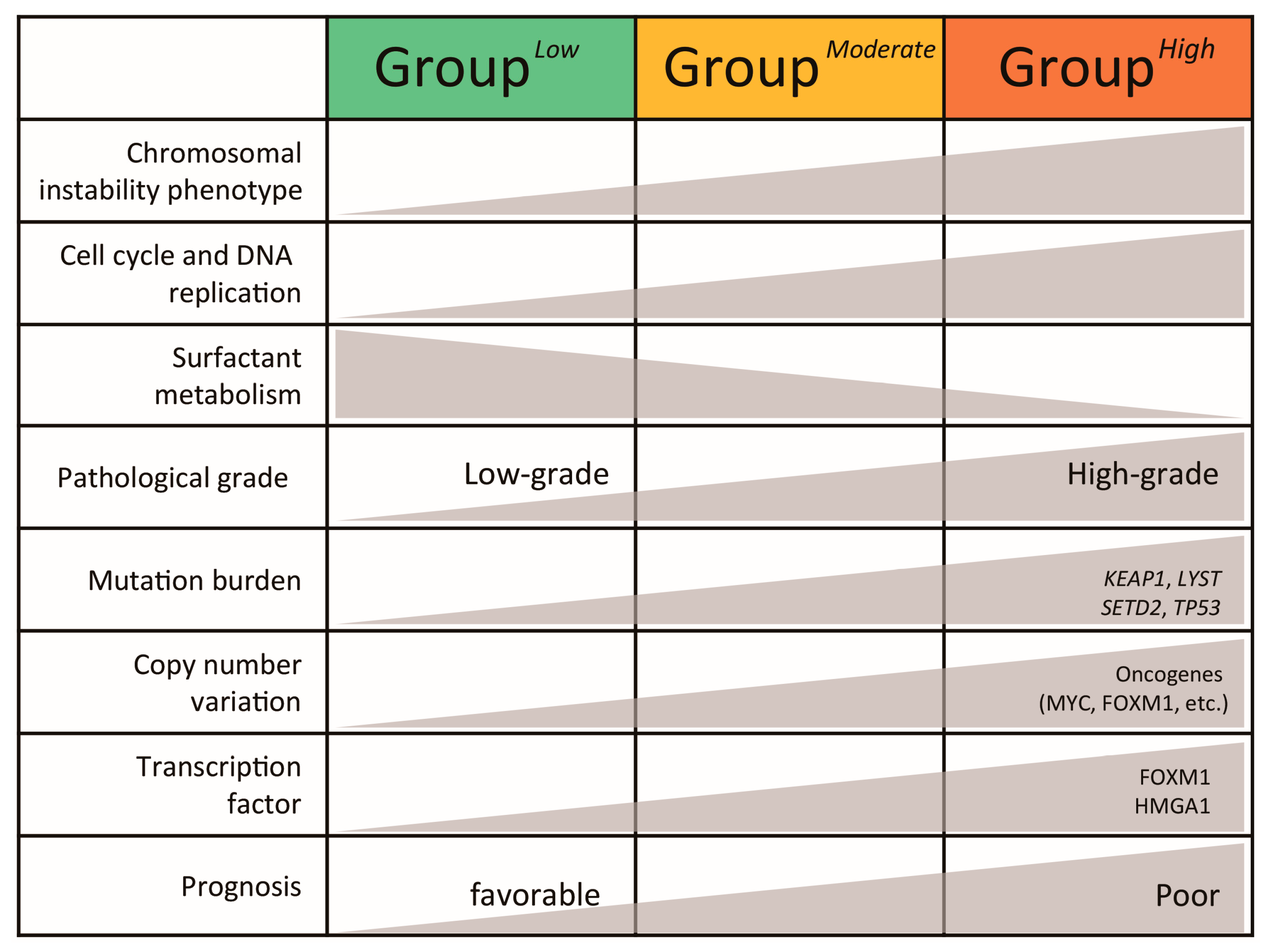
3. Results
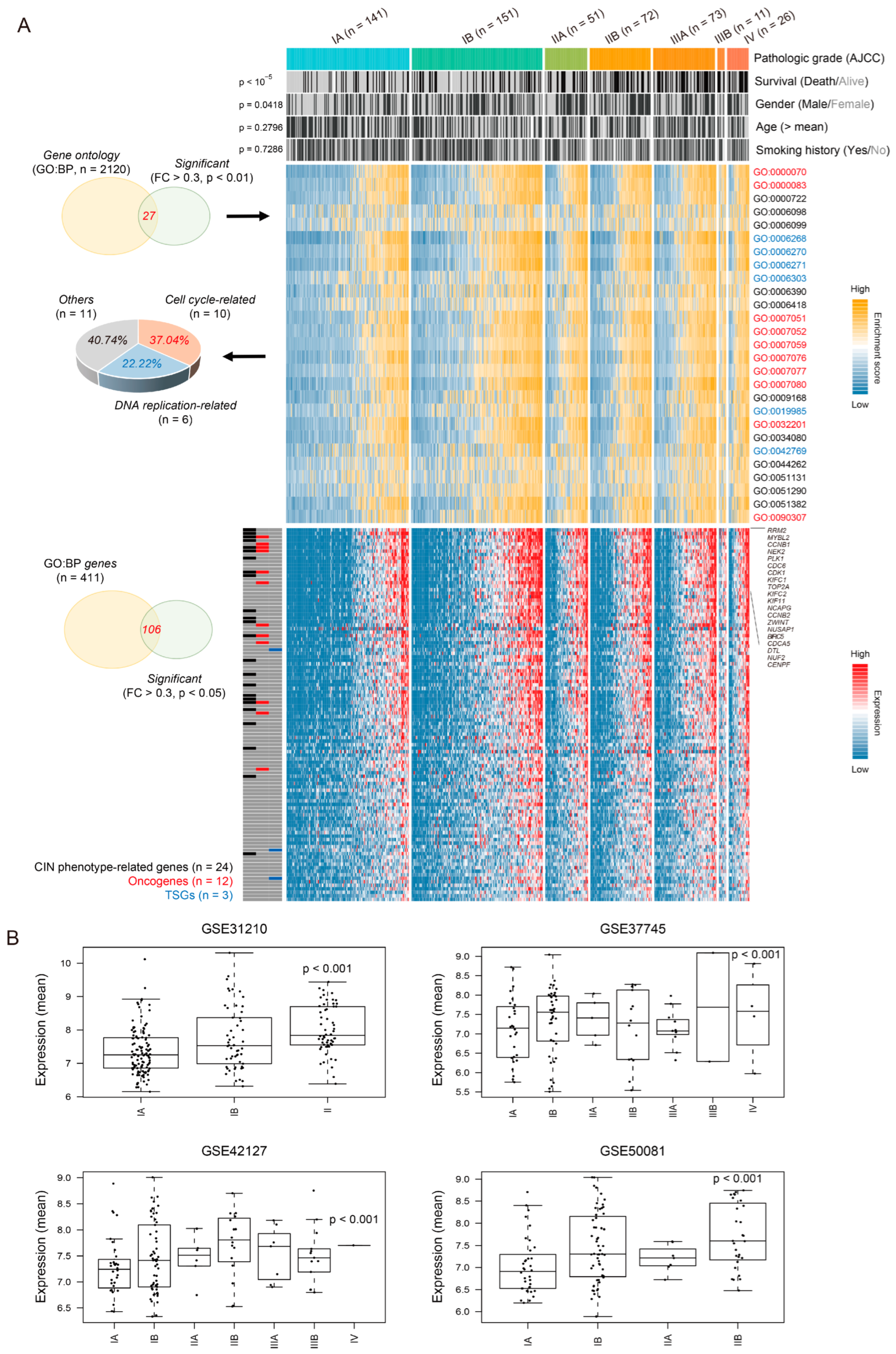
3.1. Chromosomal Instability Phenotype-Related Genes Are Associated with Pathologic Stage in Lung Adenocarcinoma
3.2. LUAD-Significant CIN Phenotype-Related Gene Status Revealed an Association with Transcriptomic Patterns and Prognostic Predictions
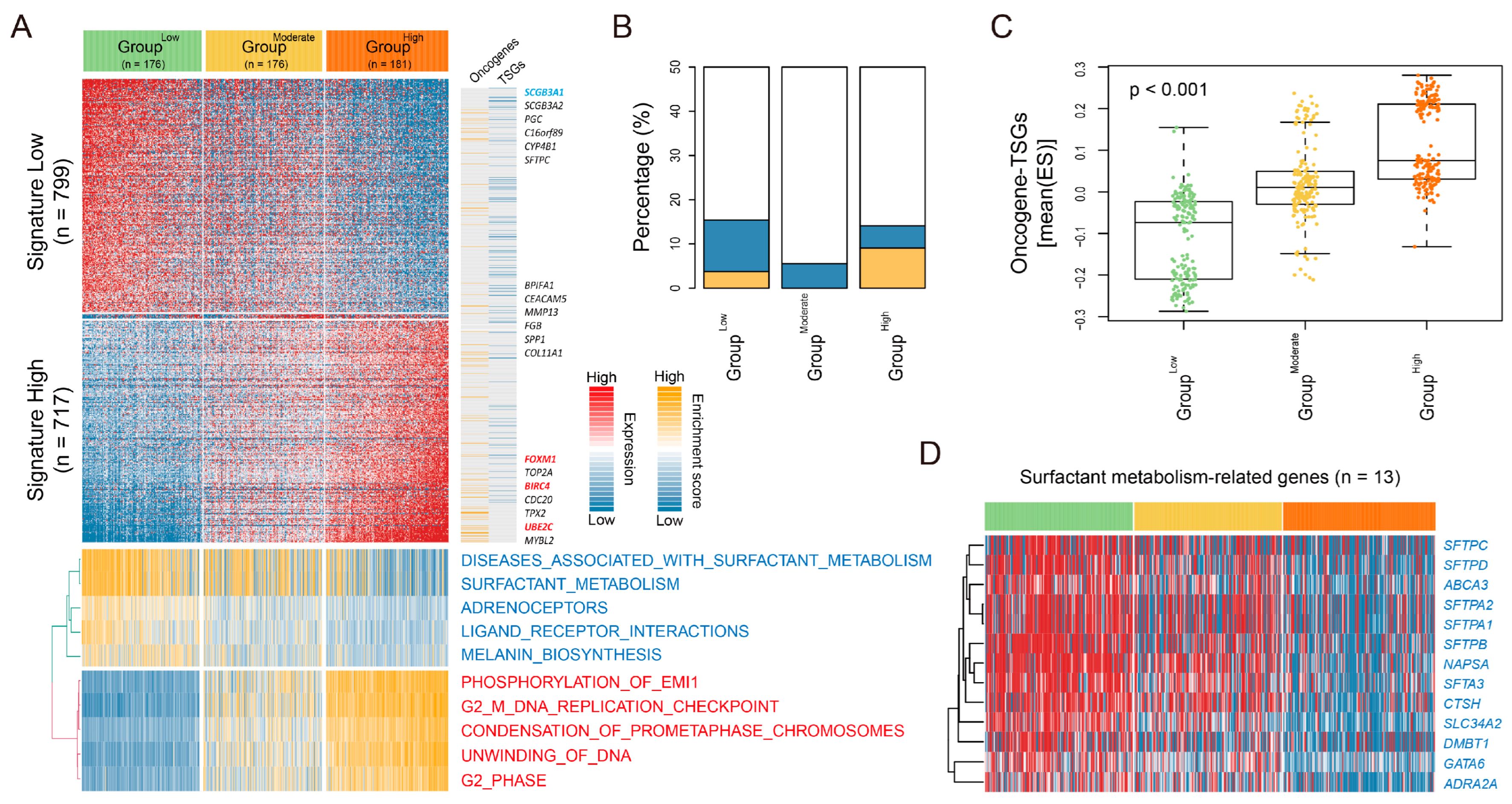
3.3. Characterization of Transcriptomic Subtypes According to LUAD-Significant CIN Phenotype-Related Gene Status
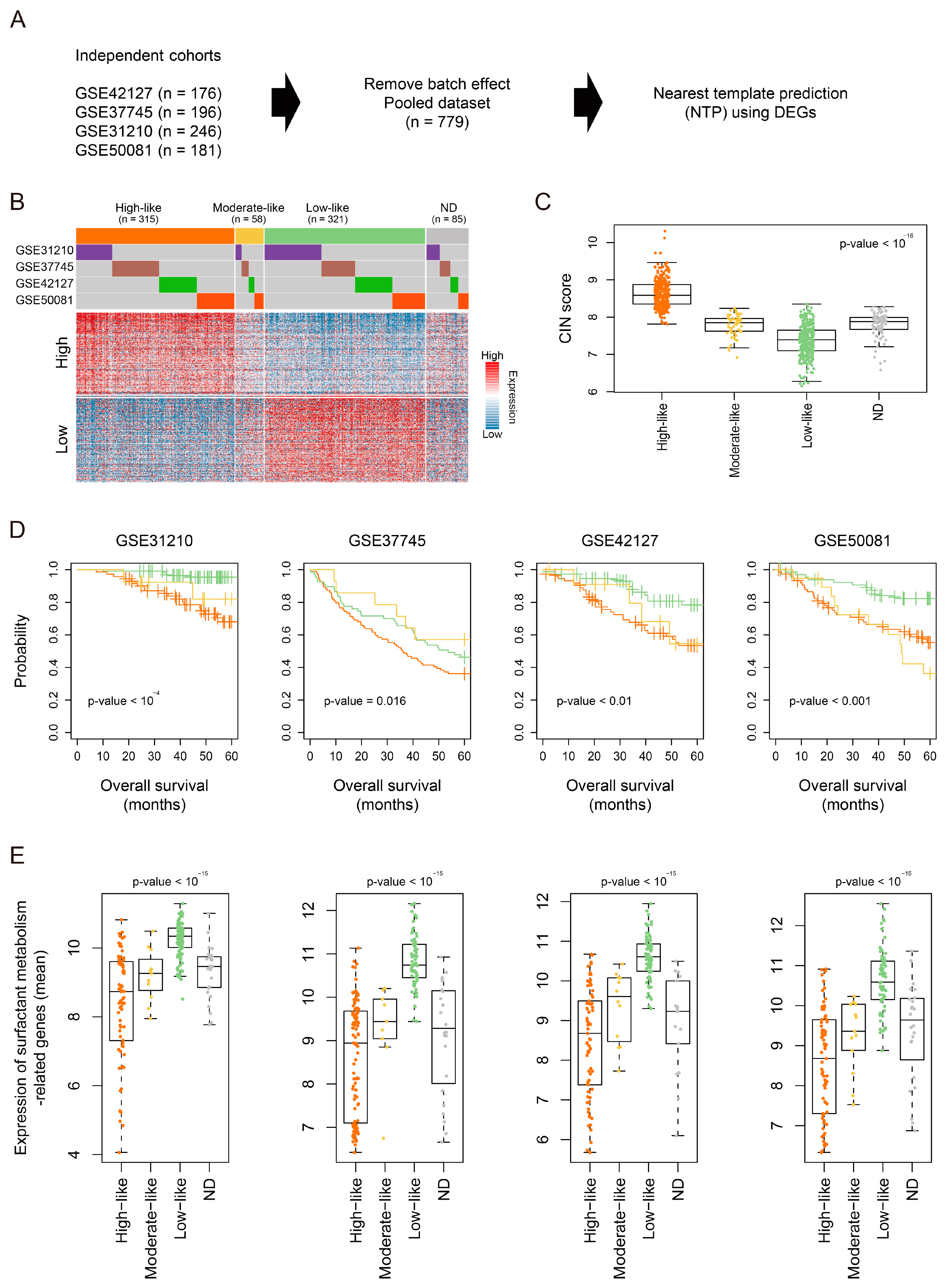
3.4. Validation of the Molecular and Clinical Significance of LUAD-Significant CIN Phenotype-Related Gene Status in a Pooled LUAD Dataset
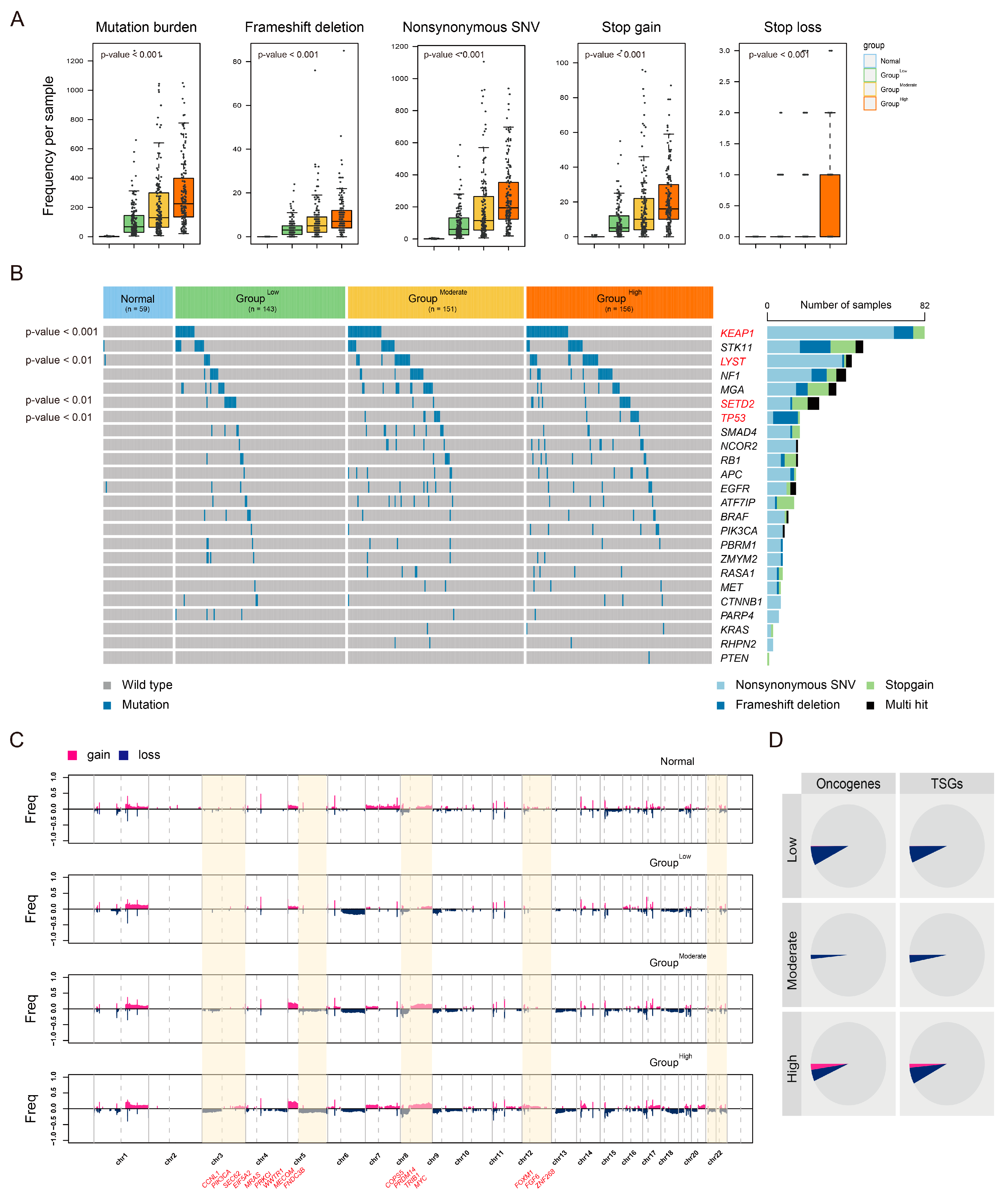
3.5. Distinct Genomic Profiles According to LUAD-Significant CIN Phenotype-Related Gene Status
3.6. Transcription Factor Profiles According to LUAD-Significant CIN Phenotype-Related Gene Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550, Erratum in Nature 2018, 559, E12. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Budczies, J.; Romanovsky, E.; Kirchner, M.; Neumann, O.; Blasi, M.; Schnorbach, J.; Shah, R.; Bozorgmehr, F.; Savai, R.; Stiewe, T.; et al. KRAS and TP53 co-mutation predicts benefit of immune checkpoint blockade in lung adenocarcinoma. Br. J. Cancer 2024, 131, 524–533. [Google Scholar] [CrossRef]
- Zavitsanou, A.M.; Pillai, R.; Hao, Y.; Wu, W.L.; Bartnicki, E.; Karakousi, T.; Rajalingam, S.; Herrera, A.; Karatza, A.; Rashidfarrokhi, A.; et al. KEAP1 mutation in lung adenocarcinoma promotes immune evasion and immunotherapy resistance. Cell Rep. 2023, 42, 113295. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Cantley, L.C. The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell 2018, 174, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Burrell, R.A.; Endesfelder, D.; Novelli, M.R.; Swanton, C. Cancer chromosomal instability: Therapeutic and diagnostic challenges. EMBO Rep. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, C.I.; Coleman, M.C.; Spitz, D.R.; Smith, B.J.; Knudson, C.M. Manganese superoxide dismutase gene dosage affects chromosomal instability and tumor onset in a mouse model of T cell lymphoma. Free Radic. Biol. Med. 2008, 44, 1677–1686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.; Agustinus, A.S.; Li, J.; DiBona, M.; Bakhoum, S.F. Chromosomal instability as a driver of cancer progression. Nat. Rev. Genet. 2024, 1–16. [Google Scholar] [CrossRef]
- Hoshida, Y. Nearest template prediction: A single-sample-based flexible class prediction with confidence assessment. PLoS ONE 2010, 5, e15543. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Yoo, J.E.; Rhee, H.; Kim, Y.J.; Il Kim, G.; Chung, T.; Yoon, S.; Shin, B.; Woo, H.G.; Park, Y.N. YAP inactivation in estrogen receptor alpha-positive hepatocellular carcinoma with less aggressive behavior. Exp. Mol. Med. 2021, 53, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet. 2006, 38, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Ma, Y.; Meng, N.; Li, D.; Wang, D.; Lian, J.; Hu, C. Identification of Crucial Genes and Signaling Pathways in Alectinib-Resistant Lung Adenocarcinoma Using Bioinformatic Analysis. Mol. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, X.; Zhang, L.; Yang, M.; Wang, X.; Huang, W.; Pan, H.; Wu, Y.; Huang, J.; Liang, W.; et al. SFTPA1 is a potential prognostic biomarker correlated with immune cell infiltration and response to immunotherapy in lung adenocarcinoma. Cancer Immunol. Immunother. 2022, 71, 399–415. [Google Scholar] [CrossRef]
- Zuo, B.; Wang, L.; Li, X.; Li, X.; Wang, J.; Xiong, Y.; Lei, J.; Zhang, X.; Chen, Y.; Liu, Q.; et al. Abnormal low expression of SFTPC promotes the proliferation of lung adenocarcinoma by enhancing PI3K/AKT/mTOR signaling transduction. Aging 2023, 15, 12451–12475. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Ye, X.; Zhang, L.; Wang, Z.; Xu, X.; Shen, Y.; Zheng, C. Loss-of-function mutations in KEAP1 drive lung cancer progression via KEAP1/NRF2 pathway activation. Cell Commun. Signal 2020, 18, 98. [Google Scholar] [CrossRef]
- Jeong, Y.; Hoang, N.T.; Lovejoy, A.; Stehr, H.; Newman, A.M.; Gentles, A.J.; Kong, W.; Truong, D.; Martin, S.; Chaudhuri, A.; et al. Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov. 2017, 7, 86–101. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Sadasivam, S.; DeCaprio, J.A. The DREAM complex: Master coordinator of cell cycle-dependent gene expression. Nat. Rev. Cancer 2013, 13, 585–595. [Google Scholar] [CrossRef]
- Musa, J.; Aynaud, M.M.; Mirabeau, O.; Delattre, O.; Grunewald, T.G. MYBL2 (B-Myb): A central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis. 2017, 8, e2895. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X. Comprehensive Analysis of the Expression and Prognosis for the DREAM Complex in Human Cancers. Front. Genet. 2022, 13, 814725. [Google Scholar] [CrossRef] [PubMed]
- Pocha, K.; Mock, A.; Rapp, C.; Dettling, S.; Warta, R.; Geisenberger, C.; Jungk, C.; Martins, L.R.; Grabe, N.; Reuss, D.; et al. Surfactant Expression Defines an Inflamed Subtype of Lung Adenocarcinoma Brain Metastases that Correlates with Prolonged Survival. Clin. Cancer Res. 2020, 26, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Luo, Z.; Xu, M.; Ye, S.; Lei, Y.; Xi, Y. HMGA1 and FOXM1 Cooperate to Promote G2/M Cell Cycle Progression in Cancer Cells. Life 2023, 13, 1225. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Choi, K.-C.; Park, Y.N.; Kim, Y.-J. Identification of Molecular Subtypes and Prognostic Traits Based on Chromosomal Instability Phenotype-Related Genes in Lung Adenocarcinoma. Cancers 2024, 16, 3818. https://doi.org/10.3390/cancers16223818
Jeon Y, Choi K-C, Park YN, Kim Y-J. Identification of Molecular Subtypes and Prognostic Traits Based on Chromosomal Instability Phenotype-Related Genes in Lung Adenocarcinoma. Cancers. 2024; 16(22):3818. https://doi.org/10.3390/cancers16223818
Chicago/Turabian StyleJeon, Youngsic, Kyung-Chul Choi, Young Nyun Park, and Young-Joo Kim. 2024. "Identification of Molecular Subtypes and Prognostic Traits Based on Chromosomal Instability Phenotype-Related Genes in Lung Adenocarcinoma" Cancers 16, no. 22: 3818. https://doi.org/10.3390/cancers16223818
APA StyleJeon, Y., Choi, K.-C., Park, Y. N., & Kim, Y.-J. (2024). Identification of Molecular Subtypes and Prognostic Traits Based on Chromosomal Instability Phenotype-Related Genes in Lung Adenocarcinoma. Cancers, 16(22), 3818. https://doi.org/10.3390/cancers16223818






