Multiparametric Approach to the Colorectal Cancer Phenotypes Integrating Morphofunctional Assessment and Computer Tomography
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric and Morphofunctional Assessment
2.2.1. BIVA
2.2.2. Nutritional Ultrasound
2.2.3. Functional Assessment
2.2.4. CT FocusedOn®
2.3. Assessment of Sarcopenia and Low Muscle Mass
2.4. Statistical Analyses
3. Results
3.1. Body Composition Parameters and Functional Status: BIVA, NU, HGS, and CT
3.2. Comparison with Reference Value of Sarcopenia
3.3. Correlation Analysis between Muscle Measures: CT, BIVA, NU, and Functional Test (HGS)
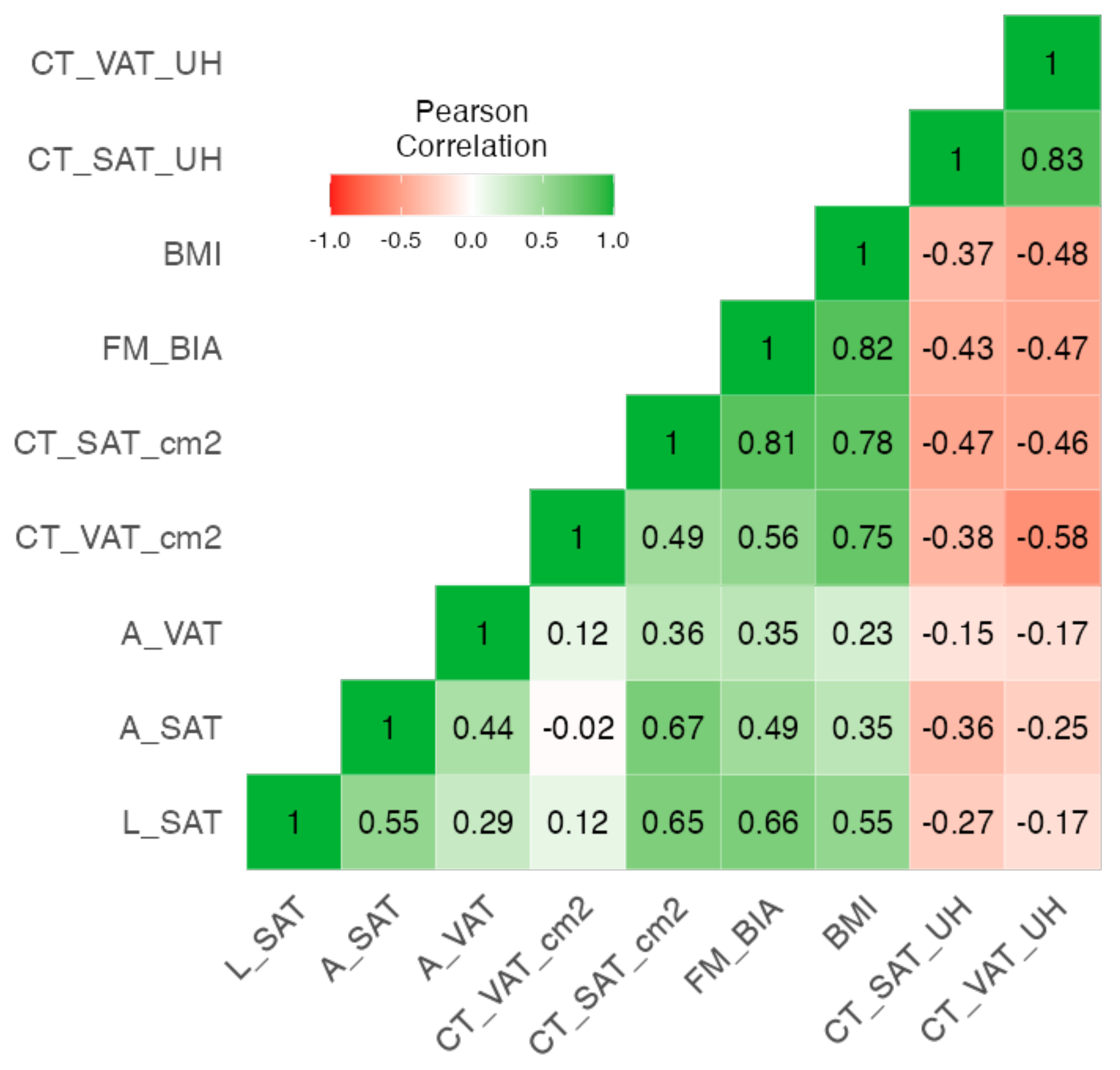
3.4. Correlation Analysis between Adipose Measures: CT, BIVA, and NU
3.5. Regression Model Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aran, V.; Victorino, A.P.; Thuler, L.C.; Ferreira, C.G. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin. Color. Cancer 2016, 15, 195–203. [Google Scholar]
- Kocarnik, J.M.; Hua, X.; Hardikar, S.; Robinson, J.; Lindor, N.M.; Win, A.K.; Hopper, J.L.; Figueiredo, J.C.; Potter, J.D.; Campbell, P.T.; et al. Long-term weight loss after colorectal cancer diagnosis is associated with lower survival: The Colon Cancer Family Registry. Cancer 2017, 123, 4701–4708. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; García-Almeida, J.M.; Bellido-Guerrero, D.; Talluri, A.; Lukaski, H.; Tinahones, F.J. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: A longitudinal cohort study. Clin. Nutr. 2022, 41, 3106–3114. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7886631/ (accessed on 28 October 2021).
- Grundmann, O.; Yoon, S.L.; Williams, J.J. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—A comprehensive review. Eur. J. Clin. Nutr. 2015, 69, 1290–1297. [Google Scholar]
- Paiva, S.I.; Borges, L.R.; Halpern-Silveira, D.; Assunção, M.C.F.; Barros, A.J.D.; Gonzalez, M.C. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support. Care Cancer 2011, 19, 187–192. [Google Scholar]
- Sánchez-Torralvo, F.J.; González-Poveda, I.; García-Olivares, M.; Porras, N.; Gonzalo-Marín, M.; Tapia, M.J.; Mera-Velasco, S.; Toval-Mata, J.A.; Ruiz-López, M.; Carrasco-Campos, J.; et al. Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients 2022, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- Vegas-Aguilar, I.M.; Guirado-Peláez, P.; Fernández-Jiménez, R.; Boughanem, H.; Tinahones, F.J.; Garcia-Almeida, J.M. Exploratory Assessment of Nutritional Evaluation Tools as Predictors of Complications and Sarcopenia in Patients with Colorectal Cancer. Cancers 2023, 15, 847. [Google Scholar] [CrossRef] [PubMed]
- Golder, A.M.; Sin, L.K.E.; Alani, F.; Alasadi, A.; Dolan, R.; Mansouri, D.; Horgan, P.G.; McMillan, D.C.; Roxburgh, C.S. The relationship between the mode of presentation, CT-derived body composition, systemic inflammatory grade and survival in colon cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 2863–2874. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70, 74–84. Available online: https://www.sciencedirect.com/science/article/pii/S2530016422001471 (accessed on 28 October 2021).
- Deng, M.; Yan, L.; Tong, R.; Zhao, J.; Li, Y.; Yin, Y.; Zhang, Q.; Gao, J.; Wang, Q.; Hou, G.; et al. Ultrasound Assessment of the Rectus Femoris in Patients with Chronic Obstructive Pulmonary Disease Predicts Sarcopenia. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 2801–2810. [Google Scholar] [CrossRef]
- Sánchez-Torralvo, F.J.; Porras, N.; Ruiz-García, I.; Maldonado-Araque, C.; García-Olivares, M.; Girón, M.V.; Gonzalo-Marín, M.; Olveira, C.; Olveira, G. Usefulness of Muscle Ultrasonography in the Nutritional Assessment of Adult Patients with Cystic Fibrosis. Nutrients 2022, 14, 3377. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; Cabrera Cesar, E.; Sánchez García, A.; Espíldora Hernández, F.; Vegas-Aguilar, I.M.; Amaya-Campos, M.D.M.; Cornejo-Pareja, I.; Guirado-Peláez, P.; Simón-Frapolli, V.; Murri, M.; et al. Rectus Femoris Cross-Sectional Area and Phase Angle asPredictors of 12-Month Mortality in Idiopathic Pulmonary Fibrosis Patients. Nutrients 2023, 15, 4473. [Google Scholar] [CrossRef]
- Simón-Frapolli, V.J.; Vegas-Aguilar, I.M.; Fernández-Jiménez, R.; Cornejo-Pareja, I.M.; Sánchez-García, A.M.; Martínez-López, P.; Nuevo-Ortega, P.; Reina-Artacho, C.; Estecha-Foncea, M.A.; Gómez-González, A.M.; et al. Phase angle and rectus femoris cross-sectional area as predictors of severe malnutrition and their relationship with complications in outpatients with post-critical SARS-CoV2 disease. Front. Nutr. 2023, 10, 1218266. [Google Scholar] [CrossRef]
- Nakanishi, R.; Oki, E.; Sasaki, S.; Hirose, K.; Jogo, T.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Kudo, K.; Kurashige, J.; et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg. Today 2018, 48, 151–157. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Soler-Beunza, A.G.; Vegas-Aguilar, I.M.; Fernández-Jiménez, R.; Tinahones, F.J.; García-Almeida, J.M. Predictors of Sarcopenia in Outpatients with Post-Critical SARS-CoV2 Disease. Nutritional Ultrasound of Rectus Femoris Muscle, a Potential Tool. Nutrients 2022, 14, 4988. [Google Scholar] [CrossRef]
- Piccoli, A.; Pastori, G. BIVA software; Department of Medical and Surgical Sciences, University of Padova: Padova, Italy, 2002. [Google Scholar]
- de Luis Roman, D.; García Almeida, J.M.; Bellido Guerrero, D.; Guzmán Rolo, G.; Martín, A.; Primo Martín, D.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Tejera Pérez, C.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Coratella, G.; Cerullo, G.; Stagi, S.; Paoli, S.; Marini, S.; Grigoletto, A.; Moroni, A.; Petri, C.; Andreoli, A.; et al. New bioelectrical impedance vector references and phase angle centile curves in 4367 adults: The need for an urgent update after 30 years. Clin. Nutr. 2023, 42, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Ciudin, A.; Guerra, R.; Eiroa, D.; Espinet, C.; Roson, N.; Burgos, R.; Simó, R. Comparison of computed tomography and dual-energy X-ray absorptiometry in the evaluation of body composition in patients with obesity. Front. Endocrinol. 2023, 14, 1161116. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef]
- Martin, D.; Maeder, Y.; Kobayashi, K.; Schneider, M.; Koerfer, J.; Melloul, E.; Halkic, N.; Hübner, M.; Demartines, N.; Becce, F.; et al. Association between CT-Based Preoperative Sarcopenia and Outcomes in Patients That Underwent Liver Resections. Cancers 2022, 14, 261. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Tewari, N.; Awad, S.; Macdonald, I.A.; Lobo, D.N. A comparison of three methods to assess body composition. Nutrition 2018, 47, 1–5. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.R.; Orsso, C.E.; Gonzalez, M.C.; Sicchieri, J.M.F.; Mialich, M.S.; Jordao, A.A.; Prado, C.M. Phase angle and cellular health: Inflammation and oxidative damage. Rev. Endocr. Metab. Disord. 2022, 24, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Prior-Sánchez, I.; Herrera-Martínez, A.D.; Zarco-Martín, M.T.; Fernández-Jiménez, R.; Gonzalo-Marín, M.; Muñoz-Garach, A.; Vilchez-López, F.J.; Cayón-Blanco, M.; Villarrubia-Pozo, A.; Muñoz-Jiménez, C.; et al. Prognostic value of bioelectrical impedance analysis in head and neck cancer patients undergoing radiotherapy: A VALOR® study. Front. Nutr. 2024, 11, 1335052. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, C.S.A.; Queiroz, N.P.; Silva, D.M.; Freitas, A.T.V.S.; Costa, N.A.; Peixoto, M.R.G. Comparison of Bioelectrical Impedance Vector Analysis (BIVA) to 7-point Subjective Global Assessment for the diagnosis of malnutrition. J. Bras. Nefrol. 2022, 44, 171–178. [Google Scholar] [CrossRef]
- Castillo-Martínez, L.; Colín-Ramírez, E.; Orea-Tejeda, A.; González Islas, D.G.; Rodríguez García, W.D.; Santillán Díaz, C.; Gutiérrez Rodríguez, A.E.; Vázquez Durán, M.; Keirns Davies, C. Cachexia assessed by bioimpedance vector analysis as a prognostic indicator in chronic stable heart failure patients. Nutrition 2012, 28, 886–891. [Google Scholar] [CrossRef]
- Espinosa-Cuevas, Á.; Ch-Durán, L.-X.; Carsi, X.A.; González-Ortiz, A.; Ramos-Acevedo, S.; López-Cisneros, S.; Correa Rotter, R.; Miranda Alatriste, P.V. Agreement between vector analysis and body composition measurements by four types of bioelectrical impedance technology in hemodialysis patients. Nutr. Hosp. 2022, 39, 1047–1057. [Google Scholar]
- Lee, C.M.; Kang, J. Prognostic impact of myosteatosis in patients with colorectal cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1270–1282. [Google Scholar] [CrossRef]
- Mortellaro, S.; Triggiani, S.; Mascaretti, F.; Galloni, M.; Garrone, O.; Carrafiello, G.; Ghidini, M. Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review. J. Pers. Med. 2024, 14, 243. [Google Scholar] [CrossRef]
- Ji, W.; Liu, X.; Zhang, Y.; Zhao, Y.; He, Y.; Cui, J.; Li, W. Development of Formulas for Calculating L3 Skeletal Muscle Mass Index and Visceral Fat Area Based on Anthropometric Parameters. Front. Nutr. 2022, 9, 910771. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kyttä, J.; Cancino, W.; Rinta-Kiikka, I.; Pertuz, S.; Arponen, O. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef]


| All | Male | Female | p-Value | |
|---|---|---|---|---|
| N = 267 | N = 165 | N = 102 | ||
| Age (years) | 68.2 ± 10.9 | 68.3 ± 11.4 | 68.1 ± 9.97 | 0.87 |
| Gender | 165 (61.8%) | 102 (38.2%) | ||
| BMI (kg/m2) | 26.8 ± 4.93 | 26.5 ± 4.30 | 27.3 ± 5.80 | 0.28 |
| Malnutrition GLIM criteria Type of cancer | 99 (37.1%) | 61 (22.8%) | 38 (14.2%) | 0.96 |
| Colon | 215 (80.5%) | 131 (49.1%) | 84 (31.5%) | |
| Rectum | 52(19.5%) | 34 (12.7%) | 18 (6.7%) | |
| Stage | ||||
| Unknown at valuation | 15 (5.6%) | 8 (3.0%) | 7 (2.6%) | 0.57 |
| I | 62 (23.2%) | 37 (13.9%) | 25 (9.4%) | |
| II | 84 (31.5%) | 56 (21.0%) | 28 (10.5%) | |
| III | 91 (34.1%) | 54 (20.2%) | 37 (13.9%) | |
| IV | 15 (5.6%) | 10 (3.7%) | 5 (1.9%) | |
| Type of surgery | 0.57 | |||
| Open | 13 (4.9%) | 9 (3.4%) | 4 (1.5%) | |
| Laparoscopic | 254 (95.1%) | 156 (58.4%) | 98 (36.7%) | |
| Outcomes | ||||
| Days of admission | 7.32 ± 6.43 | 8.02 ± 7.48 | 6.19 ± 3.99 | 0.02 * |
| Éxitus | 23 (8.6%) | 17 (6.4%) | 6 (2.2%) | 0.21 |
| Immediate Complication | 65 (24.3%) | 43 (15.7%) | 23 (8.6%) | 0.59 |
| Male (n = 165) | Female (n = 102) | p-Value | |
|---|---|---|---|
| BIVA Raw Bioelectrical data | |||
| Rz | 439 ± 77.1 | 483 ± 89.1 | <0.001 |
| Xc | 43.9 ± 10.7 | 43.9 ± 10.5 | 0.955 |
| Phase angle (°) | 5.71 ± 1.15 | 5.20 ± 0.927 | <0.001 |
| BCM (kg) | 34.4 ± 6.68 | 25.9 ± 5.06 | <0.001 |
| Validate BIVA equation | |||
| FFM (kg) | 59.8 ± 9.07 | 45.4 ± 7.29 | <0.001 |
| FFMI (kg/m2) | 20.6 ± 2.81 | 18.0 ± 2.34 | <0.001 |
| FM (kg) | 18.8 ± 9.12 | 20.9 ± 9.24 | 0.080 |
| FM (%) | 24.4 ± 11.3 | 30.3 ± 10.9 | <0.001 |
| ASMM (kg) | 22.8 ± 3.48 | 17.2 ± 3.14 | <0.001 |
| ASMMI (kg/m2) | 7.89 ± 1.06 | 6.83 ± 1.16 | <0.001 |
| Nutritional Ultrasound (NU) | |||
| Rectus femoris cross-sectional area (RF-CSA) (cm2) | 4.35 ± 1.45 | 3.22 ± 1.03 | <0.001 |
| RF-X axis (cm) | 3.82 ± 0.508 | 3.74 ± 3.06 | 0.731 |
| RF-Y axis (cm) | 1.36 ± 0.348 | 1.24 ± 1.04 | 0.174 |
| Leg Subcutaneous adipose tissue (L-SAT) (cm) | 0.594 ± 0.293 | 1.49 ± 1.90 | <0.001 |
| Abdominal Subcutaneous adipose tissue (A-SAT) (cm) | 1.31 ± 0.643 | 2.19 ± 0.899 | <0.001 |
| Preperitoneal adipose tissue (A-VAT) (cm) | 0.685 ± 0.295 | 0.939 ± 0.418 | 0.001 |
| Functional test | |||
| Handgrip strength (kg) | 32.7 ± 9.06 | 19.1 ± 6.64 | <0.001 |
| Male N = 165 | Female N = 102 | p-Value | ||
|---|---|---|---|---|
| Muscle area (SMA) | mean ± SD | 130 ± 23.7 | 92.4 ±15.8 | <0.001 |
| Muscle (%) | mean ± SD | 18.0 ± 4.31 | 14.3 ± 4.21 | <0.001 |
| Muscle (HU) | mean ± SD | 41.0 ± 9.31 | 38.1 ± 9.86 | 0.015 |
| SMI-CT | mean ± SD | 44.8 ± 7.47 | 36.8 ± 5.60 | <0.001 |
| IMAT area | mean ± SD | 15.7 ± 11.4 | 17.3 ± 10.6 | 0.256 |
| IMAT (%) | mean ± SD | 2.65 ± 4.28 | 2.48 ± 1.27 | 0.703 |
| IMAT (HU) | mean ± SD | −64.4 ± 6.33 | −65.8 ± 6.74 | 0.095 |
| CT-VAT area | mean ± SD | 200 ± 117 | 143 ± 83.3 | <0.001 |
| CT-VAT (%) | mean ± SD | 31.0 ± 37.9 | 19.4 ± 8.76 | 0.003 |
| CT-VAT (UH) | mean ± SD | −93.8 ± 8.58 | −93.3 ± 9.18 | 0.650 |
| CT-SAT area | mean ± SD | 156 ± 70.4 | 232 ± 124 | <0.001 |
| CT-SAT (%) | mean ± SD | 26.3 ± 41.3 | 31.8 ± 10.5 | 0.190 |
| CT-SAT (HU) | mean ± SD | −96.6 ± 11.5 | −99.8 ± 11.3 | 0.027 |
| Reference Value | Total | n = 267 |
|---|---|---|
| Sarcopenia CT | ||
| SMI (kg/m2) | ||
| Low SMI (Martin) | Total | 117 (43.8%) |
| Male | n (%) | 75 (28.1%) |
| Female | n (%) | 42 (15.7%) |
| Low SMI (Prado) | Total | 133 (49.8%) |
| Male | n (%) | 109 (40.8%) |
| Female | n (%) | 24 (9%) |
| Sarcopenia (EWGSOP2 criteria) | n (%) | 9 (3.7%) |
| Handgrip strength | ||
| Low HGS | Total | 60 (27.5%) |
| Male | n (%) | 39 (15.3%) |
| Female | n (%) | 31 (12.2%) |
| ASMMI (kg) | ||
| Low ASMMI | Total | 27 (11.2%) |
| Male | n (%) | 22 (9.1%) |
| Female | n (%) | 5 (2.1%) |
| SAT-CT cm2 | Total | n = 267 |
|---|---|---|
| Hight fat mass CT (Caan) | Total | 77 (28.8%) |
| Male | n (%) | 41 (15.4%) |
| Female | n (%) | 36 (13.5%) |
| Muscle Quality (UH) | ||
| Myoesteatosis CT (Dolan) | Total | 65 (24.3%) |
| Male | n (%) | 29 (10.9%) |
| Female | n (%) | 36 (13.5%) |
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | Lower | Upper | t | p |
| Intercept | 23.211 | 23.7882 | −23.681 | 70.102 | 0.976 | 0.330 |
| Gender: | ||||||
| Male Female | 14.821 | 2.6860 | 9.526 | 20.116 | 5.518 | <0.001 |
| Age | −0.332 | 0.0919 | −0.513 | −0.151 | −3.618 | <0.001 |
| Weight | 0.547 | 0.0811 | 0.387 | 0.707 | 6.743 | <0.001 |
| Hight | 9.834 | 14.6346 | −19.014 | 38.682 | 0.672 | 0.502 |
| RF_CSA | 2.298 | 0.8618 | 0.600 | 3.997 | 2.667 | 0.008 |
| HGS | 0.524 | 0.1428 | 0.242 | 0.805 | 3.668 | <0.001 |
| BCM | 0.808 | 0.1944 | 0.425 | 1.191 | 4.155 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guirado-Peláez, P.; Fernández-Jiménez, R.; Sánchez-Torralvo, F.J.; Mucarzel Suárez-Arana, F.; Palmas-Candia, F.X.; Vegas-Aguilar, I.; Amaya-Campos, M.d.M.; Martínez Tamés, G.; Soria-Utrilla, V.; Tinahones-Madueño, F.; et al. Multiparametric Approach to the Colorectal Cancer Phenotypes Integrating Morphofunctional Assessment and Computer Tomography. Cancers 2024, 16, 3493. https://doi.org/10.3390/cancers16203493
Guirado-Peláez P, Fernández-Jiménez R, Sánchez-Torralvo FJ, Mucarzel Suárez-Arana F, Palmas-Candia FX, Vegas-Aguilar I, Amaya-Campos MdM, Martínez Tamés G, Soria-Utrilla V, Tinahones-Madueño F, et al. Multiparametric Approach to the Colorectal Cancer Phenotypes Integrating Morphofunctional Assessment and Computer Tomography. Cancers. 2024; 16(20):3493. https://doi.org/10.3390/cancers16203493
Chicago/Turabian StyleGuirado-Peláez, Patricia, Rocío Fernández-Jiménez, Francisco José Sánchez-Torralvo, Fernanda Mucarzel Suárez-Arana, Fiorella Ximena Palmas-Candia, Isabel Vegas-Aguilar, María del Mar Amaya-Campos, Gema Martínez Tamés, Virginia Soria-Utrilla, Francisco Tinahones-Madueño, and et al. 2024. "Multiparametric Approach to the Colorectal Cancer Phenotypes Integrating Morphofunctional Assessment and Computer Tomography" Cancers 16, no. 20: 3493. https://doi.org/10.3390/cancers16203493
APA StyleGuirado-Peláez, P., Fernández-Jiménez, R., Sánchez-Torralvo, F. J., Mucarzel Suárez-Arana, F., Palmas-Candia, F. X., Vegas-Aguilar, I., Amaya-Campos, M. d. M., Martínez Tamés, G., Soria-Utrilla, V., Tinahones-Madueño, F., García-Almeida, J. M., Burgos-Peláez, R., & Olveira, G. (2024). Multiparametric Approach to the Colorectal Cancer Phenotypes Integrating Morphofunctional Assessment and Computer Tomography. Cancers, 16(20), 3493. https://doi.org/10.3390/cancers16203493






