Radiotherapy Effects on Airway Management in Patients with Nasopharyngeal Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Radiotherapy Treatment Characteristics
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mond, H.G.; Irwin, M.; Ector, H.; Proclemer, A. The world survey of cardiac pacing and cardioverter-defibrillators: Calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin. Electrophysiol. 2008, 31, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.F.; Evans, L.M.; Yousef, Z.; Penney, A.; Brown, P.N.; Perks, R. A national audit of current cardiac device policies from radiotherapy centres across the UK. Clin. Oncol. (R Coll. Radiol.) 2014, 26, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hashii, H.; Hashimoto, T.; Okawa, A.; Shida, K.; Isobe, T.; Hanmura, M.; Nishimura, T.; Aonuma, K.; Sakae, T.; Sakurai, H. Comparison of the effects of high-energy photon beam irradiation (10 and 18 MV) on 2 types of implantable cardioverter-defibrillators. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Pan, J.J.; Ng, W.T.; Zong, J.F.; Chan, L.L.; O’Sullivan, B.; Lin, S.J.; Sze, H.C.; Chen, Y.B.; Choi, H.C.; Guo, Q.J.; et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016, 122, 546–558. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal "unity of ecology and evolution" pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Lee, A.W.; Sze, W.M.; Au, J.S.; Leung, S.F.; Leung, T.W.; Chua, D.T.; Zee, B.C.; Law, S.C.; Teo, P.M.; Tung, S.Y.; et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1107–1116. [Google Scholar] [CrossRef]
- Chua, D.T.; Sham, J.S.; Kwong, D.L.; Au, G.K. Treatment outcome after radiotherapy alone for patients with Stage I-II nasopharyngeal carcinoma. Cancer 2003, 98, 74–80. [Google Scholar] [CrossRef]
- Blanchard, P.; Lee, A.; Marguet, S.; Leclercq, J.; Ng, W.T.; Ma, J.; Chan, A.T.; Huang, P.Y.; Benhamou, E.; Zhu, G.; et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015, 16, 645–655. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; LeBlanc, M.; Giri, P.G.; Fu, K.K.; Cooper, J.; Vuong, T.; Forastiere, A.A.; Adams, G.; Sakr, W.A.; Schuller, D.E.; et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998, 16, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Grégoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. S5), v187–v189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wen, Y.F.; Guo, L.; Liu, H.; Huang, P.Y.; Mo, H.Y.; Li, N.W.; Xiang, Y.Q.; Luo, D.H.; Qiu, F.; et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: Phase III randomized trial. J. Natl. Cancer Inst. 2011, 103, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Ribassin-Majed, L.; Marguet, S.; Lee, A.W.M.; Ng, W.T.; Ma, J.; Chan, A.T.C.; Huang, P.Y.; Zhu, G.; Chua, D.T.T.; Chen, Y.; et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis. J. Clin. Oncol. 2017, 35, 498–505. [Google Scholar] [CrossRef]
- Peng, G.; Wang, T.; Yang, K.Y.; Zhang, S.; Zhang, T.; Li, Q.; Han, J.; Wu, G. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother. Oncol. 2012, 104, 286–293. [Google Scholar] [CrossRef]
- Lee, A.W.; Ng, W.T.; Chan, L.L.; Hung, W.M.; Chan, C.C.; Sze, H.C.; Chan, O.S.; Chang, A.T.; Yeung, R.M. Evolution of treatment for nasopharyngeal cancer—success and setback in the intensity-modulated radiotherapy era. Radiother. Oncol. 2014, 110, 377–384. [Google Scholar] [CrossRef]
- Kam, M.K.; Leung, S.F.; Zee, B.; Chau, R.M.; Suen, J.J.; Mo, F.; Lai, M.; Ho, R.; Cheung, K.Y.; Yu, B.K.; et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J. Clin. Oncol. 2007, 25, 4873–4879. [Google Scholar] [CrossRef]
- Pow, E.H.; Kwong, D.L.; McMillan, A.S.; Wong, M.C.; Sham, J.S.; Leung, L.H.; Leung, W.K. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int. J. Radiat. Oncol. Biol. Phys. S 2006, 66, 981–991. [Google Scholar] [CrossRef]
- Groenewold, M.D.; Olthof, C.G.; Bosch, D.J. Anaesthesia after neoadjuvant chemotherapy, immunotherapy or radiotherapy. BJA Educ. 2022, 22, 12–19. [Google Scholar] [CrossRef]
- Ristau, J.; Thiel, M.; Katayama, S.; Schlampp, I.; Lang, K.; Häfner, M.F.; Herfarth, K.; Debus, J.; Koerber, S.A. Simultaneous integrated boost concepts in definitive radiation therapy for esophageal cancer: Outcomes and toxicity. Radiat. Oncol. 2021, 16, 23. [Google Scholar] [CrossRef]
- Gudaitytė, J.; Dvylys, D.; Šimeliūnaitė, I. Anaesthetic challenges in cancer patients: Current therapies and pain management. Acta Med. Litu. 2017, 24, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.M.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Uzun, D.D.; Tryjanowski, T.; Arians, N.; Mohr, S.; Schmitt, F.C.F.; Michalski, C.W.; Weigand, M.A.; Debus, J.; Lang, K. Impact of Radiotherapy on Endotracheal Intubation Quality Metrics in Patients with Esophageal Cancer: A Challenge for Advanced Airway Management? Cancers 2024, 16, 2540. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.E.; Camiré, D.; Hwang, P.H.; Nekhendzy, V. Difficult Tracheal Intubation and Airway Outcomes after Radiation for Nasopharyngeal Carcinoma. Laryngoscope 2024, 134, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Oglesby, F.; Kane, A.D.; Armstrong, R.A.; Kursumovic, E.; Soar, J. Airway and respiratory complications during anaesthesia and associated with peri-operative cardiac arrest as reported to the 7th National Audit Project of the Royal College of Anaesthetists. Anaesthesia 2024, 79, 368–379. [Google Scholar] [CrossRef]
- Cook, T.M.; Woodall, N.; Frerk, C. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br. J. Anaesth. 2011, 106, 617–631. [Google Scholar] [CrossRef]
- Uzun, D.D.; Hezel, F.; Weigand, M.A.; Schmitt, F.C.F. Apnoische Oxygenierung—Sicherheit durch Standard! Notfall+ Rettungsmedizin 2024, 1–2. [Google Scholar] [CrossRef]
- Jain, D.; Khan Joad, A.S. Head and neck radiotherapy—A risk factor for anaesthesia? Indian J. Anaesth. 2020, 64, 488–494. [Google Scholar] [CrossRef]
- Jayaraj, A.K.; Siddiqui, N.; Abdelghany, S.M.O.; Balki, M. Management of difficult and failed intubation in the general surgical population: A historical cohort study in a tertiary care centre. Can. J. Anaesth. 2022, 69, 427–437. [Google Scholar] [CrossRef]
- Schnittker, R.; Marshall, S.D.; Berecki-Gisolf, J. Patient and surgery factors associated with the incidence of failed and difficult intubation. Anaesthesia 2020, 75, 756–766. [Google Scholar] [CrossRef]
- Yen, T.T.; Lin, C.H.; Jiang, R.S.; Shih, Y.T.; Yen, H.R.; Liang, K.L. Incidence of late-onset pneumonia in patients after treatment with radiotherapy for nasopharyngeal carcinoma: A nationwide population-based study. Head Neck 2015, 37, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Apfelbaum, J.L.; Hagberg, C.A.; Caplan, R.A.; Blitt, C.D.; Connis, R.T.; Nickinovich, D.G.; Hagberg, C.A.; Caplan, R.A.; Benumof, J.L.; Berry, F.A.; et al. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013, 118, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Ezri, T.; Warters, R.D.; Szmuk, P.; Saad-Eddin, H.; Geva, D.; Katz, J.; Hagberg, C. The incidence of class "zero" airway and the impact of Mallampati score, age, sex, and body mass index on prediction of laryngoscopy grade. Anesth. Analg. 2001, 93, 1073–1075, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, J.B.; Lemmens, H.J.; Brock-Utne, J.G.; Vierra, M.; Saidman, L.J. Morbid obesity and tracheal intubation. Anesth. Analg. 2002, 94, 732–736, table of contents. [Google Scholar] [CrossRef]
- Bond, A. Obesity and difficult intubation. Anaesth. Intensive Care 1993, 21, 828–830. [Google Scholar] [CrossRef]
- Rose, D.K.; Cohen, M.M. The airway: Problems and predictions in 18,500 patients. Can. J. Anaesth. 1994, 41, 372–383. [Google Scholar] [CrossRef]
- Rocke, D.A.; Murray, W.B.; Rout, C.C.; Gouws, E. Relative Risk Analysis of Factors Associated with Difficult Intubation in Obstetric Anesthesia. Anesthesiology 1992, 77, 67–73. [Google Scholar] [CrossRef]
- Kheterpal, S.; Han, R.; Tremper, K.K.; Shanks, A.; Tait, A.R.; O’Reilly, M.; Ludwig, T.A. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 2006, 105, 885–891. [Google Scholar] [CrossRef]
- Martin, L.D.; Mhyre, J.M.; Shanks, A.M.; Tremper, K.K.; Kheterpal, S. 3423 emergency tracheal intubations at a university hospital: Airway outcomes and complications. Anesthesiology 2011, 114, 42–48. [Google Scholar] [CrossRef]
- Mort, T.C. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg 2004, 99, 607–613, table of contents. [Google Scholar] [CrossRef]
- Sakles, J.C.; Chiu, S.; Mosier, J.; Walker, C.; Stolz, U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad. Emerg. Med. 2013, 20, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Noppens, R.R.; Turkstra, T.; Payne, S.; Kunitz, O.; Tzanova, I.; Schmidtmann, I. A multicentre randomised controlled trial of the McGrath™ Mac videolaryngoscope versus conventional laryngoscopy. Anaesthesia 2023, 78, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Lang, P.; Lang, C.; Schmidtmann, I.; Kunitz, O.; Roth, M.; Strate, M.; Schmutz, A.; Vits, E.; Balogh, O.; et al. A comparison of the McGrath videolaryngoscope with direct laryngoscopy for rapid sequence intubation in the operating theatre: A multicentre randomised controlled trial. Anaesthesia 2024, 79, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Köhl, V.; Wünsch, V.A.; Müller, M.C.; Sasu, P.B.; Dohrmann, T.; Peters, T.; Tolkmitt, J.; Dankert, A.; Krause, L.; Zöllner, C.; et al. Hyperangulated vs. Macintosh videolaryngoscopy in adults with anticipated difficult airway management: A randomised controlled trial. Anaesthesia 2024, 79, 957–966. [Google Scholar] [CrossRef]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85–94. [Google Scholar] [CrossRef]
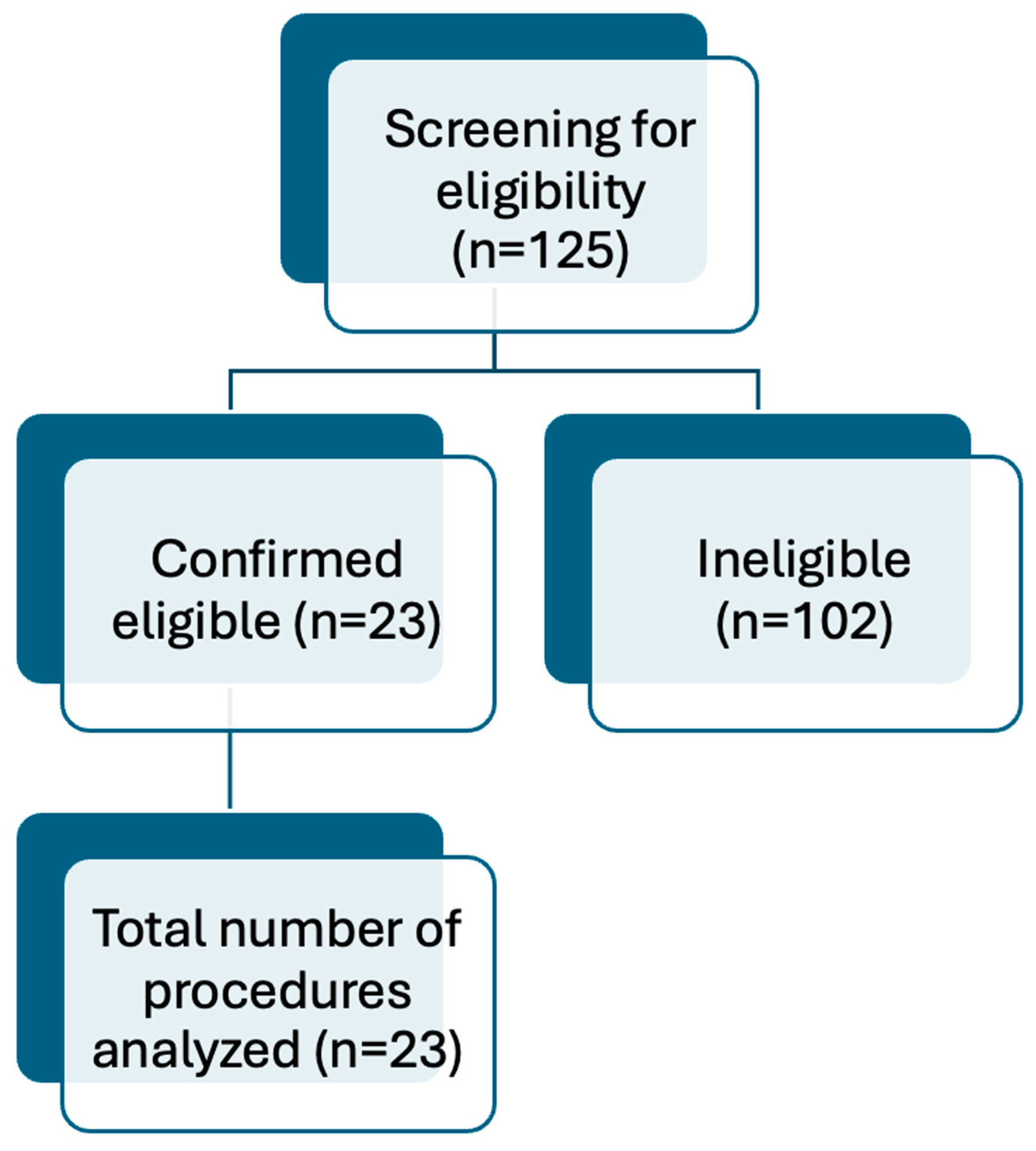
| Characteristics | No. of Patients (%) |
|---|---|
| Gender | |
| Female | 6 (26.1%) |
| Male | 17 (73.9%) |
| Body size | |
| Mean (range) | 172 cm (153–187 cm) |
| Body weight | |
| Mean (range) | 75.8 kg (47–110 kg) |
| Body Mass Index | |
| Mean (range) | 25.7 kg/m2 (17–35.9 kg/m2) |
| Age at RT | |
| Mean (range) | 52.9 (32–85 years) |
| Histology | |
| Squamous cell carcinoma | 23 (100%) |
| T-stage | |
| T1 | 5 (21.7%) |
| T2 | 4 (17.4%) |
| T3 | 4 (17.4%) |
| T4 | 10 (43.5%) |
| N-stage | |
| N0 | 4 (17.4%) |
| N+ | 19 (82.6%) |
| M-stage | |
| M0 | 23 (100%) |
| M1 | 0 (0%) |
| Cardiovascular risk factors | |
| Hypertension | 9 (39.1%) |
| Pulmonary diseases | 1 (4.3%) |
| Diabetes mellitus | 2 (8.7%) |
| Smoking | 9 (39.1%) |
| Coronary artery disease | 2 (8.7%) |
| Heart failure | 2 (8.7%) |
| Radiotherapy Treatment Characteristics | n (%) |
|---|---|
| RT technique | |
| Bimodal radiotherapy | 12 (52.2%) |
| IMRT | 11 (47.8%) |
| Therapy regimes | |
| Median total dose main plan and boost plan | 68.15 Gy (range: 51.0–74.0 Gy) |
| Median single dose main plan and boost plan | 1.9 Gy (range: 1.8–3.0 Gy) |
| Irradiation cervical lymph nodes | |
| Yes | 23 (100.0%) |
| No | 0 (0%) |
| Mean PTV volume | 918.01 ccm (range 656.3 ccm–1852.0 ccm) |
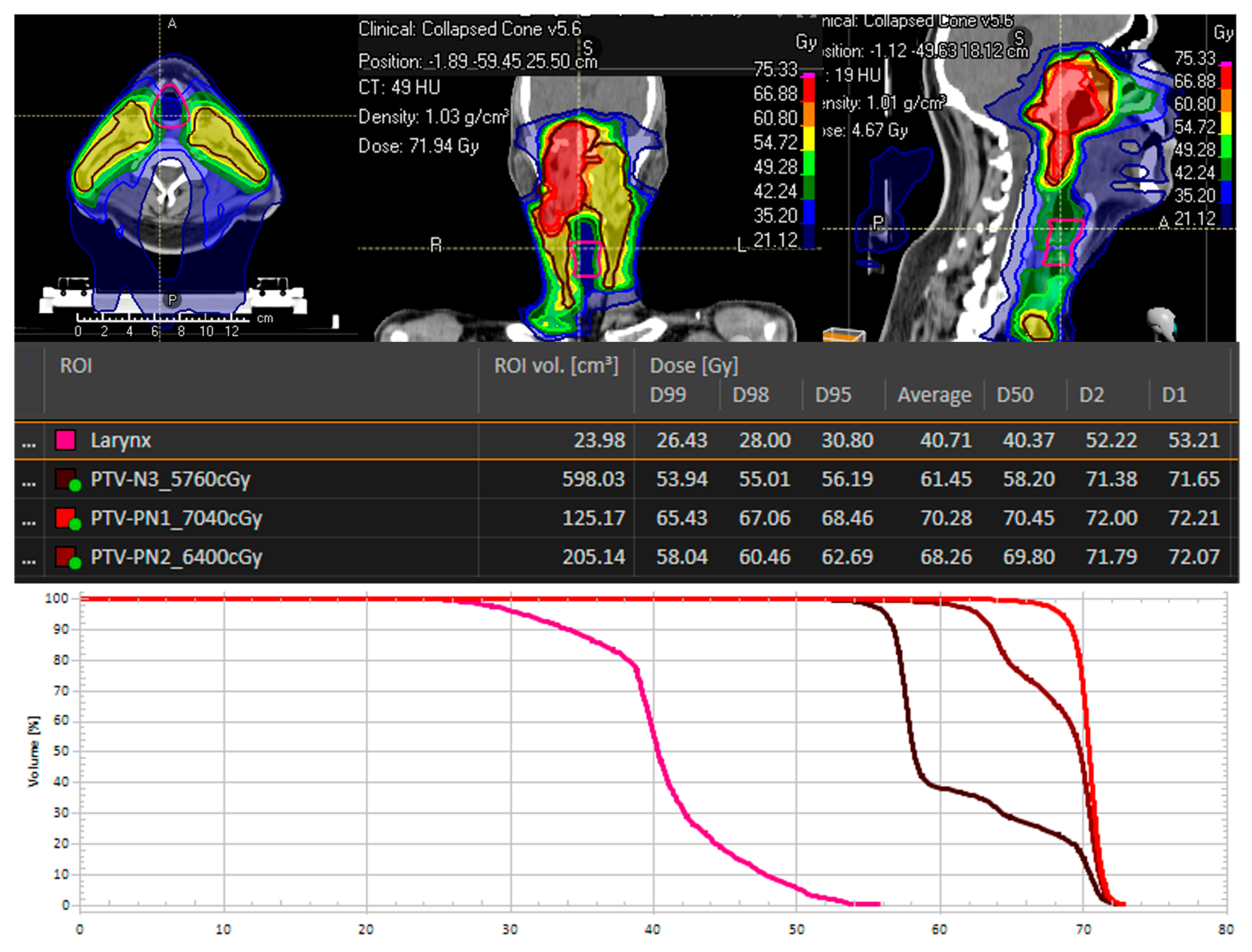
| Mean total dose laryngeal | 53.53 Gy (range 37.67–61.1 Gy) |
| Dmax laryngeal | 66.61 Gy (range 62.9–68.9 Gy) |
| Dmin laryngeal | 50.62 Gy (range 26.69–58.3 Gy) |
| Mean time RT until surgery | 170.7 weeks (range 15–497 days) |
| Reason for surgery | |
| Local recurrence resection | 15 (65.2%) |
| Metastasis resection liver, brain, distant lymph nodes | 6 (26.1%) |
| Cerebral biopsy | 2 (8.7%) |
| Anesthesia Characteristics | n (%) |
|---|---|
| ASA physical status | |
| I (Healthy) | 0 (0.0%) |
| II (Mild systemic illness) | 7 (30.4%) |
| III (Severe systemic illness) | 15 (65.2%) |
| IV (Life-threatening systemic illness) | 1 (4.4%) |
| Mallampati score | |
| I (Soft palate, uvula, pillars visible) | 6 (26.1%) |
| II (Soft palate, major part of uvula visible) | 12 (52.2%) |
| III (Soft palate, base of uvula visible) | 4 (17.3%) |
| IV (Only hard palate visible) | 1 (4.4%) |
| Mouth opening | |
| <3 cm | 2 (8.7%) |
| >3 cm | 21 (91.3%) |
| Neck range of motion | |
| Full | 22 (95.6%) |
| Limited | 1 (4.4%) |
| Mask ventilation | |
| Easy | 21 (95.5%) |
| Difficult | 1 (4.5%) |
| Intubation technique | |
| Direct laryngoscopy | 16 (69.6%) |
| Video laryngoscopy | 6 (26.1%) |
| Fiberoptic | 1 (4.3%) |
| Tracheostomy | 0 (0.0%) |
| First-pass intubation success | |
| Yes | 20 (87.0%) |
| No | 3 (13.0%) |
| Cormack/Lehane classification | |
| I | 11 (47.8%) |
| II | 10 (43.5%) |
| III | 2 (8.7%) |
| IV | 0 (0.0%) |
| Hypnotic drugs | |
| Propofol | 23 (100%) |
| Analgetic drugs | |
| Fentanyl | 4 (17.4%) |
| Sufentanil | 19 (82.6%) |
| Neuromuscular blocking drugs | |
| Rocuronium | 9 (39.1%) |
| Atracurium | 8 (34.8%) |
| Mivacurium | 6 (26.1%) |
| Anesthesia type | |
| Volatile anesthetics | 15 (65.2%) |
| Total intravenous anesthesia | 8 (34.8%) |
| Complications During Anesthesia | n (%) |
|---|---|
| Oropharyngeal bleeding | |
| Yes | 1 (4.3%) |
| No | 22 (95.7%) |
| Severe hypoxemia | |
| Yes | 0 (0.0%) |
| No | 23 (100%) |
| Aspiration | |
| Yes | 0 (0.0%) |
| No | 23 (100%) |
| Peri-operative mortality | |
| Yes | 0 (0.0%) |
| No | 23 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzun, D.D.; Zimmermann, T.N.; Schmitt, F.C.F.; Plinkert, P.K.; Weigand, M.A.; Debus, J.; Held, T.; Uzun-Lang, K. Radiotherapy Effects on Airway Management in Patients with Nasopharyngeal Cancer. Cancers 2024, 16, 3781. https://doi.org/10.3390/cancers16223781
Uzun DD, Zimmermann TN, Schmitt FCF, Plinkert PK, Weigand MA, Debus J, Held T, Uzun-Lang K. Radiotherapy Effects on Airway Management in Patients with Nasopharyngeal Cancer. Cancers. 2024; 16(22):3781. https://doi.org/10.3390/cancers16223781
Chicago/Turabian StyleUzun, Davut D., Timo N. Zimmermann, Felix C. F. Schmitt, Peter K. Plinkert, Markus A. Weigand, Juergen Debus, Thomas Held, and Kristin Uzun-Lang. 2024. "Radiotherapy Effects on Airway Management in Patients with Nasopharyngeal Cancer" Cancers 16, no. 22: 3781. https://doi.org/10.3390/cancers16223781
APA StyleUzun, D. D., Zimmermann, T. N., Schmitt, F. C. F., Plinkert, P. K., Weigand, M. A., Debus, J., Held, T., & Uzun-Lang, K. (2024). Radiotherapy Effects on Airway Management in Patients with Nasopharyngeal Cancer. Cancers, 16(22), 3781. https://doi.org/10.3390/cancers16223781









