Simple Summary
The response to neoadjuvant treatment in rectal cancer varies among patients, while the evidence of benefit from additional adjuvant chemotherapy, especially in patients with persistent tumor and/or lymph node metastases, is unclear. In our cohort study of 1778 patients, we showed that persistent tumor and/or lymph node metastasis after intensified neoadjuvant treatment, e.g., total neoadjuvant treatment (TNT), was not more strongly associated with an extensive risk of treatment failure (TF, local recurrence and/or distant metastasis) than after less intensive neoadjuvant treatment, e.g., 5-flurouracil-based chemoradiotherapy (5-FU CRT). These results may help clinicians to individualize their surveillance strategies, but do not provide an argument for adding additional chemotherapy in non-responders after TNT.
Abstract
Objectives: Additional adjuvant treatment in patients with rectal cancer with limited response to neoadjuvant treatment to mitigate their higher risk of treatment failure remains controversial. Methods: This is a post hoc analysis of a cohort study of 3 randomized phase 2 or 3 trials (CAO/ARO/AIO-94, -04, and -12 trial) that included 1948 patients with locally advanced rectal adenocarcinoma. After excluding patients with missing information, 1788 patients (1254 men and 524 women; median age: 62.6 years, age range: 19–84 years) were eligible. We analyzed the extent of tumor response and its association with the incidence of treatment failure after different neoadjuvant treatment approaches. Results: Tumor response was significantly enhanced with more intensive neoadjuvant treatment. After a median follow-up of 55 months for the entire cohort (IQR: 37 months–62 months), the incidence of treatment failure (TF) stratified by tumor response or post-neoadjuvant pathological outcome was not significantly affected by the intensity of neoadjuvant treatment, whereas the ypTNM stage was significantly associated with the risk of treatment failure. Conclusions: In this cohort study, we provide evidence that limited or no response to intensified neoadjuvant treatment protocols is not likely to be more strongly associated with an extensive risk of TF after 5-FU CRT+/− adjuvant chemotherapy.
1. Introduction
The multimodal treatment of locally advanced rectal cancer has evolved over the past decades. The introduction of neoadjuvant radiotherapy, neoadjuvant chemoradiotherapy (CRT), and total mesorectal excision (TME) has improved locoregional control [1,2,3]. The intensification of neoadjuvant CRT and/or adjuvant CT with the addition of oxaliplatin provided inconsistent results [4]. More recently, total neoadjuvant treatment (TNT) led to enhanced pathologic complete response rates, improved disease-free survival (DFS), and, partly, improved overall survival (OS) [5,6,7]. Nevertheless, ypN+ was reported in 25% of patients treated with TNT compared to 32% in the standard of care group, and in 17% of patients treated with TNT compared to 32% in the standard of care group in the RAPIDO and PRODIGE23 trials [5,6].
The management of patients with minor or no downstaging or no substantial tumor response after (intensified) neoadjuvant treatment remains challenging. Considering the inconsistent results of adjuvant chemotherapy after standard 5-FU CRT, it is unclear whether additional adjuvant chemotherapy might improve survival in these patients [8]. It has been hypothesized that patients with ypN+ after neoadjuvant treatment reflect a subgroup with aggressive, therapy-resistant tumor biology at high risk of developing local recurrence or distant metastasis [9].
Here, we examined the extent of the response to neoadjuvant treatment and its impact on long-term treatment failure, stratified by the intensity of neoadjuvant treatment. We hypothesized that, in rectal cancer patients without downstaging or with only minor tumor response despite more intensive TNT, which may reveal an extremely aggressive phenotype of rectal cancer, the incidence of treatment failure may be even higher compared to (standard) neoadjuvant CRT.
2. Materials and Methods
The CAO/ARO/AIO-94 trial recruited patients between February 1995 and September 2002, the CAO/ARO/AIO-04 trial between July 2006 and February 2010, and the CAO/ARO/AIO-12 trial between June 2015 to January 2018. The design and oncologic outcomes have been previously published [1,10,11,12,13,14]. Eligibility criteria for this post hoc analysis excluded patients with no curative post-surgical status (R2, ypM+, no data on surgery). With respect to the CAO/ARO/AIO-94 trial, only patients treated within the neoadjuvant CRT arm were included in this analysis. A graphical abstract of the different treatment schemes is provided as Supplementary Figure S1. All sites obtained medical ethics committee approval and written patient informed consent.
Tumor response was defined by the extent of downstaging: post-neoadjuvant tumor stage according to UICC (ypTNM or ycTNM) minus pre-treatment clinical tumor stage according to UICC (cTNM): Progress: >0, No response: =0, Limited downstaging: =−1, Intermediate downstaging: =−2, Extensive downstaging: =−3. TRG was recorded in the study cohort according to Dworak et al. [15] The five-tier classification included TRG 4 (no vital tumor cells detectable), TRG 3 (only scattered tumor cells amidst fibrosis with or without acellular mucin), TRG 2 (predominant fibrosis with scattered but easily recognizable tumor cells), TRG 1 (predominant tumor with prominent fibrosis or vasculopathy), and TRG 0 (no regression) and it was grouped into a three-tier TRG system [15,16].
Treatment failure (TF) events were defined as occurrences of local recurrence or distant metastasis, whichever occurred first. The time to TF was defined as the time from curative surgery to the TF event. To note, we decided not to use disease-free survival because the definition differed between the trials, and we did not use overall survival because we have previously shown a significant difference in survival between the cohorts after treatment failure [17]. The latter highlights the limitation of OS as a clinical endpoint for the assessment of the efficacy of primary treatments in trial cohorts recruiting patients over a longer period of time. The Pearson’s chi-squared test was used to assess differences in clinical characteristics. Kruskal–Wallis tests and Dunn–Bonferroni post hoc tests were used to examine the association of neoadjuvant treatment approach with time to surgery and extent of treatment response. The “rma” function in the “metafor” package of the R statistical software was used to examine differences in the association of treatment response and risk for TF stratified by the intensity of the neoadjuvant treatment approach. This moderation effect analysis examined whether the relationship between pathological tumor stage or TRG and risk of treatment failure changed depending on the intensity of neoadjuvant treatment approach, which can help to understand if and how different neoadjuvant treatment approaches influence the effect size [18]. The median follow-up was calculated with the reverse Kaplan–Meier approach. DFS was examined with the log-rank test and incidences of TF were analyzed by Fine–Gray regression models with death as the competing risk. The proportional hazard assumptions (PHA) were tested with the “cox.zph” function in the “survival” package and revealed no PHA violations. A competing risk regression model with an interaction term was used to examine whether the effect of treatment on hazard rates depended on the level of tumor response as assessed by pathological stage or TRG, thus allowing an assessment of whether the relationship between tumor response and outcome changes with different treatments [19,20]. Analyses were performed using the R4.2.1 software. A p-value < 0.05 was considered significant. Data in the analyses reported here were analyzed between October 2023 and June 2024.
3. Results
Of the 1948 patients treated within the three trials, 19 patients were excluded because of incomplete patient data, while 151 were excluded because they did not meet the eligibility criteria of this post hoc analysis. Of the remaining 1778 patients (1254 males and 524 females; median age: 62.6 years, age range: 19–84 years) with locally advanced rectal cancer (cT3/4 or cN+), 934 were treated with 5U-CRT, 560 with concurrent 5-FU/Ox CRT +/− adjuvant CT, and 284 with TNT. Figure S2 shows the flow diagram of the present analysis and the distribution of pre-treatment clinical characteristics. Surgical characteristics and pathological characteristics are reported in Table 1.

Table 1.
Distribution of clinical, surgical and pathological characteristics.
Patients treated with TNT had slightly more advanced tumor stages, whereas the distribution of age, sex, and tumor location did not differ. The median neoadjuvant treatment time was longer in patients treated with TNT (median: 139 days TNT vs. 91 days 5-FU CRT/5-FU/Ox CRT, p < 0.001). Surgical methods did not differ significantly between treatment approaches (p = 0.276), although R1 resections were slightly higher in the TNT cohort (4.1% vs. 2.2%) (Table 1).
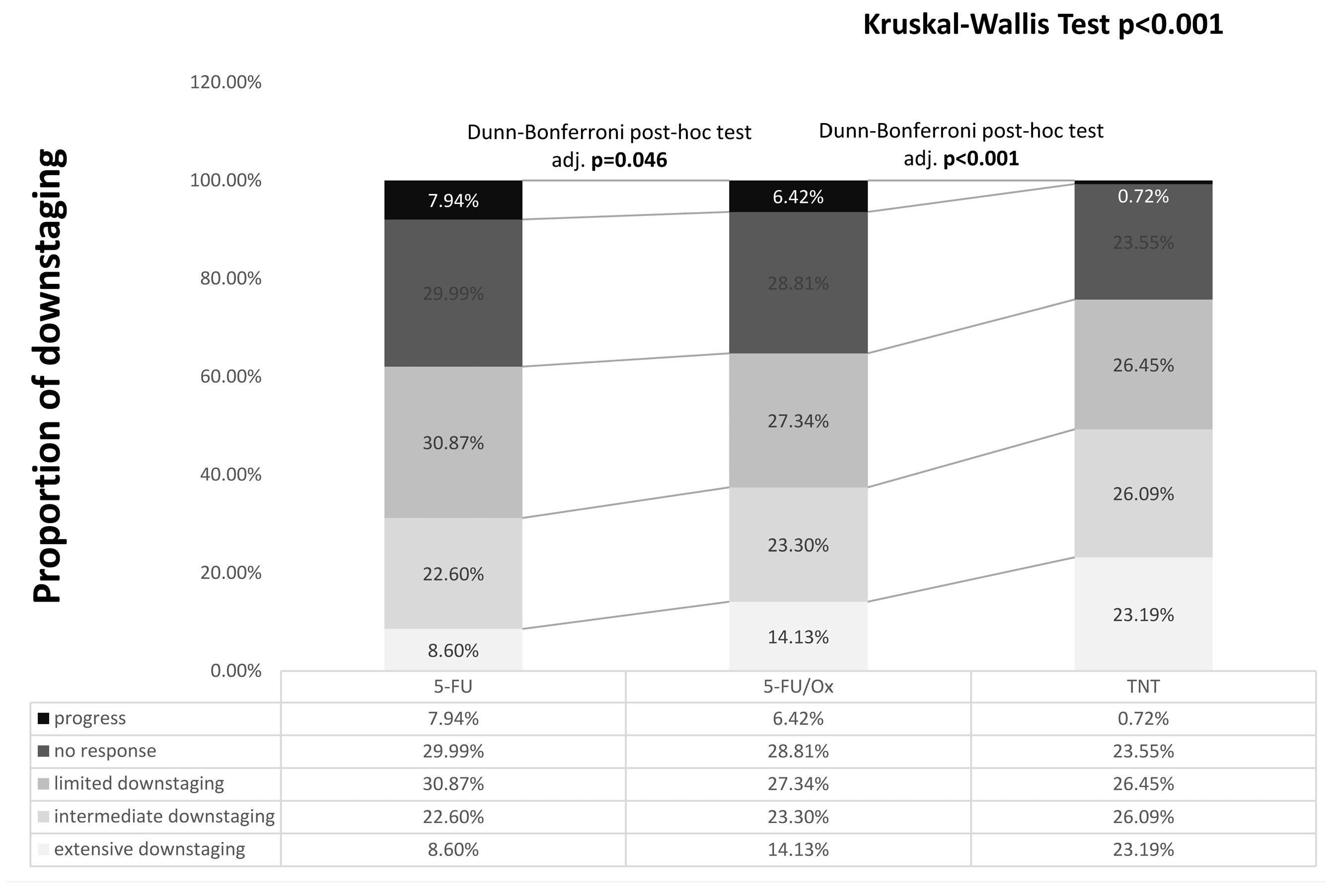
The tumor response was significantly enhanced by 5-FU/Ox CRT compared to 5-FU CRT (p = 0.046) and further after TNT (p < 0.001), with less patients having no response or local progression (24.65% after TNT vs. 35.2% after 5-FU/Ox vs. 37.9% after 5-FU, Figure 1). Pathological parameters are summarized in Table 1.
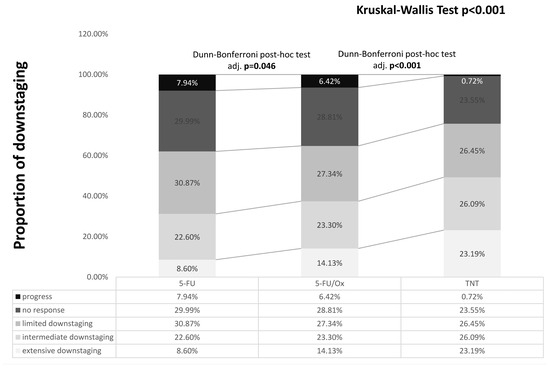
Figure 1.
Downstaging after different neoadjuvant treatment. Differences were assessed with the Kruskal–Wallis test and Dunn–Bonferroni post hoc tests. Calculation: post-neoadjuvant tumor stage according to UICC (ypTNM or ycTNM) minus clinical baseline tumor stage according to UICC (cTNM): Progress: >0, No response: =0, Limited downstaging: =−1, Intermediate downstaging: =−2, Extensive downstaging: =−3.
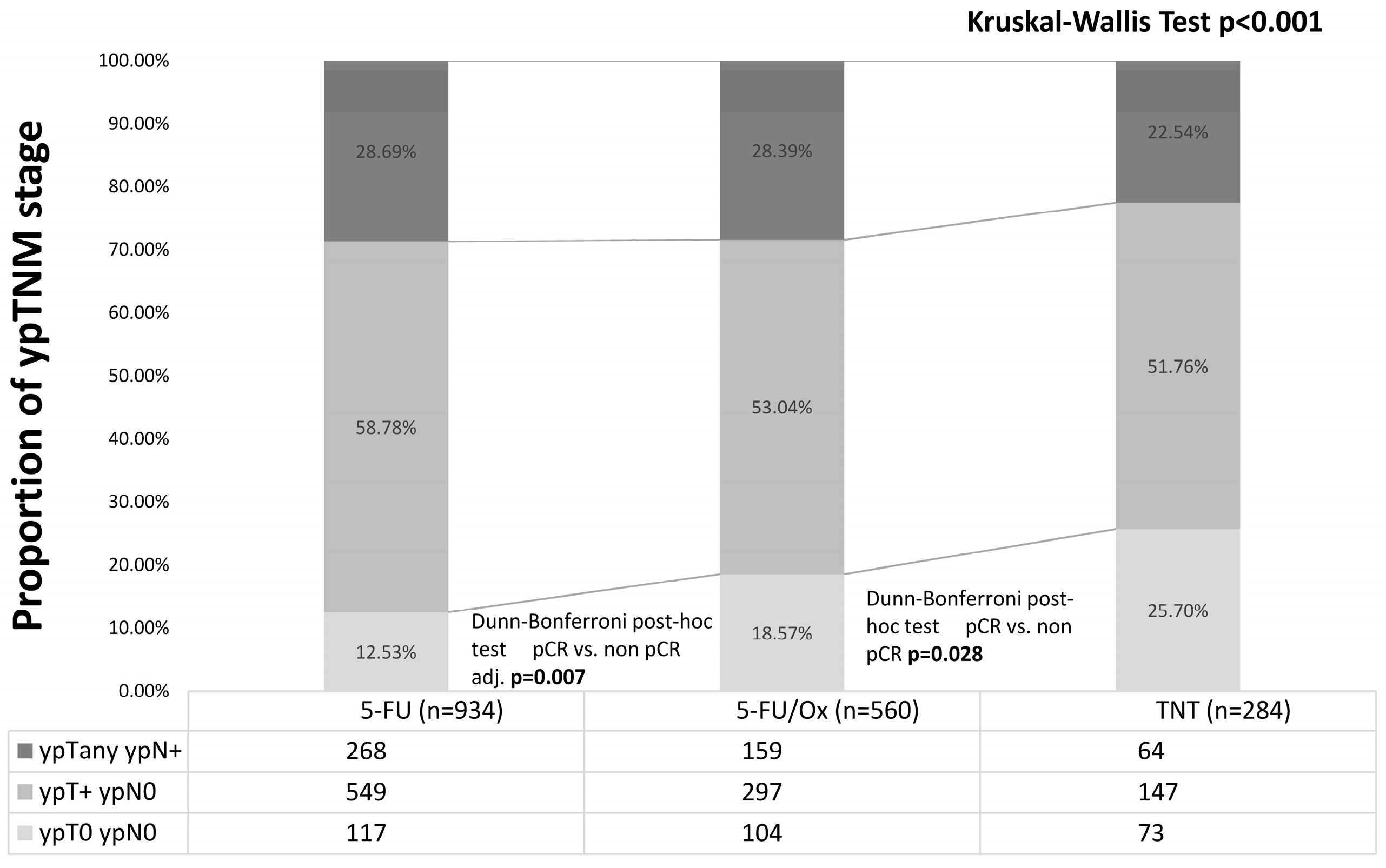
Of all patients, 25.7% had a pCR (including 8 patients with cCR who refused surgery) after TNT vs. 18.6% after 5-FU/Ox (p = 0.03) vs. 12.5% after 5-FU CRT (p = 0.007, Figure 2).
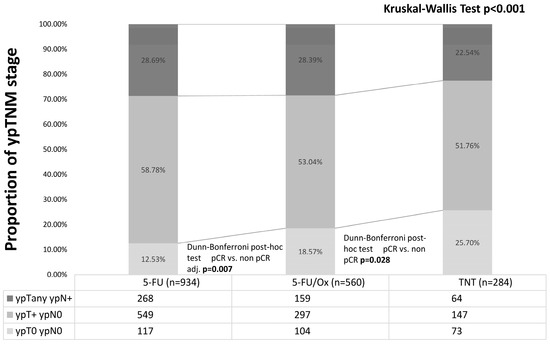
Figure 2.
Post-neoadjuvant tumor stage after neoadjuvant 5-FU CRT, 5-FU/Ox CRT, or TNT. Differences were assessed with the Kruskal–Wall test and Dunn–Bonferroni post hoc tests.
Regardless of the neoadjuvant treatment approach, distant metastases were the predominant reason for TF (5-FU CRT: 84%, 5-FU/OX CRT: 88%, TNT: 83%). The median time to treatment failure ranged from 16 months (5-FU CRT) to 19 months (5-FU/Ox CRT) with no clinically meaningful differences in the median time to distant metastasis, locoregional recurrence, and treatment approach (Table S1).
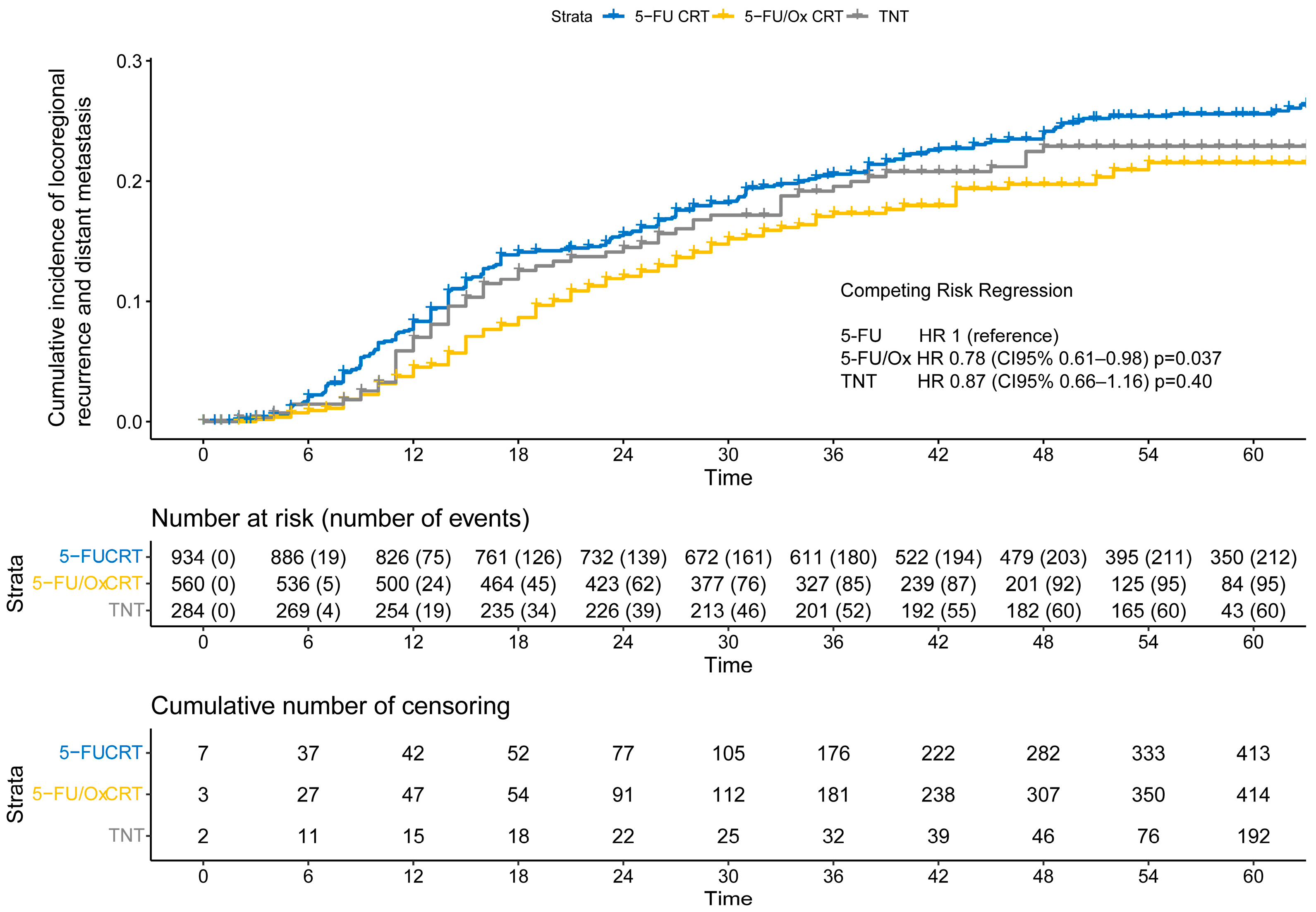
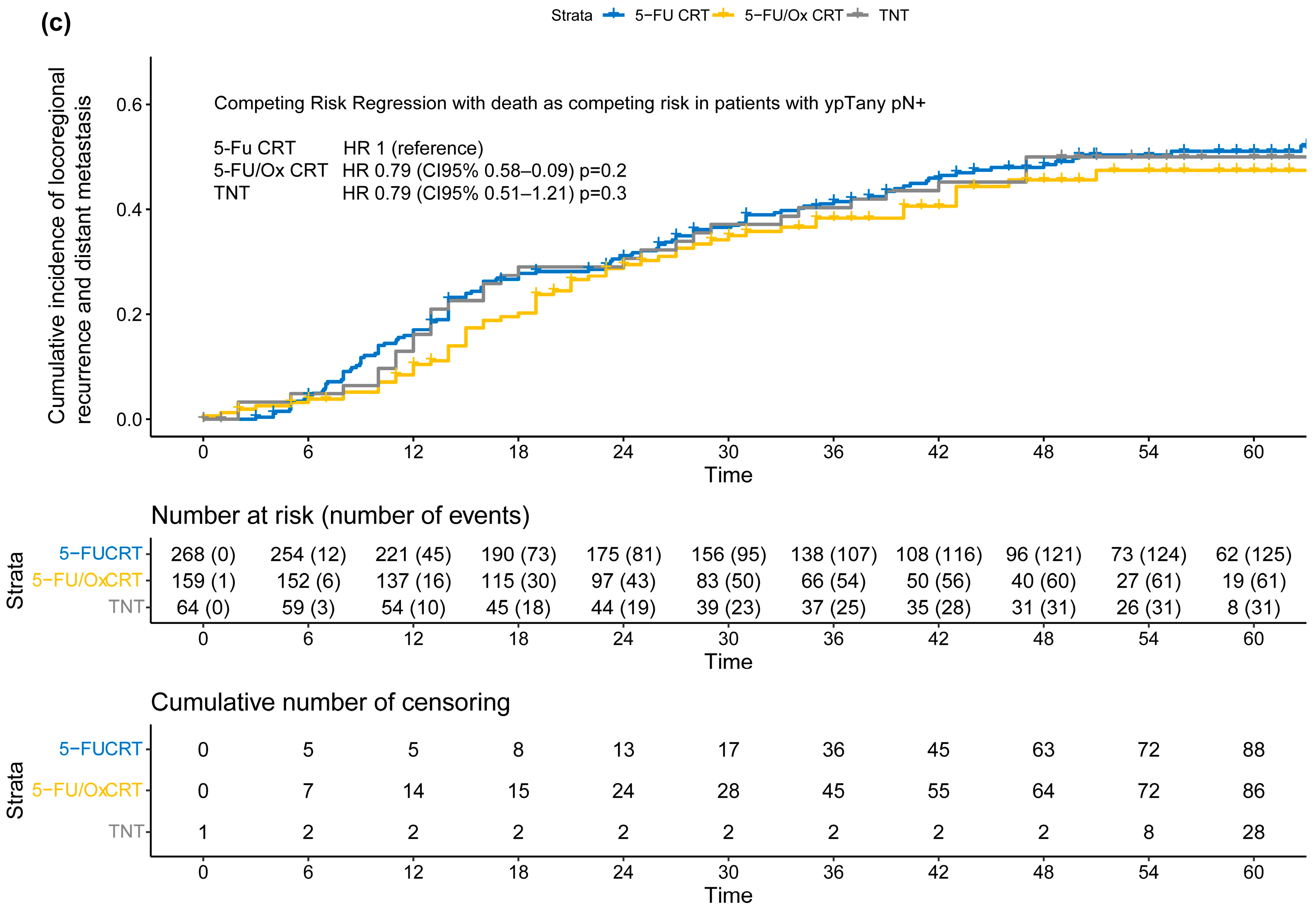
After a median follow-up of 55 months for the entire cohort (IQR: 37 months–62 months), the cumulative incidence of TF was significantly lower after 5-FU/Ox CRT (HR 0.78, CI 95% 0.61–0.98, p = 0.037) but not after TNT (HR 0.87, CI 95% 0.66–1.16, p = 0.40) compared to 5-FU CRT (Figure 3), while the competitive risk of death slightly increased throughout the follow-up period (Figure S3). The study cohort was further analyzed to explore the association of clinical, surgical, and pathological parameters with the risk of TF. R1 resection was associated with a higher risk only after 5-FU/Ox CRT (HR 3.24 (CI 9%% 1.28–8.22), p = 0.01)), whereas ypN+ and TRG 4 were associated with a higher and lower risk of TF, respectively, regardless of the neoadjuvant treatment approach. No clinical, surgical, or pathological parameter showed an inverse association with the risk of TK between the different neoadjuvant treatment approaches (Table S2).
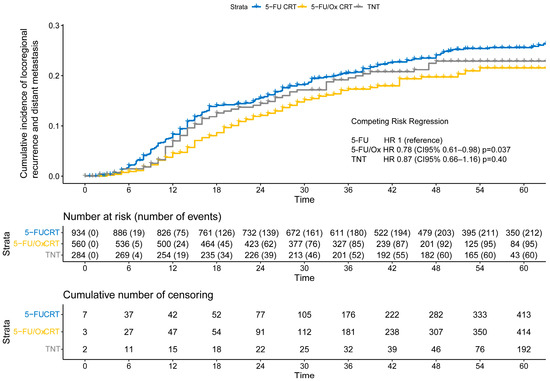
Figure 3.
The cumulative incidence of locoregional recurrence and distant metastasis (treatment failure) after neoadjuvant 5-FU CRT, 5-FU/Ox CRT, or TNT. A competing risk regression, a model with death as the competing risk was used to assess statistical differences between treatment approaches.
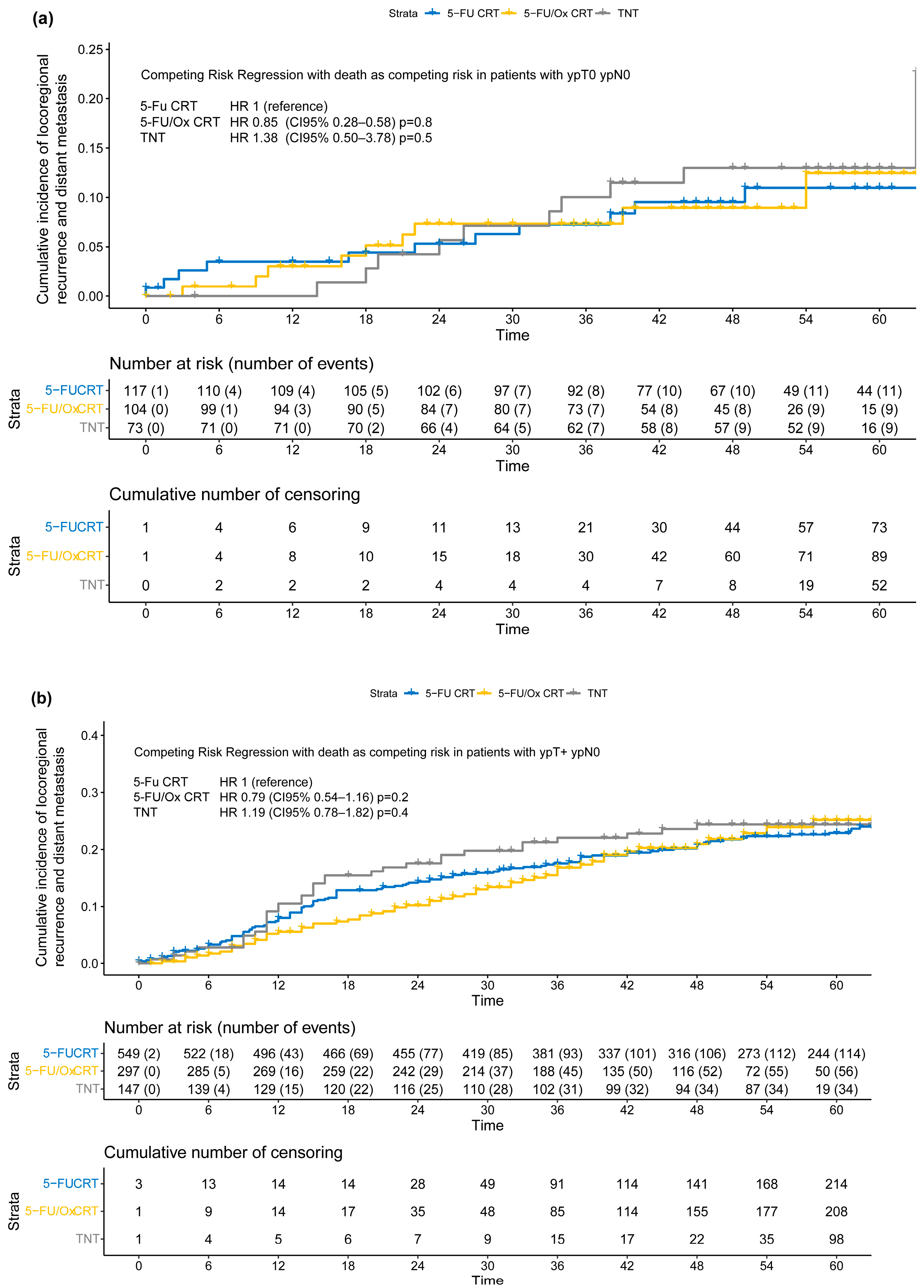
The extent of tumor response was significantly associated with the incidence of TF in the entire study cohort (HR no response vs. downstaged: 3.44 [CI 95% 2.75–4.30, p < 0.001], HR progress vs. downstaged: HR 5.00 [CI 95% 3.64–6.75, p < 0.001] Figure S4), and when grouped by neoadjuvant treatment approach (5-FU CRT: Figure S5, 5-FU/Ox: Figure S6, and TNT: Figure S7). However, the incidence of TF stratified by the pathological outcome (ypTNM stage) did not differ significantly between neoadjuvant treatment approaches (Figure 4). In addition, tumor response as assessed by TRG according to Dworak et al. [15] was significantly associated with the incidence of TF in the overall cohort and when grouped by neoadjuvant treatment approach (Table S2, Figures S8–S11), and did not differ significantly between neoadjuvant treatment approaches, either (Table S3, Figures S12–S14).
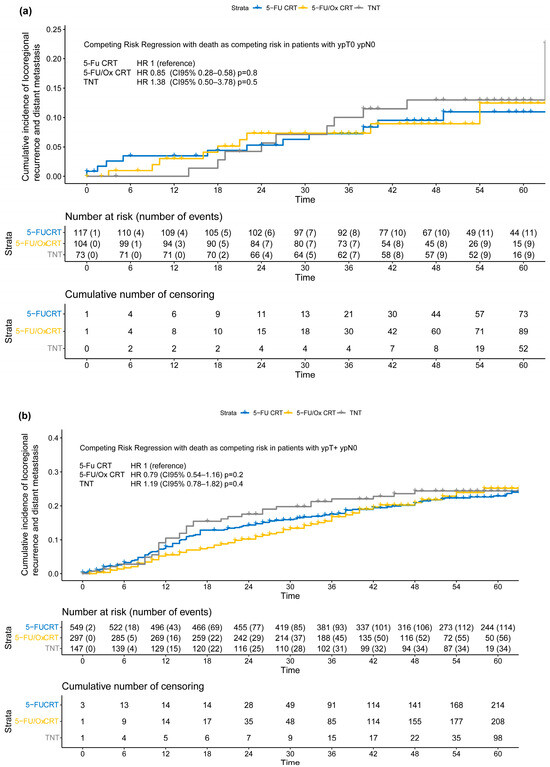
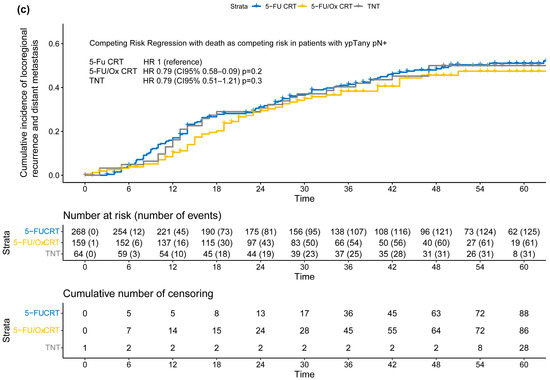
Figure 4.
(a–c) Cumulative incidence of locoregional recurrence and distant metastasis (treatment failure) according to post-neoadjuvant tumor stage (a) ypT0 ypN0 or ycT0 ycN0 or (b) ypT+ ypN0 or (c) ypTany ypN+ after neoadjuvant 5-FU CRT, 5-FU/Ox CRT, or TNT. A competing risk regression model with death as the competing risk was used to assess statistical differences between treatment approaches.
Moderation analyses did not reveal significant differences, after stratification by neoadjuvant treatment approach, in the incidence of TF in patients with ypT+ ypN0 (5-FU CRT: HR 2.05 (CI 95% 1.21–3.47), 5-FU/Ox CRT HR 2.24 (CI 95% 1.10–4.54) and TNT 2.20 (CI 95% 0.97–4.97), test for moderation effect: p = 0.98) and with ypTany ypN+ (5-FU CRT 5.08 (CI 95% 2.50–3.78), 5-FU/Ox CRT 5.59 (CI 95% 2.76–11.3), and TNT 4.86 (CI 95% 2.15–11.0), test for moderation effect: p= 0.96). In addition, moderation analyses did not reveal significant differences, after stratification by TRG, in the incidence of TF in patients with TRG 0/1 (5-FU CRT HR 6.40 (CI 95% 3.11–13.2), 5-FU/Ox HR 4.12 (CI 95% 1.71–9.94) and TNT HR 2.63 (1.03–6.70), test for moderation effect: p= 0.31) and with TRG 2/3 (5-FU CRT HR 3.69 (CI 95% 1.87–7.44), 5-FU/Ox HR 3.16 (CI 95% 1.44–6.93), and TNT HR 2.47 (1.03–6.70), test for moderation effect: p = 0.74).
Furthermore, in a competing risk regression model, the association between tumor response, assessed by calculated downstaging or by TRG, and risk for treatment failure, adjusted for treatment and including an interaction term between downstaging or TRG and treatment approach, confirms the significant association between downstaging or TRG and risk of TF, but neither treatment approach nor the interaction terms showed a significant association with treatment failure (Table 2 and Table 3).

Table 2.
Competing risk regression model including downstaging, neoadjuvant treatment approach, and interaction term downstaging x treatment approach.

Table 3.
Competing risk regression model including TRG, neoadjuvant treatment approach, and interaction term TRG × treatment approach.
4. Discussion
The optimal adjuvant treatment and surveillance strategy after limited tumor response in patient with locally advanced rectal cancer after various forms of neoadjuvant treatment remains unclear. Here, we examined the impact of standard 5-FU-CRT versus intensified 5-FU/Ox-CRT versus TNT on pathological outcomes and downstaging and assessed its implications for long-term TF. We did not observe that enhanced tumor response after TNT was associated with a lower incidence of TF compared to 5-FU CRT, whereas, after 5-FU/Ox CRT, enhanced tumor response correlated with a lower incidence of TF in our cohort. Time to surgery (139 days after randomization in patients treated with TNT) was significantly longer compared to 91 days after 5-FU or 5-Fu/Ox CRT. This prolonged time to surgery may also allow for increased tumor response and should be considered when interpreting the impact of intensified neoadjuvant treatment on pathological outcomes [21].
Regardless of neoadjuvant treatment intensity, tumor responses assessed by the extent of downstaging and post-neoadjuvant tumor stage were significantly associated with TF. We hypothesized that intensified neoadjuvant treatment approaches could (1) shift more patients into more favorable, early pathological stages, whereas (2) patients without downstaging may have a particularly dismal prognosis, an effect we previously described as a reverse stage migration (Will Rogers) phenomenon [9]. However, in our analysis, the relative risk for TF stratified by post-neoadjuvant tumor stage or the extent of tumor response was not significantly altered by different neoadjuvant treatment approaches. Especially in patients with ypN+ after TNT, we did not find an extensive increase in the incidence of TF compared to patients with ypN+ treated with 5-FU CRT or 5-FU/Ox CRT. Thus, we could not confirm our hypothesis.
The management of patients with positive pathological lymph nodes after neoadjuvant 5-FU +/− oxaliplatin CRT, or TNT, respectively, remains challenging. Previous analyses by our group indicated that adherence to adjuvant CT does not impact on disease-free survival when performed unstratified [4]. In a subgroup analysis of patients treated in the standard of care group (i.e., Capecitabine-based CRT) of the RAPIDO trial, which was not stratified by pathologic outcomes, adjuvant CT appeared to improve long-term oncologic outcomes as a function of treatment adherence, but this did not reach statistical significance [22]. Further evidence that patients with poor response to neoadjuvant treatment may benefit from adjuvant chemotherapy is lacking [23]. In addition, there is limited evidence on whether non-adherence to TNT might influence the benefit of adjuvant treatment or which adjuvant treatment protocol (doublets vs. triplets vs. targeted therapy) might improve patient outcomes. The ESMO guidelines (2017) recommend adjuvant CT after CRT in patients with yp stage III (and “high-risk” yp stage II), whereas the NCCN Guideline recommends adjuvant chemotherapy for patients with stage II/III rectal cancer treated with neoadjuvant chemoradiotherapy, but not following the TNT approach [24]. In the RAPIDO trial, no additional adjuvant chemotherapy was considered after TNT, irrespective of treatment response [25]. In the Prodige23 trial, even patients treated with induction mFolFIRINOX received adjuvant CAPOX/FOLFOX for 8 weeks [6].
Our study has several limitations. First, post hoc analyses and comparisons between three independent trial cohorts can be biased by a wide variety of factors. Differences in recruiting periods, treatment centers, and physicians may have led to bias. Second, inclusion and exclusion criteria were not the same in the three trials. For example, MRI-defined inclusion criteria were not required in the CAO/ARO/AIO-94 trial, which may have impacted our results even if we excluded non-curative post-surgical patients in all trials and stratified all patients based on pathological outcome. Third, a post-treatment surveillance program in all three trials was performed in accordance with the Guidelines of the German Cancer Society for colorectal carcinomas, but surveillance recommendations have changed over the years [26]. In addition, MRI or CT examinations may have been increasingly performed in recent years, particularly in high-risk patients, beyond the recommendations of the guidelines by individual physicians’ choice. Fourth, comparing the pre-treatment clinical stage with the post-treatment pathological outcome is biased by the potential of overstaging of T stage, or the inaccuracy of MRI for nodal staging [27,28]. These challenges in assessing the clinical node status in rectal cancer patients emphasize the need for better strategies in radiology staging in rectal cancer [29,30]. Fifth, TRG was not available in all patients, no central pathology review was performed, and no analysis of inter- and intra-observer variability of TRG scoring was conducted [16].
5. Conclusions
In conclusion, this post hoc cross-trial comparison of patients treated within three randomized clinical trials with neoadjuvant 5-FU CRT or 5-FU/Ox CRT, or TNT showed significant differences in the incidence of TF with death as a competing risk when analyzed by post-neoadjuvant tumor stage or tumor response, but no relative differences when stratified by the intensity of neoadjuvant treatment. An extensive risk of TF, and therefore the need for additional adjuvant treatment after TNT in patients with limited tumor response or ypN+ stage after TNT, cannot be inferred from the data used in this analysis. Without prospective evidence of benefit from additional adjuvant chemotherapy in these patients, we would suggest that surveillance protocols should be more individualized based on the highly significant association between pathological outcome and the incidence of TF.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16213673/s1, Figure S1. Treatment schedules of the CAO/ARO/AIO-94, CAO/ARO/AIO-04 and CAO/ARO/AIO-12 trials. Figure S2. Study flow diagram of the present post hoc analysis. Table S1. Association of neoadjuvant treatment approach and reasons for treatment failure and median time to treatment failure. Table S2. Competing risk regression with death as competing risk (CI 95%) of clinical, surgical, pathological characteristics and risk of treatment failure according to neoadjuvant treatment approach. Figure S3. Cumulative incidence of locoregional recurrence/distant metastasis or death. Figure S4. Cumulative incidence of locoregional recurrence/distant metastasis stratified by response to neoadjuvant treatment. Figure S5. Cumulative incidence of locoregional recurrence/distant metastasis stratified by response to neoadjuvant treatment after 5-FU CRT. Figure S6. Cumulative incidence of locoregional recurrence/distant metastasis stratified by response to neoadjuvant treatment after 5-FU/Ox CRT. Figure S7. Cumulative incidence of locoregional recurrence/distant metastasis stratified by response to neoadjuvant treatment after TNT. Table S3. Competing risk regression with death as competing risk to assess statistical differences between treatment approaches stratified by TRG. Figure S8. Cumulative incidence of locoregional recurrence/distant metastasis stratified by TRG. Figure S9. Cumulative incidence of locoregional recurrence/distant metastasis stratified by TRG after 5-FU CRT. Figure S10. Cumulative incidence of locoregional recurrence/distant metastasis stratified by TRG after 5-FU/Ox CRT. Figure S11. Cumulative incidence of locoregional recurrence/distant metastasis stratified by TRG after TNT. Figure S12. Cumulative incidence of locoregional recurrence/distant metastasis according to TRG 0/1 after neoadjuvant 5-FU CRT, 5-FU/OX CRT, or TNT. Figure S13. Cumulative incidence of locoregional recurrence/distant metastasis according to TRG 2/3 after neoadjuvant 5-FU CRT, 5-FU/OX CRT, or TNT. Figure S14. Cumulative incidence of locoregional recurrence/distant metastasis according to TRG 4 after neoadjuvant 5-FU CRT, 5-FU/OX CRT, or TNT.
Author Contributions
Conceptualization, M.D., M.F., C.R. and E.F.; methodology, M.D., C.R. and E.F.; software, M.D. and D.M.; validation, R.-D.H., C.R. and E.F.; writing—original draft preparation, M.D., D.M., C.R. and E.F.; review and editing, R.-D.H. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
The CAO/ARO/AIO-94, the CAO/ARO/AIO-04 and the CAO/ARO/AIO-12 trials were funded by grants (70-587/106-757/110460) of the German Cancer Aid. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The CAO/ARO/AIO-94 trial was approved by the Ethics Committee of the University of Erlangen [1,10]. The CAO/ARO/AIO-04 trial was approved by the Ethics Committee of the University of Erlangen on 28 June 2006 (Ethic Code: 07_2006, EudraCT-Nr: 2006-002385-20). The CAO/ARO/AIO-12 trial was approved by the Ethics Committee of the University Hospital Frankfurt on 19 January 2015 (Ethic Code: 406/14, EudraCT-Nr: 2011-006310-13).
Informed Consent Statement
All sites obtained medical ethics committee approval and written patient informed consent. No additional informed consent was required for this secondary post hoc analysis.
Data Availability Statement
The data presented in this study are not available due to the data protection requirements of the trials.
Conflicts of Interest
Martin reported receiving personal fees from Astra Zeneca outside the submitted work. Hofheinz reported honoraria from Roche, Amgen, Merck Serono, Sanofi MSD, Bristol-Myers Squibb, Eli Lily Medac, Servier, and Deutsche Krebshilfe, and nonfinancial support from GSK outside the submitted work. No other disclosures were reported.
References
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Swedish Rectal Cancer, T.; Cedermark, B.; Dahlberg, M.; Glimelius, B.; Pahlman, L.; Rutqvist, L.E.; Wilking, N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.D.; Ghadimi, M.; Minsky, B.D.; Rodel, C.; Fokas, E. Association of Treatment Adherence With Oncologic Outcomes for Patients With Rectal Cancer: A Post Hoc Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1416–1421. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2023, 41, LBA3504. [Google Scholar] [CrossRef]
- Carvalho, C.; Glynne-Jones, R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017, 18, e354–e363. [Google Scholar] [CrossRef]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Sauer, R.; Wittekind, C.; Rodel, C. Downstage migration after neoadjuvant chemoradiotherapy for rectal cancer: The reverse of the Will Rogers phenomenon? Cancer 2015, 121, 1724–1727. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Rodel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.R.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Rodel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Allgauer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Color. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef]
- Fokas, E.; Strobel, P.; Fietkau, R.; Ghadimi, M.; Liersch, T.; Grabenbauer, G.G.; Hartmann, A.; Kaufmann, M.; Sauer, R.; Graeven, U.; et al. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. J. Natl. Cancer Inst. 2017, 109, djx095. [Google Scholar] [CrossRef]
- Diefenhardt, M.; Martin, D.; Fleischmann, M.; Hofheinz, R.D.; Ghadimi, M.; Rodel, C.; Fokas, E. Overall Survival After Treatment Failure Among Patients With Rectal Cancer. JAMA Netw. Open 2023, 6, e2340256. [Google Scholar] [CrossRef]
- Viechtbauer, W. The metafor Package A Meta-Analysis Package for R. Available online: https://www.metafor-project.org/doku.php/metafor (accessed on 13 June 2024).
- Gray, R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Nahhas, R.W. Introduction to Regression Methods for Public Health Using R. Available online: https://bookdown.org/rwnahhas/RMPH/ (accessed on 27 October 2024).
- Probst, C.P.; Becerra, A.Z.; Aquina, C.T.; Tejani, M.A.; Wexner, S.D.; Garcia-Aguilar, J.; Remzi, F.H.; Dietz, D.W.; Monson, J.R.; Fleming, F.J.; et al. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J. Am. Coll. Surg. 2015, 221, 430–440. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Zwart, W.H.; Nilsson, P.J.; Putter, H.; Roodvoets, A.G.H.; Meershoek-Klein Kranenbarg, E.; Frodin, J.E.; Nygren, P.; Ostergaard, L.; Kersten, C.; et al. The value of post-operative chemotherapy after chemoradiotherapy in patients with high-risk locally advanced rectal cancer-results from the RAPIDO trial. ESMO Open 2023, 8, 101158. [Google Scholar] [CrossRef]
- Collette, L.; Bosset, J.F.; den Dulk, M.; Nguyen, F.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Pierart, M.; Calais, G.; European Organisation for, R.; et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J. Clin. Oncol. 2007, 25, 4379–4386. [Google Scholar] [CrossRef] [PubMed]
- NCCN. NCCN Guidelines Version 4.2024 Rectal Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 25 August 2024).
- van Etten, B.; Glimelius, B.; Hospers, G.A.P.; Marijnen, C.A.M.; Nilsson, P.J.; van de Velde, C.J.H. RAPIDO Rectal cancer And Pre-operative Induction therapy followed by Dedicated Operation. Available online: https://dccg.nl/files/RAPIDO_protocol_3.1_signed_20160318_0.pdf (accessed on 27 October 2024).
- Vogl, T.J.; Pereira, P.L.; Helmberger, T.; Schreyer, A.G.; Schmiegel, W.; Fischer, S.; Herzog, C. Updated S3 Guidelines—Diagnosis and Treatment of Colorectal Carcinoma: Relevance for Radiological Diagnosis and Intervention. Rofo 2019, 191, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Scheele, J.; Schmidt, S.A.; Tenzer, S.; Henne-Bruns, D.; Kornmann, M. Overstaging: A Challenge in Rectal Cancer Treatment. Visc. Med. 2018, 34, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Detering, R.; van Oostendorp, S.E.; Meyer, V.M.; van Dieren, S.; Bos, A.; Dekker, J.W.T.; Reerink, O.; van Waesberghe, J.; Marijnen, C.A.M.; Moons, L.M.G.; et al. MRI cT1-2 rectal cancer staging accuracy: A population-based study. Br. J. Surg. 2020, 107, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.; D’Souza, N.; Shaw, A.; Day, N.; Brown, G. The Current Status of Nodal Staging in Rectal Cancer. Curr. Color. Cancer Rep. 2019, 15, 143–148. [Google Scholar] [CrossRef]
- Pangarkar, S.; Mistry, K.; Choudhari, A.; Smriti, V.; Ahuja, A.; Katdare, A.; Engineer, R.; Ostwal, V.; Ramadwar, M.; Saklani, A.; et al. Accuracy of MRI for nodal restaging in rectal cancer: A retrospective study of 166 cases. Abdom. Radiol. 2021, 46, 498–505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).