Usefulness of Nutritional Assessment Indicators in Predicting Treatment Discontinuation Due to Adverse Events from PARP Inhibitors in Ovarian Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
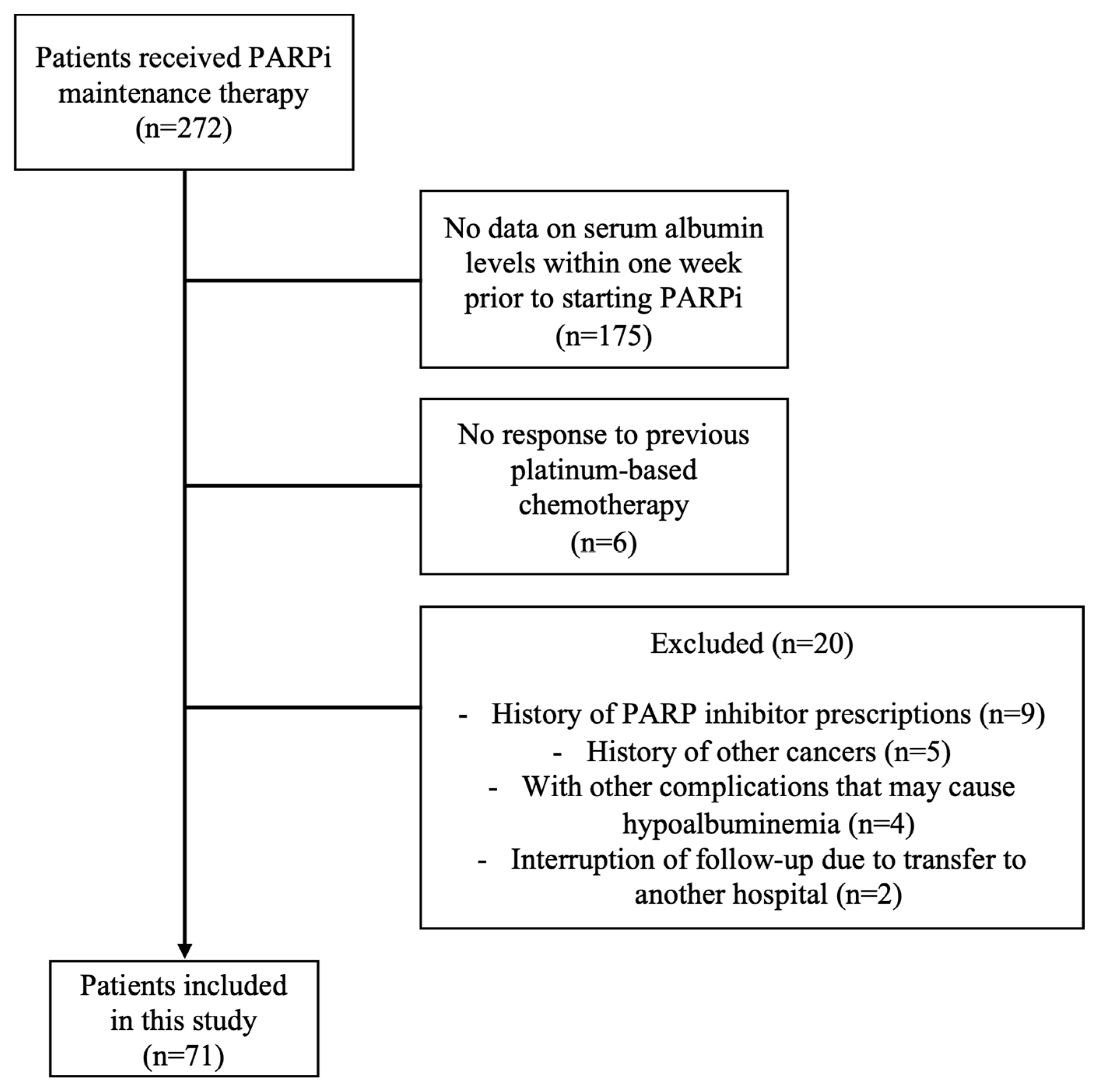
2.1. Participants and Study Design
2.2. Nutritional Assessment Indicators
2.3. Adverse Events
2.4. Statistical Analyses
2.5. Ethical Consideration
3. Results
3.1. Study Population and Patient Characteristics
3.2. Details of AEs and Cases of Discontinuation Due to AEs Caused by PARPi
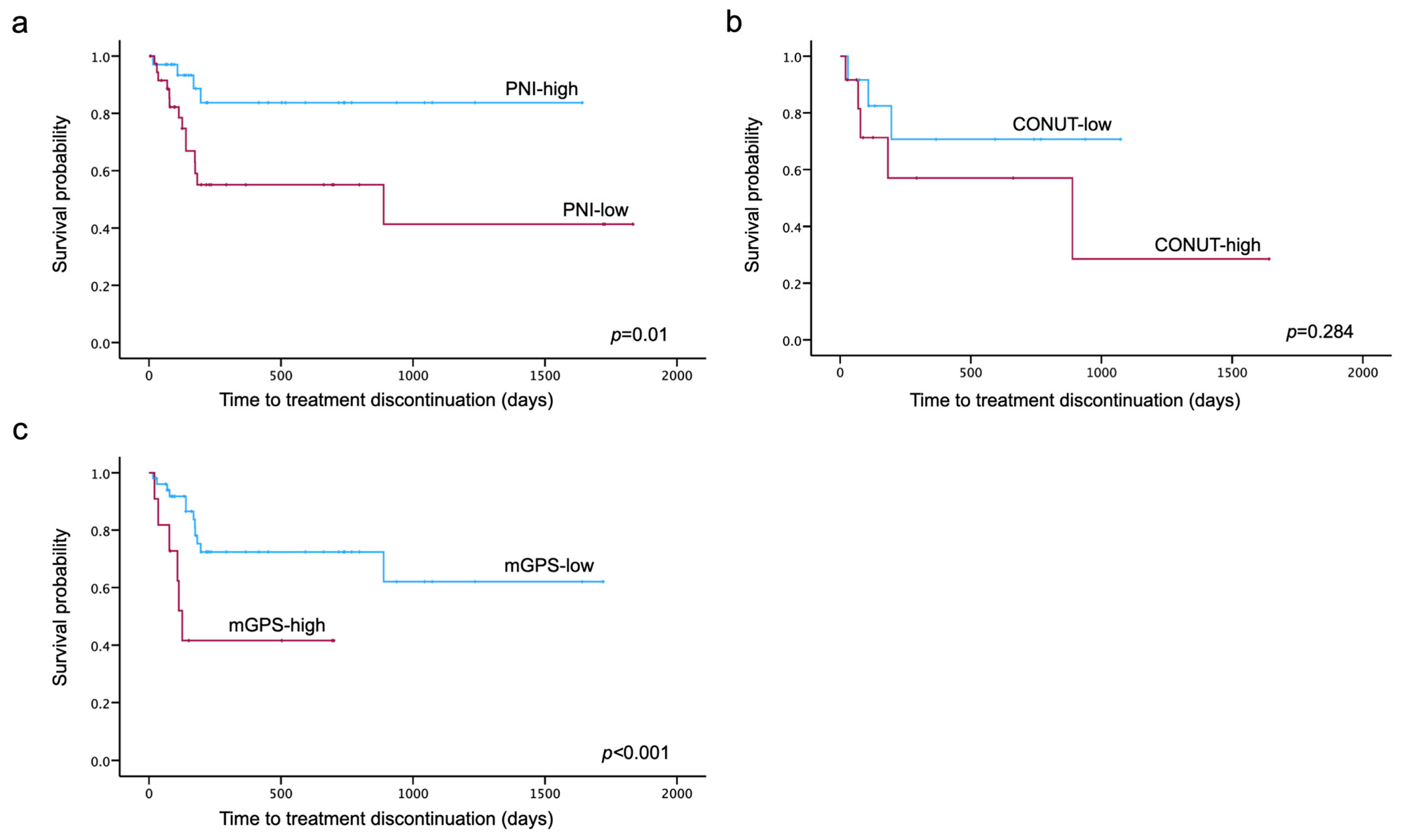
3.3. TTD
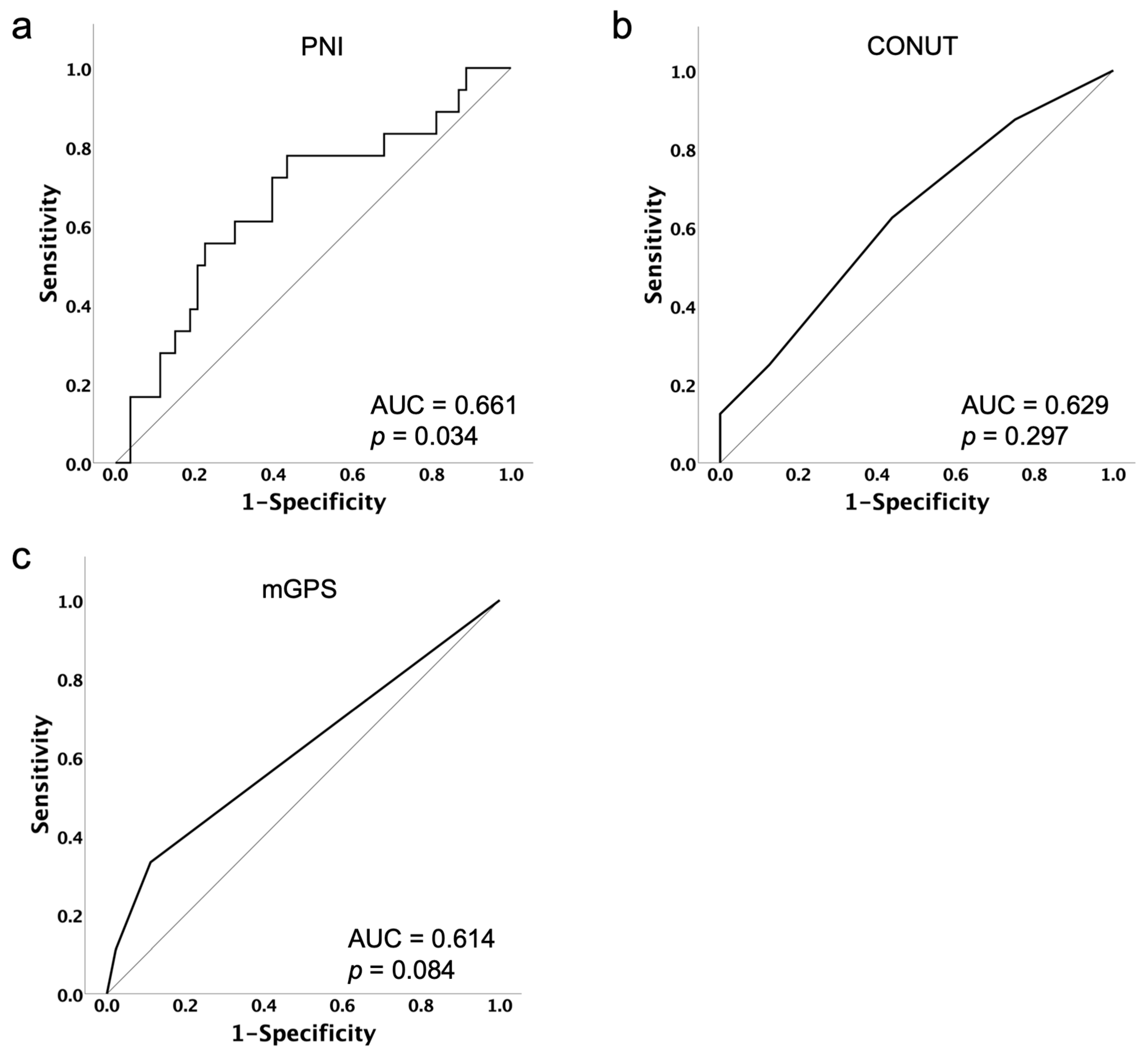
3.4. Predictors for Treatment Discontinuation Due to AEs from PARPi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan (Ministry of Health, Labour and Welfare, National Cancer Registry). Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html (accessed on 15 March 2024).
- Penn, C.A.; Alvarez, R.D. Current Issues in the Management of Patients with Newly Diagnosed Advanced-Stage High-Grade Serous Carcinoma of the Ovary. JCO Oncol. Pract. 2023, 19, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 61–85. [Google Scholar] [CrossRef] [PubMed]
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A.; et al. The Impact of Second to Sixth Line Therapy on Survival of Relapsed Ovarian Cancer after Primary Taxane/Platinum-Based Therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum Neurotoxicity Pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Xishan, Z.; Ye, Z.; Feiyan, M.; Liang, X.; Shikai, W. The Role of Prognostic Nutritional Index for Clinical Outcomes of Gastric Cancer after Total Gastrectomy. Sci. Rep. 2020, 10, 17373. [Google Scholar] [CrossRef]
- Peng, P.; Chen, L.; Shen, Q.; Xu, Z.; Ding, X. Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) Score for Predicting Outcomes of Breast Cancer: A Systematic Review and Meta-Analysis. Pak. J. Med. Sci. Q. 2023, 39, 1535–1541. [Google Scholar] [CrossRef]
- Luan, C.-W.; Kuo, L.-T.; Wang, Y.-T.; Liao, C.-T.; Kang, C.-J.; Lee, Y.-C.; Chen, K.-Y.; Lai, C.-H.; Tsai, Y.-H.; Huang, E.I.; et al. Utility of Modified Glasgow Prognostic Score for Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Head Neck 2023, 45, 1856–1867. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, B.S.; Peng, T.-C.; Peng, Y.-H. Inflammatory and Nutritional Biomarkers in Patients with Esophageal Squamous Cell Carcinoma Undergoing Neoadjuvant Chemotherapy and Radiation Therapy. Oncol. Nurs. Forum 2024, 51, 177–192. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Zhou, Q.; Kamimura, K.; Moriyama, M.; Saijo, Y. The Prognostic Nutrition Index Predicts the Development of Hematological Toxicities in and the Prognosis of Esophageal Cancer Patients Treated with Cisplatin Plus 5-Fluorouracil Chemotherapy. Nutr. Cancer 2018, 70, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Lin, H.; Wang, J.; Cao, B. Predictive Biomarkers for Immune-Related Adverse Events in Cancer Patients Treated with Immune-Checkpoint Inhibitors. BMC Immunol. 2024, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Furuno, T.; Sogawa, R.; Hashimoto, T.; Matsuo, S.; Shirahama, W.; Kamura, T.; Hosoya, K.; Senjyu, Y.; Yamashita, Y.; Inoue, T.; et al. Association between the Prognostic Nutritional Index and the Occurrence of Immune-Related Adverse Events. Biol. Pharm. Bull. 2024, 47, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- de Ulíbarri, J.I.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A Tool for Controlling Nutritional Status. First Validation in a Hospital Population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Toiyama, Y.; Miki, C.; Inoue, Y.; Tanaka, K.; Mohri, Y.; Kusunoki, M. Evaluation of an Inflammation-Based Prognostic Score for the Identification of Patients Requiring Postoperative Adjuvant Chemotherapy for Stage II Colorectal Cancer. Exp. Ther. Med. 2011, 2, 95–101. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events. Version 5.0. Published 27 November 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 15 March 2024).
- Zhao, M.; Qiu, S.; Wu, X.; Miao, P.; Jiang, Z.; Zhu, T.; Xu, X.; Zhu, Y.; Zhang, B.; Yuan, D.; et al. Efficacy and Safety of Niraparib as First-Line Maintenance Treatment for Patients with Advanced Ovarian Cancer: Real-World Data from a Multicenter Study in China. Target. Oncol. 2023, 18, 869–883. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, Y.; Huang, F.; Wei, X.; Wei, Q.; Hao, X.; Zhang, Y.; Feng, R. Low Prognostic Nutritional Index Predicts Poor Outcome in Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Int. J. Hematol. 2016, 104, 485–490. [Google Scholar] [CrossRef]
- Draeger, D.-L.; Groh, S.; Buchholz, T.; Woehl, M.; Nolting, J.; Hakenberg, O.W. Prediction of Treatment Response and Survival with Chemotherapy for Metastatic Penile Cancer by the Modified Glasgow Prognostic Score. Urol. Int. 2023, 107, 489–495. [Google Scholar] [CrossRef]
- Kono, T.; Sakamoto, K.; Shinden, S.; Ogawa, K. Pre-Therapeutic Nutritional Assessment for Predicting Severe Adverse Events in Patients with Head and Neck Cancer Treated by Radiotherapy. Clin. Nutr. 2017, 36, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Go, S.-I.; Park, S.; Kang, M.H.; Kim, H.-G.; Kim, H.R.; Lee, G.-W. Clinical Impact of Prognostic Nutritional Index in Diffuse Large B Cell Lymphoma. Ann. Hematol. 2019, 98, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bai, P.; Kong, X.; Huang, S.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Prognostic Nutritional Index (PNI) in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy as a Useful Prognostic Indicator. Front. Cell Dev. Biol. 2021, 9, 656741. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Jacob, M.; Tavares, N.; Cruz-Martins, N.; Souto-Moura, C.; Araújo, D.; Novais-Bastos, H.; Santos, V.; Fernandes, G.; Magalhães, A.; et al. Modified Glasgow Prognostic Score Predicts Survival among Advanced Non-Small Cell Lung Carcinoma Patients Treated with Anti-PD1 Agents. Anticancer. Drugs 2021, 32, 567–574. [Google Scholar] [CrossRef]
- Qusairy, Z.; Gangloff, A.; Leung, S.O.A. Dysregulation of Cholesterol Homeostasis in Ovarian Cancer. Curr. Oncol. 2023, 30, 8386–8400. [Google Scholar] [CrossRef]
- Francis, K.E.; Kim, S.I.; Friedlander, M.; Gebski, V.; Ray-Coquard, I.; Clamp, A.; Penson, R.T.; Oza, A.; Perri, T.; Huzarski, T.; et al. The Impact of Olaparib Dose Reduction and Treatment Interruption on Treatment Outcome in the SOLO2/ENGOT-Ov21 Platinum-Sensitive Recurrent Ovarian Cancer. Ann. Oncol. 2022, 33, 593–601. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; González-Navarro, I.; Remón-Ruíz, P.J.; Pereira-Cunill, J.L.; García-Luna, P.P. Perioperative Nutritional Support: A Review of Current Literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef]
- Dale, W.; Klepin, H.D.; Williams, G.R.; Alibhai, S.M.H.; Bergerot, C.; Brintzenhofeszoc, K.; Hopkins, J.O.; Jhawer, M.P.; Katheria, V.; Loh, K.P.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Systemic Cancer Therapy: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4293–4312. [Google Scholar] [CrossRef]
- Berek, J.S.; Matulonis, U.A.; Peen, U.; Ghatage, P.; Mahner, S.; Redondo, A.; Lesoin, A.; Colombo, N.; Vergote, I.; Rosengarten, O.; et al. Safety and Dose Modification for Patients Receiving Niraparib. Ann. Oncol. 2018, 29, 1784–1792. [Google Scholar] [CrossRef]
| n = 71 | |
|---|---|
| Age, median [IQR] | 63 [56–69.5] |
| Age, n (%) | |
| <65 | 41 (57.7) |
| ≥65 | 30 (42.3) |
| BMI (kg/m2), median [IQR] | 21.91 [19.6–24.6] |
| BMI (kg/m2), n (%) | |
| <18.5 | 12 (16.9) |
| ≥18.5 | 59 (83.1) |
| FIGO stage, n (%) | |
| I or II | 5 (7.0) |
| III or IV | 66 (93.0) |
| Primary tumor location, n (%) | |
| Ovary | 51 (71.8) |
| Fallopian tube | 14 (19.7) |
| Peritoneum | 6 (8.5) |
| Previous lines of platinum therapy, n (%) | |
| <3 | 52 (73.2) |
| ≥3 | 19 (26.8) |
| PARP inhibitor a, n (%) | |
| Olaparib | 53 (74.6) |
| Niraparib | 18 (25.4) |
| Initial dose b, n (%) | |
| Standard | 66 (93.0) |
| Reduction | 5 (7.0) |
| Adverse Events | Any Grade (n, %) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Total | 59 (83.1) | 36 (50.7) | 19 (26.8) | 22 (31.0) | 4 (5.6) | 1 (1.4) |
| Anemia | 16 (22.5) | 1 (1.4) | 4 (5.6) | 11 (15.5) | 1 (1.4) | - |
| Neutropenia | 11 (15.5) | - | 1 (1.4) | 9 (12.7) | 1 (1.4) | - |
| Thrombocytopenia | 14 (19.7) | 5 (7.0) | 3 (4.2) | 5 (7.0) | 1 (1.4) | - |
| Creatinine increased | 8 (11.3) | 4 (5.6) | 4 (5.6) | - | - | - |
| Elevated ALT | 2 (2.8) | 1 (1.4) | 1 (1.4) | |||
| Elevated AST | 2 (2.8) | 1 (1.4) | 1 (1.4) | |||
| Hypertension | 2 (2.8) | - | - | 2 (2.8) | - | - |
| Pruritus | 1 (1.4) | 1 (1.4) | - | - | - | - |
| Rash maculo-papular | 1 (1.4) | 1 (1.4) | - | - | - | - |
| Fatigue | 17 (23.9) | 13 (18.3) | 2 (2.8) | 2 (2.8) | - | - |
| Nausea | 18 (25.4) | 15 (21.1) | 3 (4.2) | - | - | - |
| Diarrhea | 1 (1.4) | 1 (1.4) | - | - | - | - |
| Dysgeusia | 6 (8.5) | 4 (5.6) | 2 (2.8) | - | - | - |
| Dizziness | 2 (2.8) | - | 2 (2.8) | - | - | - |
| Peripheral sensory neuropathy | 1 (1.4) | 1 (1.4) | - | - | - | - |
| Stomach pain | 4 (5.6) | 2 (2.8) | 1 (1.4) | 1 (1.4) | - | - |
| Arthralgia | 2 (2.8) | 1 (1.4) | - | 1 (1.4) | - | - |
| Cough | 1 (1.4) | 1 (1.4) | - | - | - | - |
| Pneumonitis | 1 (1.4) | 1 (1.4) | - | - | - | - |
| Leukemia | 1 (1.4) | - | - | - | - | 1 (1.4) |
| No | Age | BMI | FIGO Stage | Histology | Previous Lines of Platinum Therapy | Response to Previous Platinum Therapy | PARP Inhibitor | Treatment Duration (Days) | Adverse Events Leading to Discontinuation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 20.9 | II | Serous carcinoma | 3 | PR | Olaparib | 78 | Stomach pain |
| 2 | 71 | 26.1 | III | Serous carcinoma | 5 | PR | Olaparib | 889 | AML |
| 3 | 69 | 24.8 | IV | SCC | 2 | PR | Olaparib | 30 | Anemia Thrombocytopenia |
| 4 | 68 | 21.0 | IV | HGSC | 2 | CR | Olaparib | 196 | Creatinine increased |
| 5 | 52 | 24.3 | IV | Serous carcinoma | 2 | CR | Niraparib | 174 | Fatigue Arthralgia |
| 6 | 69 | 18.1 | IV | HGSC | 2 | PR | Olaparib | 21 | Creatinine increased |
| 7 | 61 | 20.1 | III | HGSC | 1 | CR | Niraparib | 69 | Elevated ALT and AST |
| 8 | 63 | 21.7 | I | Mucinous carcinoma | 2 | CR | Olaparib | 108 | Anemia |
| 9 | 66 | 22.5 | III | HGSC | 2 | CR | Olaparib | 175 | Anemia Thrombocytopenia |
| 10 | 63 | 17.8 | III | HGSC | 2 | CR | Olaparib | 140 | Anemia Neutropenia |
| 11 | 51 | 17.2 | IV | Clear cell carcinoma | 3 | PR | Niraparib | 77 | Thrombocytopenia |
| 12 | 74 | 26.7 | IV | LGSC | 2 | PR | Olaparib | 126 | Fatigue |
| 13 | 69 | 22.1 | III | Adenocarcinoma | 3 | PR | Olaparib | 183 | Anemia |
| 14 | 53 | 21.2 | III | HGSC | 1 | CR | Olaparib | 169 | Pneumonitis |
| 15 | 70 | 28.2 | III | HGSC | 1 | PR | Niraparib | 35 | Anemia |
| 16 | 55 | 26.5 | III | HGSC | 1 | PR | Olaparib | 15 | Fatigue |
| 17 | 64 | 19.6 | III | Adenocarcinoma | 1 | PR | Olaparib | 113 | Anemia |
| 18 | 79 | 20.6 | IV | HGSC | 1 | CR | Olaparib | 140 | Creatinine increased |
| Factors | Univariate | p-Value | Multivariate | p-Value |
|---|---|---|---|---|
| HR [95%CI] | HR [95%CI] | |||
| Age ≥ 65 | 1.307 [0.517–3.301] | 0.571 | ||
| BMI < 18.5 | 0.959 [0.275–3.337] | 0.947 | ||
| FIGO stage III or IV | 0.436 [0.098–1.935] | 0.275 | ||
| Previous lines of platinum therapy > 2 | 0.809 [0.264–2.478] | 0.710 | ||
| Initial standard dose | 0.043 [0.000–57.142] | 0.391 | ||
| PNI ≤ 48.44 | 3.867 [1.272–11.761] | 0.017 | 4.232 [1.358–13.191] | 0.013 |
| CONUT ≥ 2 | 2.153 [0.511–9.069] | 0.296 | ||
| mGPS ≥1 | 3.554 [1.301–9.711] | 0.013 | 3.678 [1.309–10.333] | 0.013 |
| Factors | Univariate | p-Value | Multivariate | p-Value |
|---|---|---|---|---|
| HR [95%CI] | HR [95%CI] | |||
| Age ≥ 65 | 1.881 [0.594–5.961] | 0.283 | ||
| BMI < 18.5 | 0.335 [0.042–2.639] | 0.299 | ||
| FIGO stage III or IV | 0.410 [0.088–1.920] | 0.258 | ||
| Previous lines of platinum therapy > 2 | 0.538 [0.144–2.009] | 0.356 | ||
| Initial standard dose | 0.044 [0.000–858.143] | 0.535 | ||
| PNI ≤ 48.44 | 1.805 [0.543–6.003] | 0.336 | ||
| CONUT ≥ 2 | 1.290 [0.284–5.861] | 0.741 | ||
| mGPS ≥1 | 6.935 [1.669–28.817] | 0.008 | 8.626 [1.946–38.239] | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Inoue, D.; Tsuyoshi, H.; Nakamura, Y.; Kato, M.; Kato, M.; Niwa, K.; Yashiro, K.; Orisaka, M.; Yoshida, Y. Usefulness of Nutritional Assessment Indicators in Predicting Treatment Discontinuation Due to Adverse Events from PARP Inhibitors in Ovarian Cancer Patients. Cancers 2024, 16, 3602. https://doi.org/10.3390/cancers16213602
Tanaka Y, Inoue D, Tsuyoshi H, Nakamura Y, Kato M, Kato M, Niwa K, Yashiro K, Orisaka M, Yoshida Y. Usefulness of Nutritional Assessment Indicators in Predicting Treatment Discontinuation Due to Adverse Events from PARP Inhibitors in Ovarian Cancer Patients. Cancers. 2024; 16(21):3602. https://doi.org/10.3390/cancers16213602
Chicago/Turabian StyleTanaka, Yoshiaki, Daisuke Inoue, Hideaki Tsuyoshi, Yuriko Nakamura, Masato Kato, Masataka Kato, Kentaro Niwa, Kenji Yashiro, Makoto Orisaka, and Yoshio Yoshida. 2024. "Usefulness of Nutritional Assessment Indicators in Predicting Treatment Discontinuation Due to Adverse Events from PARP Inhibitors in Ovarian Cancer Patients" Cancers 16, no. 21: 3602. https://doi.org/10.3390/cancers16213602
APA StyleTanaka, Y., Inoue, D., Tsuyoshi, H., Nakamura, Y., Kato, M., Kato, M., Niwa, K., Yashiro, K., Orisaka, M., & Yoshida, Y. (2024). Usefulness of Nutritional Assessment Indicators in Predicting Treatment Discontinuation Due to Adverse Events from PARP Inhibitors in Ovarian Cancer Patients. Cancers, 16(21), 3602. https://doi.org/10.3390/cancers16213602





