Asymmetry of the Frontal Aslant Tract and Development of Supplementary Motor Area Syndrome
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Outcome Measures
2.3. Imaging and Tractography Data
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Sample Size and Exclusion Report
3.2. Patient Variables and SMA Syndrome Incidence
3.3. Anatomical, Surgical, and Pathological Variables
3.4. Functional Outcomes
3.5. Interhemispheric Differences of FAT
3.6. Verbal SMA Syndrome and Interhemispheric FAT Asymmetry
3.7. FAT Variables Associated with SMA Syndrome Onset
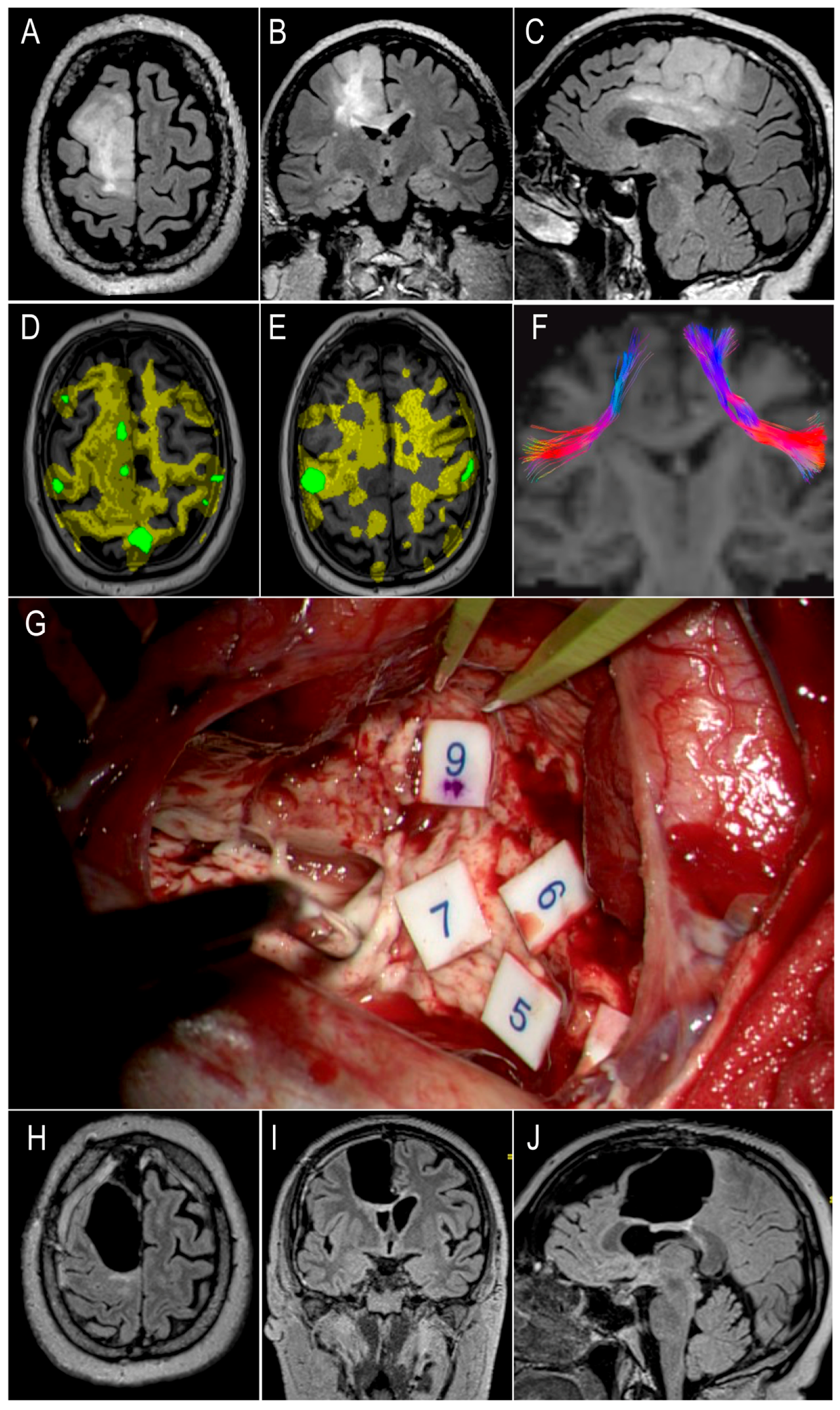
3.8. Illustrative Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffau, H.; Capelle, L. Preferential Brain Locations of Low-Grade Gliomas: Comparison with Glioblastomas and Review of Hypothesis. Cancer 2004, 100, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Pinson, H.; Van Lerbeirghe, J.; Vanhauwaert, D.; Van Damme, O.; Hallaert, G.; Kalala, J.-P. The Supplementary Motor Area Syndrome: A Neurosurgical Review. Neurosurg. Rev. 2022, 45, 81–90. [Google Scholar] [CrossRef]
- Vergani, F.; Lacerda, L.; Martino, J.; Attems, J.; Morris, C.; Mitchell, P.; de Schotten, M.T.; Dell’Acqua, F. White Matter Connections of the Supplementary Motor Area in Humans. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Freyschlag, C.F.; Rech, F. Motor Control. In Intraoperative Mapping of Cognitive Networks: Which Tasks for Which Locations; Mandonnet, E., Herbet, G., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–19. ISBN 978-3-030-75071-8. [Google Scholar]
- Satoer, D.; De Witte, E.; Dragoy, O. Spontaneous Speech. In Intraoperative Mapping of Cognitive Networks: Which Tasks for Which Locations; Mandonnet, E., Herbet, G., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 95–114. ISBN 978-3-030-75071-8. [Google Scholar]
- Penfield, W. The Supplementary Motor Area of The Cerebral Cortex: A Clinical and Experimental Study. AMA Arch. NeurPsych 1951, 66, 289. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; Haider, A.S.; Balasubramanian, K.; Dadario, N.B.; Robertson, F.C.; Silverstein, J.W.; D’Amico, R.S. Supplementary Motor Area Syndrome after Brain Tumor Surgery: A Systematic Review. World Neurosurg. 2022, 165, 160–171.e2. [Google Scholar] [CrossRef]
- Laplane, D.; Talairach, J.; Meininger, V.; Bancaud, J.; Orgogozo, J.M. Clinical Consequences of Corticectomies Involving the Supplementary Motor Area in Man. J. Neurol. Sci. 1977, 34, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Kinoshita, M.; Yahata, T.; Nakada, M. Recovery Time from Supplementary Motor Area Syndrome: Relationship to Postoperative Day 7 Paralysis and Damage of the Cingulum. J. Neurosurg. 2020, 132, 865–874. [Google Scholar] [CrossRef]
- Krainik, A.; Lehericy, S.; Duffau, H.; Vlaicu, M.; Poupon, F.; Capelle, L.; Cornu, P.; Clemenceau, S.; Sahel, M.; Valery, C.-A.; et al. Role of the Supplementary Motor Area in Motor Deficit Following Medial Frontal Lobe Surgery. Neurology 2001, 57, 871–878. [Google Scholar] [CrossRef]
- Kinoshita, M.; de Champfleur, N.M.; Deverdun, J.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Role of Fronto-Striatal Tract and Frontal Aslant Tract in Movement and Speech: An Axonal Mapping Study. Brain Struct. Funct. 2015, 220, 3399–3412. [Google Scholar] [CrossRef]
- Dragoy, O.; Zyryanov, A.; Bronov, O.; Gordeyeva, E.; Gronskaya, N.; Kryuchkova, O.; Klyuev, E.; Kopachev, D.; Medyanik, I.; Mishnyakova, L.; et al. Functional Linguistic Specificity of the Left Frontal Aslant Tract for Spontaneous Speech Fluency: Evidence from Intraoperative Language Mapping. Brain Lang. 2020, 208, 104836. [Google Scholar] [CrossRef]
- Catani, M.; Dell’Acqua, F.; Vergani, F.; Malik, F.; Hodge, H.; Roy, P.; Valabregue, R.; Thiebaut de Schotten, M. Short Frontal Lobe Connections of the Human Brain. Cortex 2012, 48, 273–291. [Google Scholar] [CrossRef] [PubMed]
- La Corte, E.; Eldahaby, D.; Greco, E.; Aquino, D.; Bertolini, G.; Levi, V.; Ottenhausen, M.; Demichelis, G.; Romito, L.M.; Acerbi, F.; et al. The Frontal Aslant Tract: A Systematic Review for Neurosurgical Applications. Front. Neurol. 2021, 12, 641586. [Google Scholar] [CrossRef] [PubMed]
- Landers, M.J.F.; Meesters, S.P.L.; van Zandvoort, M.; de Baene, W.; Rutten, G.-J.M. The Frontal Aslant Tract and Its Role in Executive Functions: A Quantitative Tractography Study in Glioma Patients. Brain Imaging Behav. 2022, 16, 1026–1039. [Google Scholar] [CrossRef]
- Young, J.S.; Gogos, A.J.; Aabedi, A.A.; Morshed, R.A.; Pereira, M.P.; Lashof-Regas, S.; Mansoori, Z.; Luks, T.; Hervey-Jumper, S.L.; Villanueva-Meyer, J.E.; et al. Resection of Supplementary Motor Area Gliomas: Revisiting Supplementary Motor Syndrome and the Role of the Frontal Aslant Tract. J. Neurosurg. 2022, 136, 1278–1284. [Google Scholar] [CrossRef]
- Oda, K.; Yamaguchi, F.; Enomoto, H.; Higuchi, T.; Morita, A. Prediction of Recovery from Supplementary Motor Area Syndrome after Brain Tumor Surgery: Preoperative Diffusion Tensor Tractography Analysis and Postoperative Neurological Clinical Course. Neurosurg. Focus 2018, 44, E3. [Google Scholar] [CrossRef]
- Dell’Acqua, F.; Tournier, J.D. Modelling White Matter with Spherical Deconvolution: How and Why? NMR Biomed. 2019, 32, e3945. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, A.S.; Beyh, A.; Lavrador, J.P.; Howells, H.; Dell’Acqua, F.; Vergani, F. Altered Corticospinal Microstructure and Motor Cortex Excitability in Gliomas: An Advanced Tractography and Transcranial Magnetic Stimulation Study. J. Neurosurg. 2021, 134, 1368–1376. [Google Scholar] [CrossRef]
- Al-Khawaja, I.; Wade, D.T.; Collin, C.F. Bedside Screening for Aphasia: A Comparison of Two Methods. J. Neurol. 1996, 243, 201–204. [Google Scholar] [CrossRef]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; VandenBerg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. JCO 2008, 26, 1338–1345. [Google Scholar] [CrossRef]
- Veraart, J.; Fieremans, E.; Novikov, D.S. Diffusion MRI Noise Mapping Using Random Matrix Theory. Magn. Reson. Med. 2016, 76, 1582–1593. [Google Scholar] [CrossRef]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-ringing Artifact Removal Based on Local Subvoxel-shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.R.; Sotiropoulos, S.N. An Integrated Approach to Correction for Off-Resonance Effects and Subject Movement in Diffusion MR Imaging. NeuroImage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Komaitis, S.; Koutsarnakis, C.; Lani, E.; Kalamatianos, T.; Drosos, E.; Skandalakis, G.P.; Liakos, F.; Liouta, E.; Kalyvas, A.V.; Stranjalis, G. Deciphering the Frontostriatal Circuitry through the Fiber Dissection Technique: Direct Structural Evidence on the Morphology and Axonal Connectivity of the Fronto-Caudate Tract. J. Neurosurg. 2021, 135, 815–827. [Google Scholar] [CrossRef]
- Bozkurt, B.; Yagmurlu, K.; Middlebrooks, E.H.; Karadag, A.; Ovalioglu, T.C.; Jagadeesan, B.; Sandhu, G.; Tanriover, N.; Grande, A.W. Microsurgical and Tractographic Anatomy of the Supplementary Motor Area Complex in Humans. World Neurosurg. 2016, 95, 99–107. [Google Scholar] [CrossRef]
- Dell’Acqua, F.; Simmons, A.; Williams, S.C.R.; Catani, M. Can Spherical Deconvolution Provide More Information than Fiber Orientations? Hindrance Modulated Orientational Anisotropy, a True-Tract Specific Index to Characterize White Matter Diffusion: Hindrance Modulated Orientational Anisotropy. Hum. Brain Mapp. 2013, 34, 2464–2483. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and Molecular Epidemiology of Adult Diffuse Glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24, v1–v95. [Google Scholar] [CrossRef]
- Ibe, Y.; Tosaka, M.; Horiguchi, K.; Sugawara, K.; Miyagishima, T.; Hirato, M.; Yoshimoto, Y. Resection Extent of the Supplementary Motor Area and Post-Operative Neurological Deficits in Glioma Surgery. Br. J. Neurosurg. 2016, 30, 323–329. [Google Scholar] [CrossRef]
- Russell, S.M.; Kelly, P.J. Incidence and Clinical Evolution of Postoperative Deficits after Volumetric Stereotactic Resection of Glial Neoplasms Involving the Supplementary Motor Area. Neurosurgery 2003, 52, 506–516. [Google Scholar] [CrossRef]
- Young, J.S.; Morshed, R.A.; Gogos, A.J.; Amara, D.; Villanueva-Meyer, J.E.; Berger, M.S.; Hervey-Jumper, S.L. The Glioma-Network Interface: A Review of the Relationship Between Glioma Molecular Subtype and Intratumoral Function. Neurosurgery 2020, 87, 1078–1084. [Google Scholar] [CrossRef]
- De Benedictis, A.; Duffau, H. Brain Hodotopy: From Esoteric Concept to Practical Surgical Applications. Neurosurgery 2011, 68, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Rech, F.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Somatotopic Organization of the White Matter Tracts Underpinning Motor Control in Humans: An Electrical Stimulation Study. Brain Struct. Funct. 2016, 221, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Forkel, S.J.; Thiebaut de Schotten, M.; Dell’Acqua, F.; Kalra, L.; Murphy, D.G.M.; Williams, S.C.R.; Catani, M. Anatomical Predictors of Aphasia Recovery: A Tractography Study of Bilateral Perisylvian Language Networks. Brain 2014, 137, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
| Variable | SMA Syndrome (N = 12) | No Syndrome (N = 13) | Total (N = 25) | Statistics | ||
|---|---|---|---|---|---|---|
| Mean Age (SD) | 45 yrs (14 yrs) | 43 yrs (15 yrs) | 44 yrs (14 yrs) | p = 0.682 | ||
| Gender | Female | 7 (58%) | 6 (46%) | 13 (52%) | p = 0.695 | |
| Male | 5 (42%) | 7 (54%) | 12 (48%) | |||
| Handedness | Left | 1 (8%) | 1 (8%) | 2 (8%) | p = 1.000 | |
| Right | 11 (92%) | 12 (92%) | 23 (92%) | |||
| Tumor Side | Dominant | 6 (50%) | 2 (15%) | 8 (32%) | p = 0.097 | |
| Nondominant | 6 (50%) | 11 (85%) | 17 (68%) | |||
| SMA-Proper Involvement | 9 (75%) | 9 (75%) | 18 (72%) | p = 1.000 | ||
| Pre-SMA Involvement | 11 (92%) | 11 (92%) | 22 (88%) | p = 1.000 | ||
| Global SMA Involvement | 8 (67%) | 8 (67%) | 15 (60%) | p = 0.688 | ||
| Cingulate Involvement | 9 (75%) | 9 (75%) | 19 (76%) | p = 1.000 | ||
| Awake Craniotomy | 9 (75%) | 9 (75%) | 18 (72%) | OR 1.33 95% CI 0.23–7.74 | ||
| Median Preoperative Volume (IR) | 66.15 cm3 (89.3 cm3) | 46.10 cm3 (27.90 cm3) | 46.80 cm3 (54.65 cm3) | p = 0.320 | ||
| Median Residue Volume (IR) | 1.30 cm3 (8.8 cm3) | 0.70 cm3 (4.25 cm3) | 0.70 cm3 (6.35 cm3) | p = 0.852 | ||
| Median Resection Volume (IR) | 50.25 cm3 (74.82 cm3) | 43.80 cm3 (25.40 cm3) | 43.80 cm3 (44.95 cm3) | p = 0.503 | ||
| Median Eor (IR) | 97.27% (9.66%) | 96.51% (9.60%) | 96.51% (8.70%) | p = 0.936 | ||
| Median Postoperative LOS (IR) | 6.5 days (4 days) | 3 days (3 days) | 5 days (5 days) | p = 0.007 | ||
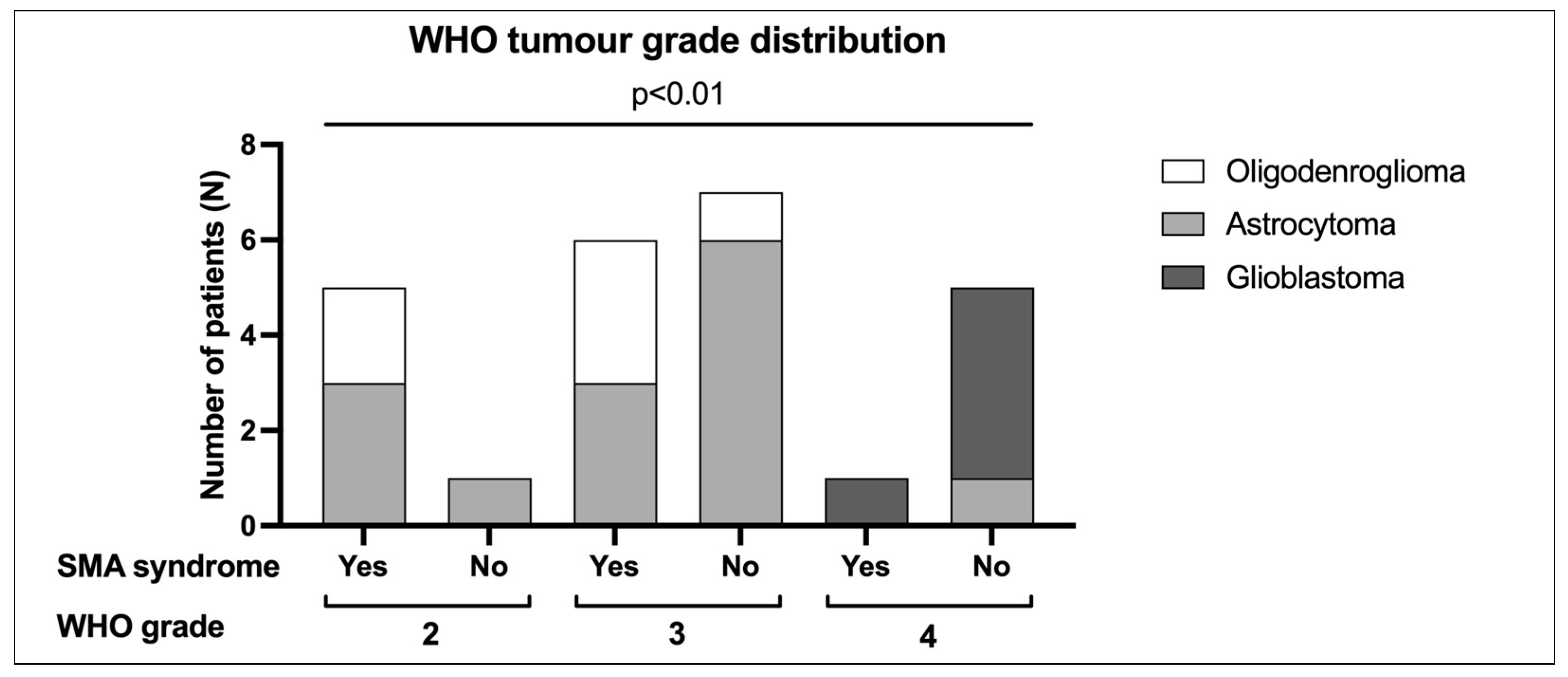
| IDH-Mut, 1p/19q Codel Oligodendroglioma | Grade 2 | 2 (17%) | 0 (0%) | 2 (8%) | p = 0.005 | |
| Grade 3 | 3 (25%) | 1 (8%) | 4 (16%) | |||
| IDH-Mut Astrocytoma | Grade 2 | 3 (25%) | 1 (8%) | 4 (16%) | ||
| Grade 3 | 3 (25%) | 6 (46%) | 9 (36%) | |||
| Grade 4 | 0 (0%) | 1 (8%) | 1 (4%) | |||
| IDH-Wt Glioblastoma | Grade 4 | 1 (8%) | 4 (31%) | 5 (20%) | ||
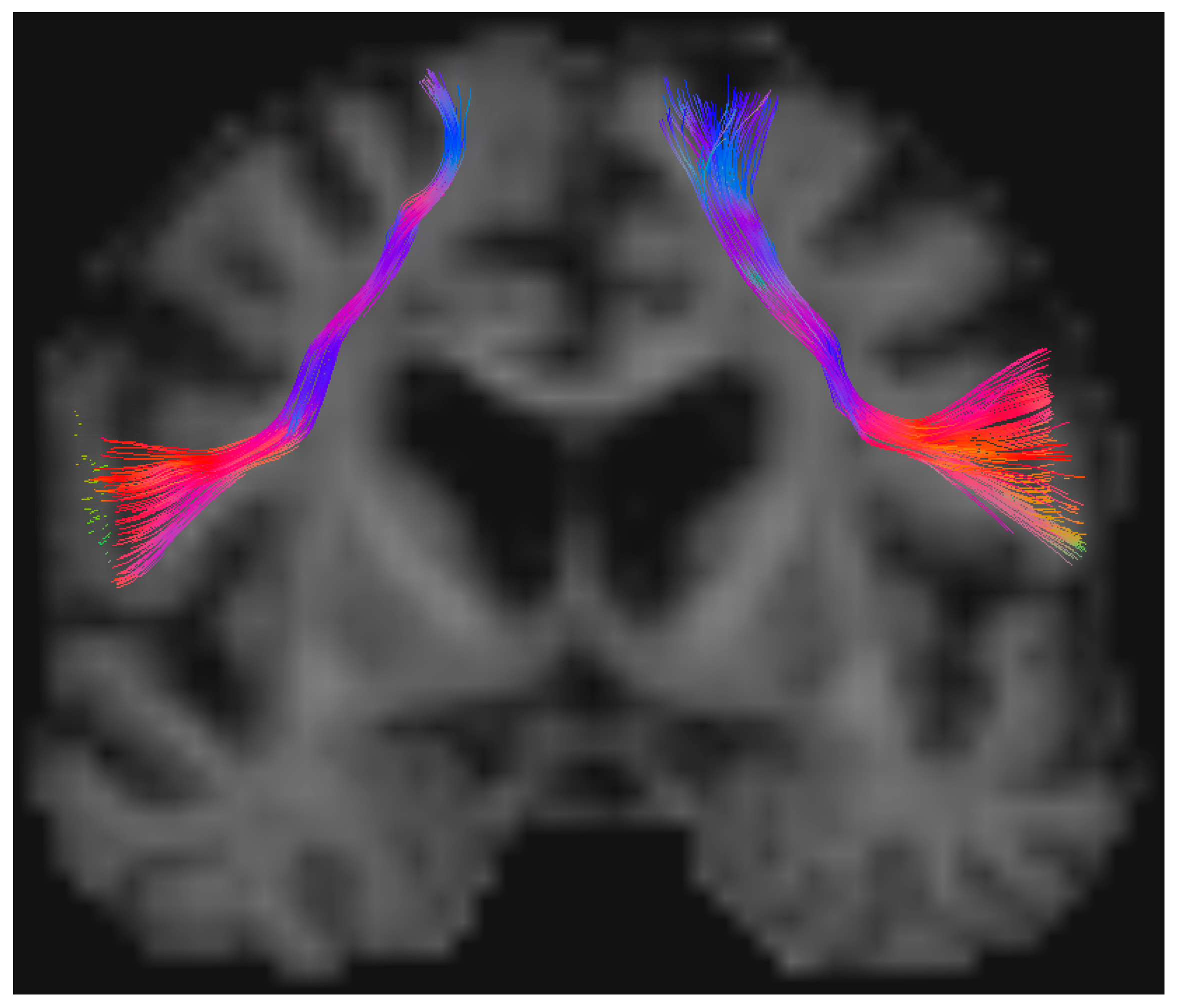
| Spherical Deconvolution FAT Variables | Contralateral Side (SD) | Tumor Side (SD) | Statistics |
|---|---|---|---|
| Mean Volume (mm3) | 13.33 (4.46) | 6.53 (4.72) | p < 0.001 |
| Mean Length (mm) | 69.90 (4.07) | 75.24 (13.06) | p = 0.043 |
| Mean HMOA | 0.013 (0.002) | 0.011 (0.002) | p = 0.001 |
| Mean ADC (×10−3 mm2/s) | 0.682 (0.045) | 0.770 (0.117) | p < 0.001 |
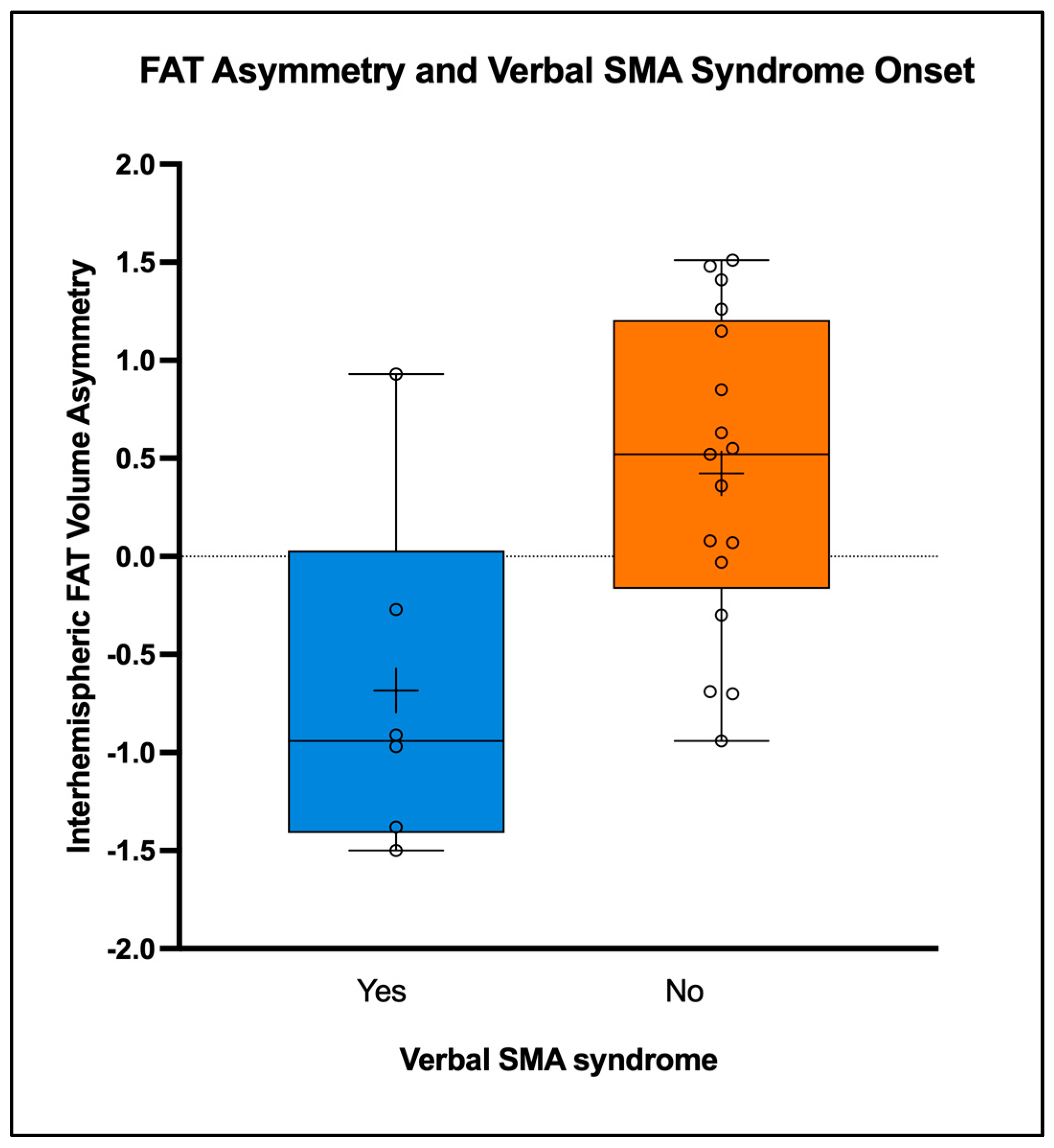
| Interhemispheric Spherical Deconvolution Value | Verbal SMA Syndrome (N = 6) | No Verbal Syndrome (N = 17) | Statistics |
|---|---|---|---|
| Mean FAT-VA (SD) | −0.68 (0.904) | 0.42 (0.794) | p = 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaga-Arias, J.M.; Jung, J.; Lavrador, J.P.; Rajwani, K.; Mirallave-Pescador, A.; Jones, A.; Wren, H.; Gullan, R.; Bhangoo, R.; Ashkan, K.; et al. Asymmetry of the Frontal Aslant Tract and Development of Supplementary Motor Area Syndrome. Cancers 2024, 16, 3739. https://doi.org/10.3390/cancers16223739
Aliaga-Arias JM, Jung J, Lavrador JP, Rajwani K, Mirallave-Pescador A, Jones A, Wren H, Gullan R, Bhangoo R, Ashkan K, et al. Asymmetry of the Frontal Aslant Tract and Development of Supplementary Motor Area Syndrome. Cancers. 2024; 16(22):3739. https://doi.org/10.3390/cancers16223739
Chicago/Turabian StyleAliaga-Arias, Jahard M., Josephine Jung, Jose Pedro Lavrador, Kapil Rajwani, Ana Mirallave-Pescador, Amy Jones, Hilary Wren, Richard Gullan, Ranj Bhangoo, Keyoumars Ashkan, and et al. 2024. "Asymmetry of the Frontal Aslant Tract and Development of Supplementary Motor Area Syndrome" Cancers 16, no. 22: 3739. https://doi.org/10.3390/cancers16223739
APA StyleAliaga-Arias, J. M., Jung, J., Lavrador, J. P., Rajwani, K., Mirallave-Pescador, A., Jones, A., Wren, H., Gullan, R., Bhangoo, R., Ashkan, K., Dell’Acqua, F., & Vergani, F. (2024). Asymmetry of the Frontal Aslant Tract and Development of Supplementary Motor Area Syndrome. Cancers, 16(22), 3739. https://doi.org/10.3390/cancers16223739