Evaluation of Subclinical Cancer Therapy-Related Cardiac Dysfunction in Patients Undergoing Hematopoietic Stem Cell Transplantation: An Echocardiography Study
Simple Summary
Abstract
1. Introduction
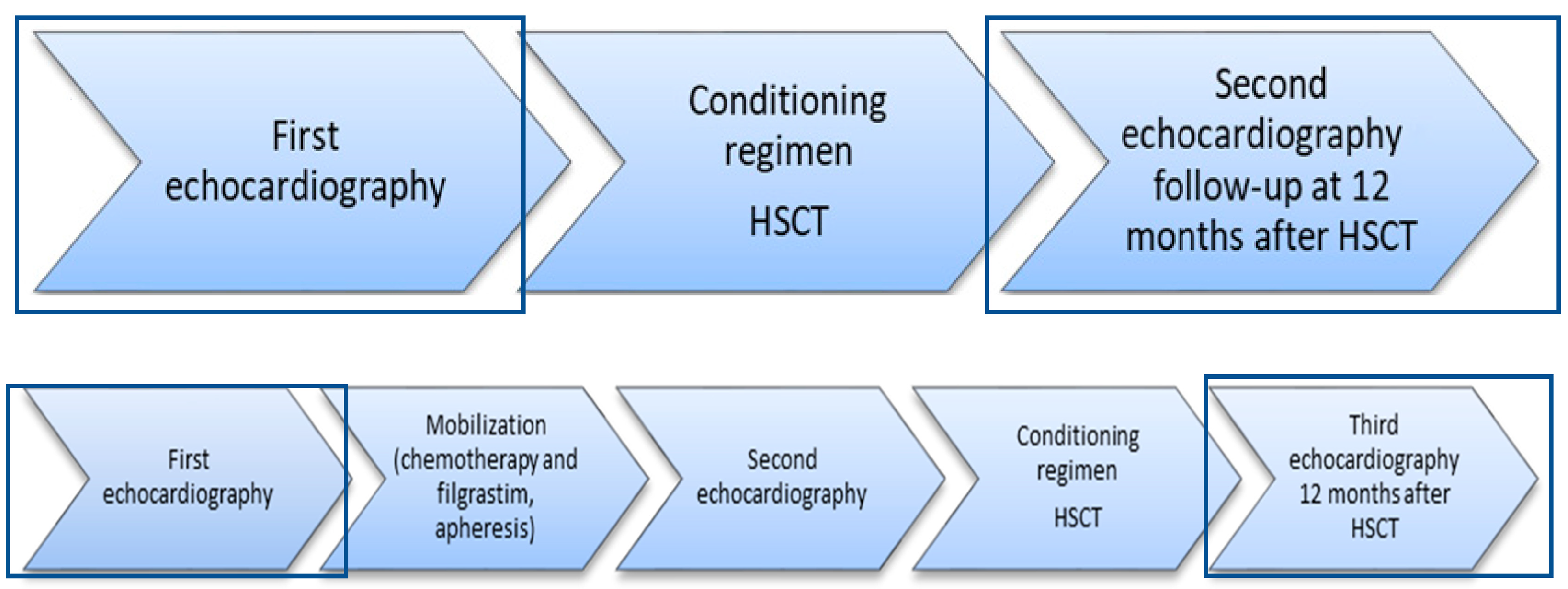
2. Materials and Methods
- Written informed consent to participate in the study;
- Patients over the age of 18 at the time of HSCT procedure.
- Previously performed HSCT;
- Refusal to participate in the study at any point.
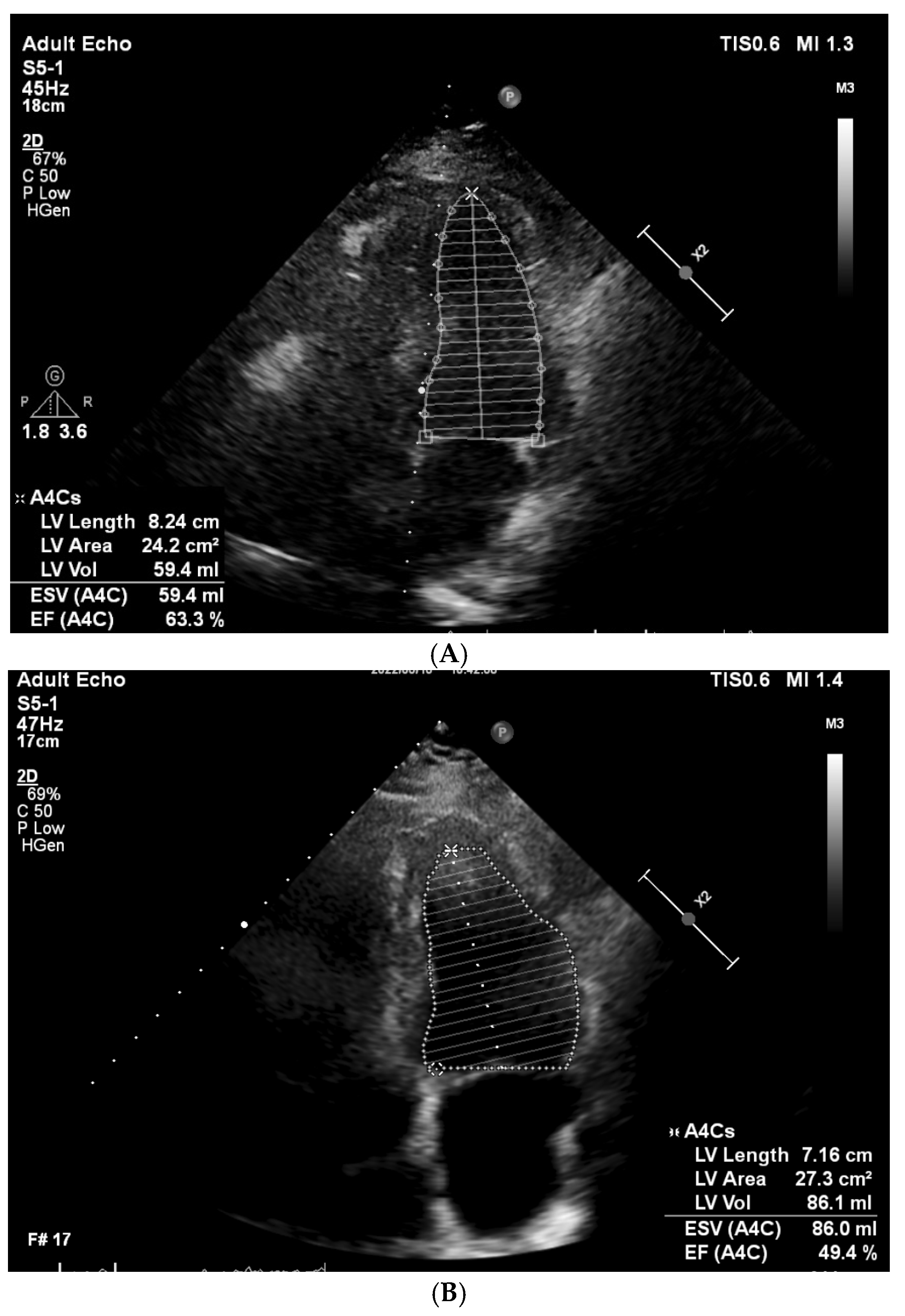
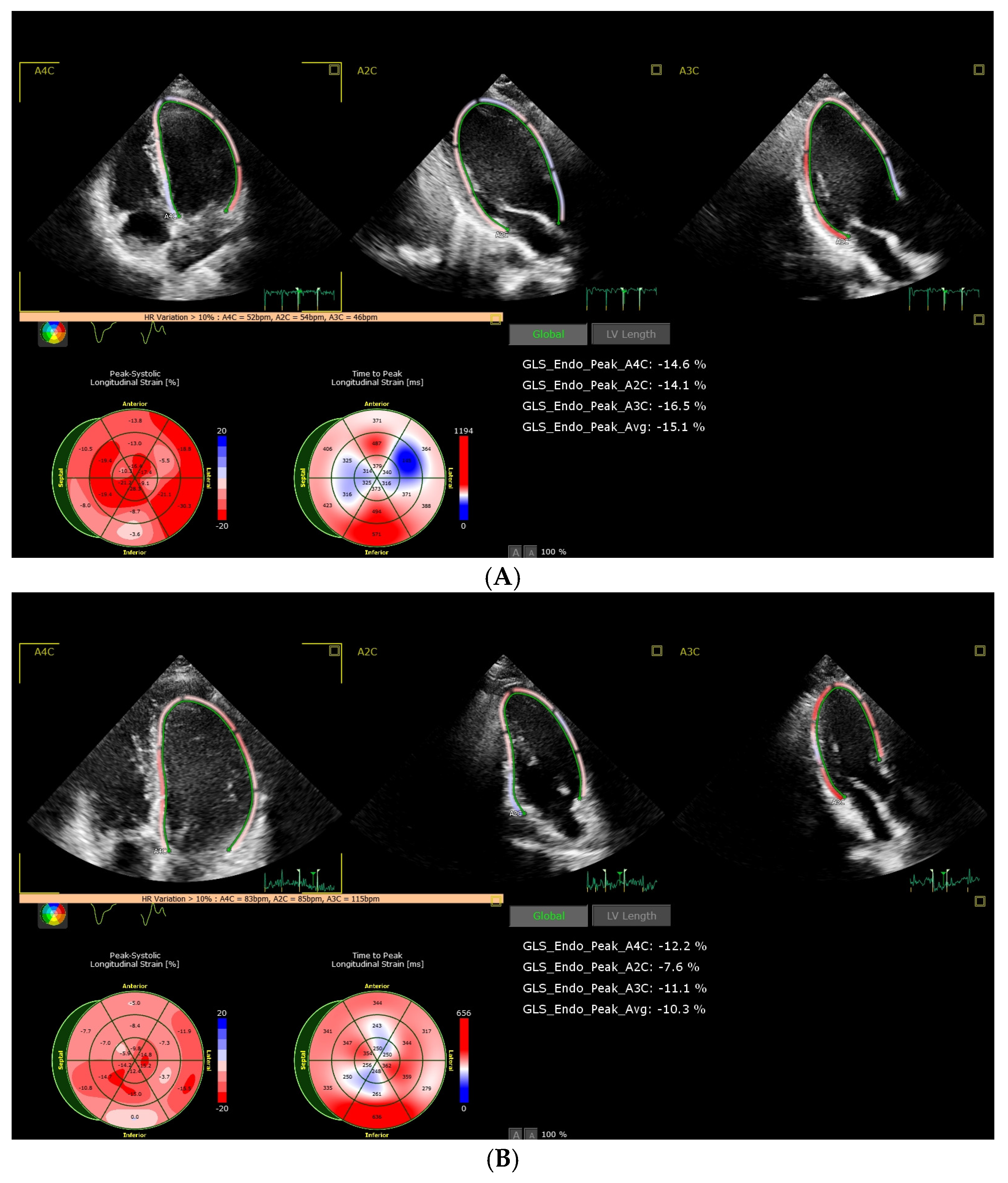
3. Results
3.1. Clinical and Demographic Characteristics
3.2. Analysis of CTRCD and Factors Influencing and Prognosing CTRCD
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; He, R.; Oerther, S.; Zhou, W.; Vosough, M.; Hassan, M. Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients. J. Pers. Med. 2022, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.D.; Hayek, S.; Rotz, S. Review of Late CV Effects After Hematopoietic Stem Cell Transplantation. Am. Coll. Cardiol. 2021. Available online: https://www.acc.org/latest-in-cardiology/articles/2021/01/25/14/28/review-of-late-cv-effects-after-hematopoietic-stem-cell-transplantation (accessed on 5 November 2024).
- Lawless, S.; Iacobelli, S.; Knelange, N.S.; Chevallier, P.; Blaise, D.; Milpied, N.; Foà, R.; Cornelissen, J.J.; Lioure, B.; Benjamin, R.; et al. Comparison of autologous and allogeneic hematopoietic cell transplantation strategies in patients with primary plasma cell leukemia, with dynamic prediction modeling. Haematologica 2022, 108, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, D.; Baldomero, H.; Bazuaye, N.; Bupp, C.; Chaudhri, N.; Corbacioglu, S.; Elhaddad, A.; Frutos, C.; Galeano, S.; Hamad, N.; et al. One and a half million hematopoietic stem cell transplants: Continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 2022, 107, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, A.; Fuji, S. Cardiac complications associated with hematopoietic stem-cell transplantation. Bone Marrow Transpl. 2021, 56, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Blaes, A.; Konety, S.; Hurley, P. Cardiovascular Complications of Hematopoietic Stem Cell Transplantation. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 25. [Google Scholar] [CrossRef]
- Rotz, S.J.; Ryan, T.D.; Hayek, S.S. Cardiovascular Disease and its Management in Children and Adults Undergoing Hematopoietic Stem Cell Transplantation. J. Thromb. Thrombolysis 2021, 51, 854. [Google Scholar] [CrossRef]
- Jacob, S.W.; de la Torre, J.C. Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacol. Rep. 2009, 61, 225–235. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Moriyama, S.; Fukata, M.; Hieda, M.; Yokoyama, T.; Yoshimoto, G.; Kusaba, H.; Nakashima, Y.; Miyamoto, T.; Maruyama, T.; Akashi, K. Early-onset cardiac dysfunction following allogeneic haematopoietic stem cell transplantation. Open Heart 2022, 9, e002007. [Google Scholar] [CrossRef] [PubMed]
- Murbraech, K.; Smeland, K.B.; Holte, H.; Loge, J.H.; Lund, M.B.; Wethal, T.; Holte, E.; Rösner, A.; Dalen, H.; Kvaløy, S.; et al. Heart failure and asymptomatic left ventricular systolic dysfunction in lymphoma survivors treated with autologous stem-cell transplantation: A national cross-sectional study. J. Clin. Oncol. 2015, 33, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, F.; Rampoldi, A.; Maxwell, J.T.; Wu, R.; Fischbach, P. Melphalan induces cardiotoxicity through oxidative stress in cardiomyocytes derived from human induced pluripotent stem cells. Stem Cell Res. Ther. 2020, 11, 470. [Google Scholar] [CrossRef]
- Thomas, S.A. Chemotherapy Agents That Cause Cardiotoxicity. US Pharm. 2017, 42, 24–33. [Google Scholar]
- Sarzhevskiy, V.; Kolesnikova, D.; Melnichenko, V.; Vakhromeeva, M. Cardiotoxicity of High-Dose Chemotherapy with Autologous Hematopoietic Stem Cells Transplantation in Patients with Malignant Lymphomas. what is Worse-Beam or Cbv? Ann. Oncol. 2014, 25, 332. [Google Scholar] [CrossRef][Green Version]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [PubMed]
- Armenian, S.H.; Sun, C.L.; Shannon, T.; Mills, G.; Francisco, L.; Venkataraman, K.; Wong, F.L.; Forman, S.J.; Bhatia, S. Incidence and Predictors of Congestive Heart Failure After Autologous Hematopoietic Cell Transplantation. Blood 2011, 118, 6023. [Google Scholar] [CrossRef]
- Fujimaki, K.; Maruta, A.; Yoshida, M.; Sakai, R.; Tanabe, J.; Koharazawa, H.; Kodama, F.; Asahina, S.; Minamizawa, M.; Matsuzaki, M.; et al. Severe Cardiac Toxicity in Hematological Stem Cell Transplantation: Predictive Value of Reduced Left Ventricular Ejection Fraction. Bone Marrow Transpl. 2001, 27, 307–310. [Google Scholar] [CrossRef][Green Version]
- Massey, R.J.; Diep, P.P.; Ruud, E.; Burman, M.M.; Kvaslerud, A.B.; Brinch, L.; Aakhus, S.; Gullestad, L.L.; Beitnes, J.O. Left Ventricular Systolic Function in Long-Term Survivors of Allogeneic Hematopoietic Stem Cell Transplantation. JACC CardioOncol. 2020, 2, 460. [Google Scholar] [CrossRef]
- Voß, F.; Nienhaus, F.; Pietrucha, S.; Ruckhäberle, E.; Fehm, T.; Melz, T.; Cramer, M.; Haberkorn, S.M.; Flögel, U.; Westenfeld, R.; et al. Anthracycline Therapy Induces an Early Decline of Cardiac Contractility in Low-Risk Patients with Breast Cancer. Cardio Oncol. 2024, 10, 43. [Google Scholar] [CrossRef]
- Di Lisi, D.; Madaudo, C.; Di Fazio, L.; Gulotta, A.; Triolo, O.F.; Galassi, A.R.; Incorvaia, L.; Russo, A.; Novo, G. Higher Incidence of Cancer Therapy-Related Cardiac Dysfunction in the COVID-19 Era: A Single Cardio-Oncology Center Experience. J. Cardiovasc. Dev. Dis. 2023, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.; Capone, V.; Cocchia, R.; Cademartiri, F.; Riccardi, F.; Arcopinto, M.; Alshahid, M.; Anwar, K.; Carafa, M.; Carbone, A.; et al. Cardiovascular Side Effects of Anthracyclines and HER2 Inhibitors among Patients with Breast Cancer: A Multidisciplinary Stepwise Approach for Prevention, Early Detection, and Treatment. J. Clin. Med. 2023, 12, 2121. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Heck, S.L.; Gulati, G. The Role of Cardioprotection in Cancer Therapy Cardiotoxicity: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 19–37. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A Review of the Pathophysiological Mechanisms of Doxorubicin-Induced Cardiotoxicity and Aging. npj Aging 2024, 10, 9. [Google Scholar] [CrossRef]
- Adão, R.; De Keulenaer, G.; Leite-Moreira, A.; Brás-Silva, C. Cardiotoxicity Associated with Cancer Therapy: Pathophysiology and Prevention. Rev. Port. Cardiol. 2013, 32, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Franco, V.I.; Lipshultz, S.E. Anthracycline-Induced Cardiotoxicity: A Review of Pathophysiology, Diagnosis, and Treatment. Curr. Treat. Options Cardio Med. 2014, 16, 315. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Fradley, M.G. Heart Failure in Patients with Cancer Treated With Anthracyclines—Revisiting the Foundation of Cardio-Oncology. JAMA Netw. Open 2023, 6, e2254677. [Google Scholar] [CrossRef] [PubMed]
- Dempke, W.C.; Zielinski, R.; Winkler, C.; Silberman, S.; Reuther, S.; Priebe, W. Anthracycline-Induced Cardiotoxicity—Are We About to Clear This Hurdle? Eur. J. Cancer 2023, 185, 94–104. [Google Scholar] [CrossRef]
- Chaulin, A.M. The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin. Life 2023, 13, 2148. [Google Scholar] [CrossRef]
- Tini, G.; Tocci, G.; Battistoni, A.; Sarocchi, M.; Pietrantoni, C.; Russo, D.; Musumeci, B.; Savoia, C.; Volpe, M.; Spallarossa, P. Role of Arterial Hypertension and Hypertension-Mediated Organ Damage in Cardiotoxicity of Anticancer Therapies. Curr. Heart Fail Rep. 2023, 20, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Vaitiekus, D.; Muckiene, G.; Vaitiekiene, A.; Maciuliene, D.; Vaiciuliene, D.; Ambrazeviciute, G.; Sereikaite, L.; Verikas, D.; Jurkevičius, R.; Juozaityte, E. Impact of arterial hypertension on Doxorubicin-Based Chemotherapy-Induced subclinical cardiac damage in breast cancer patients. Cardiovasc. Toxicol. 2020, 20, 321–327. [Google Scholar] [CrossRef] [PubMed]
| Grade | Criteria | Number of Patients |
|---|---|---|
| Severe | New LVEF reduction to <40% | 0 |
| Moderate | New LVEF reduction by ≥10% to LVEF of 40–49% | 3 |
| New LVEF reduction by <10% to LVEF of 40–49% AND new relative decline in GLS by >15% from baseline | 3 | |
| Mild | LVEF ≥50% AND new relative GLS reduction >15% from baseline | 9 |
| Sex | |
|---|---|
| Male, n (%) | 30 (54.5) |
| Female, n (%) | 25 (45.5) |
| Age, years (median (minimum–maximum)) | 61 (18–74) |
| Autologous transplantation, n (%) | 48 (87.3) |
| Main disease | |
| Multiple myeloma, n (%) | 33 (68.8) |
| Mantle cell lymphoma, n (%) | 4 (8.3) |
| Hodgkin’s lymphoma, n (%) | 2 (4.2) |
| PCNS diffuse large B cell lymphoma, n (%) | 5 (10.4) |
| Peripheral T cell lymphoma, n (%) | 1 (2.1) |
| Ewing sarcoma, n (%) | 2 (4.2) |
| NK-/T-cell lymphoma, n (%) | 1 (2.1) |
| Allogeneic transplantation, n (%) | 7 (12.7) |
| Main disease | |
| Acute myeloid leukemia, n (%) | 5 (71.4) |
| Acute myelomonocytic leukemia, n (%) | 1 (14.3) |
| MDS-EB, n (%) | 1 (14.3) |
| Characteristic | All Patients (n = 55) | Non-CTRCD (n = 40; 72.7%) | CTRCD (n = 15; 27.3%) | p |
|---|---|---|---|---|
| Age (years), median (Min–max) | 61 (18–74) | 61 (18–74) | 61 (23–74) | 0.502 |
| Sex | ||||
| Male | 30 (54.5) | 21 (52.5) | 9 (60.0) | 0.764 |
| Female | 25 (45.5) | 19 (47.5) | 6 (40.0) | |
| Disease | ||||
| Multiple myeloma | 33 (60.0) | 27 (67.5) | 6 (40.0) | 0.121 |
| Lymphoma | 12 (23.6) | 7 (17.5) | 6 (40.0) | 0.151 |
| Leukemia and MDS-EB | 7 (12.7) | 4 (10.0) | 3 (20.0) | 0.376 |
| Other diseases (Ewing sarcoma) | 2 (3.6) | 2 (5.0) | 0 (0.0) | 1.000 |
| Auto/allo | ||||
| Allogeneic HSCT | 7 (12.7) | 4 (10.0) | 3 (20.0) | 0.376 |
| Autologous HSCT | 48 (87.3) | 36 (90.0) | 12 (80.0) | |
| CVD risk factors | ||||
| CAD | 3 (5.5) | 1 (2.5) | 2 (13.3) | 0.177 |
| Arterial hypertension | 21 (38.2) | 15 (37.5) | 6 (40.0) | 1.000 |
| Diabetes mellitus | 4 (7.3) | 4 (10.0) | 0 (0.0) | 0.565 |
| Family history of CAD | 8 (14.5) | 6 (15.0) | 2 (13.3) | 1.000 |
| Dyslipidemia | 43 (78.2) | 31 (77.5) | 12 (80.0) | 1.000 |
| Previous smoking | 5 (9.1) | 4 (10.0) | 1 (6.7) | 1.000 |
| Medications | ||||
| Beta-blockers | 12 (21.8) | 10 (25.0) | 2 (13.3) | 0.477 |
| ACEis | 10 (18.2) | 7 (17.5) | 3 (20.0) | 1.000 |
| ARBs | 5 (9.1) | 3 (7.5) | 2 (13.3) | 0.606 |
| Statins | 6 (10.9) | 4 (10.0) | 2 (13.3) | 0.660 |
| Previous use of anthracyclines | 18 (32.7) | 9 (22.5) | 9 (60.0) | 0.021 |
| Conditioning regimen | ||||
| Melphalan | 35 (63.6) | 28 (70.0) | 7 (46.7) | 0.128 |
| BEAM | 7 (12.7) | 2 (5.0) | 5 (33.3) | 0.013 |
| Carmustine+TT | 5 (9.1) | 5 (12.5) | 0 (0.0) | 0.308 |
| RIC | 7 (12.7) | 4 (10.0) | 3 (20.0) | 0.376 |
| TnI, median (min–max) | 0.02 (0.02–0.64) | 0.02 (0.02–0.64) | 0.02 (0.02–0.16) | 0.958 |
| BNP, median (min–max) | 21.75 (4.00–118.70) | 19.90 (4.00–118.70) | 23.60 (9.30–56.20) | 0.719 |
| Univariate Logistic Regression | |||
|---|---|---|---|
| Covariate | OR | 95% CI | p |
| Sex (male versus female) | 1.357 | 0.407–4.529 | 0.619 |
| Multiple myeloma (myeloma versus other disease) | 0.321 | 0.094–1.095 | 0.069 |
| Lymphoma (lymphoma versus other disease) | 3.143 | 0.843–11.720 | 0.088 |
| Allogeneic HSCT (allo versus auto) | 2.250 | 0.439–11.522 | 0.330 |
| Autologous HSCT (auto versus allo) | 0.444 | 0.087–2.276 | 0.330 |
| CAD (present versus absent) | 6.000 | 0.502–71.731 | 0.157 |
| Arterial hypertension (present versus absent) | 1.111 | 0.330–3.746 | 0.865 |
| Family history of CAD (yes versus no) | 0.872 | 0.156–4.884 | 0.876 |
| Dyslipidemia (present versus absent) | 1.161 | 0.268–5.034 | 0.842 |
| Previous smoking (yes versus no) | 0.643 | 0.066–6.264 | 0.704 |
| Beta-blockers (use versus non-use) | 0.462 | 0.088–2.408 | 0.359 |
| ACEis (use versus non-use) | 1.179 | 0.262–5.310 | 0.831 |
| ARBs (use versus non-use) | 1.897 | 0.285–12.654 | 0.508 |
| Statins (use versus non-use) | 1.385 | 0.226–8.477 | 0.725 |
| Previous use of anthracyclines (use versus non-use) | 5.167 | 1.448–18.433 | 0.011 |
| Melphalan used for conditioning (use versus non-use) | 0.375 | 0.111–1.269 | 0.115 |
| BEAM used for conditioning (use versus non-use) | 9.500 | 1.599–56.426 | 0.013 |
| Multivariate Model | Bootstrap Method | |||||
|---|---|---|---|---|---|---|
| Covariate | OR | 95% CI | p | 95% Bca CI | p | |
| Risk factors | CAD | 3.701 | 0.172–79.583 | 0.403 | −20.156–38.064 | 0.085 |
| Arterial hypertension | 0.568 | 0.120–2.692 | 0.476 | −36.130–1.889 | 0.484 | |
| Family history of CAD | 0.355 | 0.035–3.642 | 0.384 | −35.882–0.743 | 0.231 | |
| Dyslipidaemia | 3.893 | 0.476–31.860 | 0.205 | −1.423–73.298 | 0.099 | |
| Previous smoking | 1.224 | 0.105–14.270 | 0.872 | −20.161–2.551 | 0.545 | |
| Previous use of anthracyclines | 3.913 | 0.712–21.501 | 0.117 | −19.701–47.749 | 0.092 | |
| BEAM | 6.654 | 0.660–67.061 | 0.108 | −0.605–42.489 | 0.039 | |
| Multivariate Model | Bootstrap Method | |||||
|---|---|---|---|---|---|---|
| Covariate | OR | 95% CI | p | 95% Bca CI | p | |
| Risk factors | CAD | 4.868 | 0.261–90.681 | 0.289 | −20.403–24.326 | 0.062 |
| Arterial hypertension | 0.614 | 0.133–2.841 | 0.533 | −35.672–2.044 | 0.552 | |
| Family history of CAD | 0.398 | 0.043–3.688 | 0.418 | −37.910–1.255 | 0.297 | |
| Dyslipidaemia | 2.729 | 0.351–21.220 | 0.337 | −1.589–55.820 | 0.257 | |
| Previous smoking | 0.858 | 0.080–9.225 | 0.900 | −20.385–1.907 | 0.549 | |
| BEAM | 14.910 | 1.764–126.038 | 0.013 | −0.469–41.661 | 0.006 | |
| Multivariate Model | Bootstrap Method | |||||
|---|---|---|---|---|---|---|
| Covariate | OR | 95% CI | p | 95% Bca CI | p | |
| Risk factors | CAD | 4.131 | 0.216–79.078 | 0.346 | −19.808–22.766 | 0.075 |
| Arterial hypertension | 0.595 | 0.133–2.662 | 0.497 | −3.286–1.312 | 0.522 | |
| Family history of CAD | 0.597 | 0.078–4.561 | 0.619 | −20.325–1.024 | 0.437 | |
| Dyslipidaemia | 2.643 | 0.419–16.692 | 0.301 | −1.072–21.330 | 0.235 | |
| Previous smoking | 1.295 | 0.112–14.945 | 0.836 | −20.105–2.645 | 0.508 | |
| Previous use of anthracyclines | 6.996 | 1.530–31.997 | 0.012 | −0.100–37.590 | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaitiekiene, A.; Kulboke, M.; Bieseviciene, M.; Kaunaite, A.; Markeviciute, A.; Bartnykaite, A.; Kireilis, B.; Rinkuniene, D.; Jankauskas, A.; Gaidamavicius, I.; et al. Evaluation of Subclinical Cancer Therapy-Related Cardiac Dysfunction in Patients Undergoing Hematopoietic Stem Cell Transplantation: An Echocardiography Study. Cancers 2024, 16, 3808. https://doi.org/10.3390/cancers16223808
Vaitiekiene A, Kulboke M, Bieseviciene M, Kaunaite A, Markeviciute A, Bartnykaite A, Kireilis B, Rinkuniene D, Jankauskas A, Gaidamavicius I, et al. Evaluation of Subclinical Cancer Therapy-Related Cardiac Dysfunction in Patients Undergoing Hematopoietic Stem Cell Transplantation: An Echocardiography Study. Cancers. 2024; 16(22):3808. https://doi.org/10.3390/cancers16223808
Chicago/Turabian StyleVaitiekiene, Audrone, Migle Kulboke, Monika Bieseviciene, Austeja Kaunaite, Auste Markeviciute, Agne Bartnykaite, Benas Kireilis, Diana Rinkuniene, Antanas Jankauskas, Ignas Gaidamavicius, and et al. 2024. "Evaluation of Subclinical Cancer Therapy-Related Cardiac Dysfunction in Patients Undergoing Hematopoietic Stem Cell Transplantation: An Echocardiography Study" Cancers 16, no. 22: 3808. https://doi.org/10.3390/cancers16223808
APA StyleVaitiekiene, A., Kulboke, M., Bieseviciene, M., Kaunaite, A., Markeviciute, A., Bartnykaite, A., Kireilis, B., Rinkuniene, D., Jankauskas, A., Gaidamavicius, I., Gerbutavicius, R., Vaitiekus, D., Sakalyte, G., & Vaskelyte, J. J. (2024). Evaluation of Subclinical Cancer Therapy-Related Cardiac Dysfunction in Patients Undergoing Hematopoietic Stem Cell Transplantation: An Echocardiography Study. Cancers, 16(22), 3808. https://doi.org/10.3390/cancers16223808







