Comparing Lenvatinib/Pembrolizumab with Atezolizumab/Bevacizumab in Unresectable Hepatocellular Carcinoma: A Real-World Experience with Propensity Score Matching Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
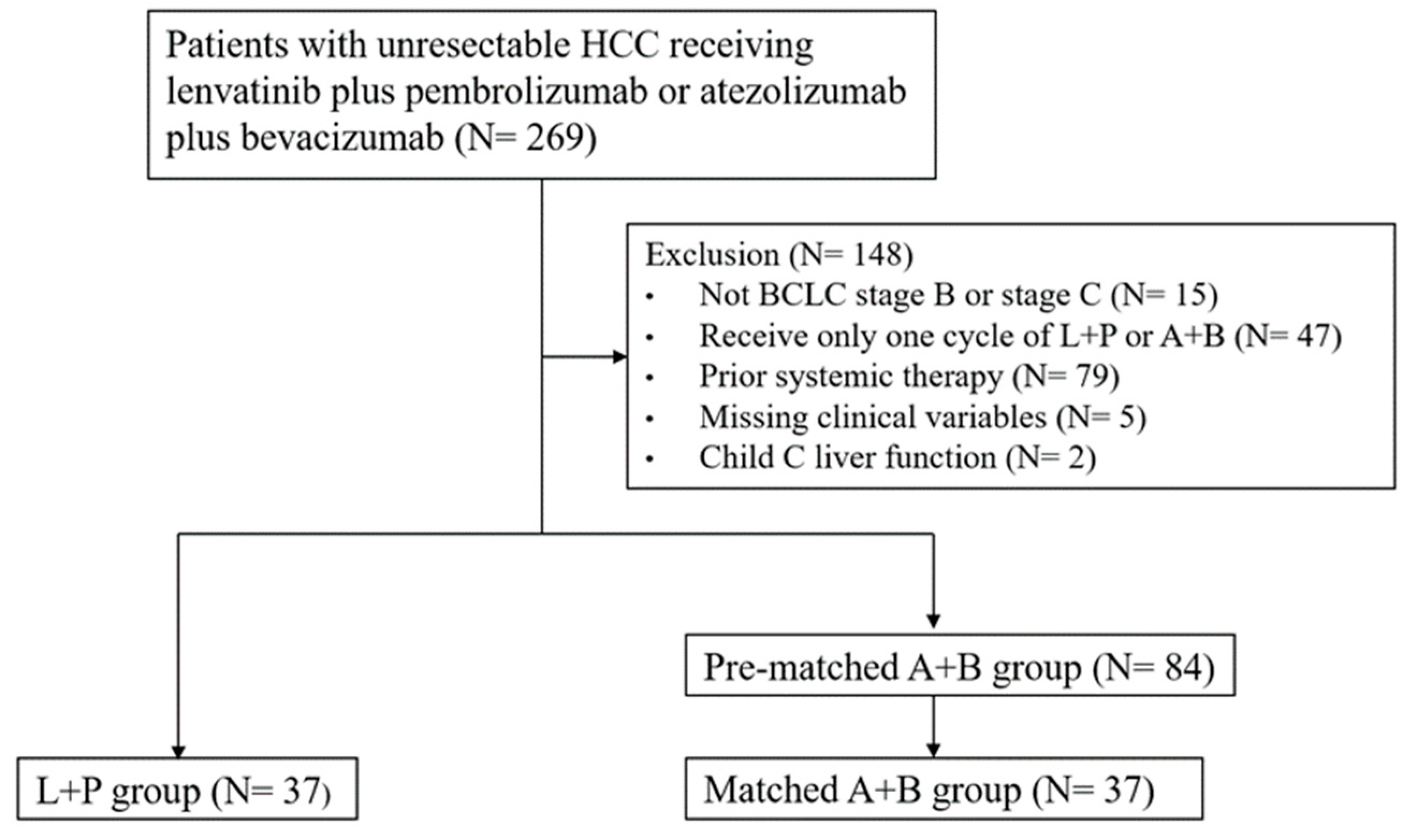
2.1. Study Design and Patients
2.2. Treatments
2.3. Assessments
2.4. Propensity Score Matching and Statistical Analyses
3. Results
3.1. Baseline Characteristics of HCC Patients Receiving Either A+B or L+P Treatment
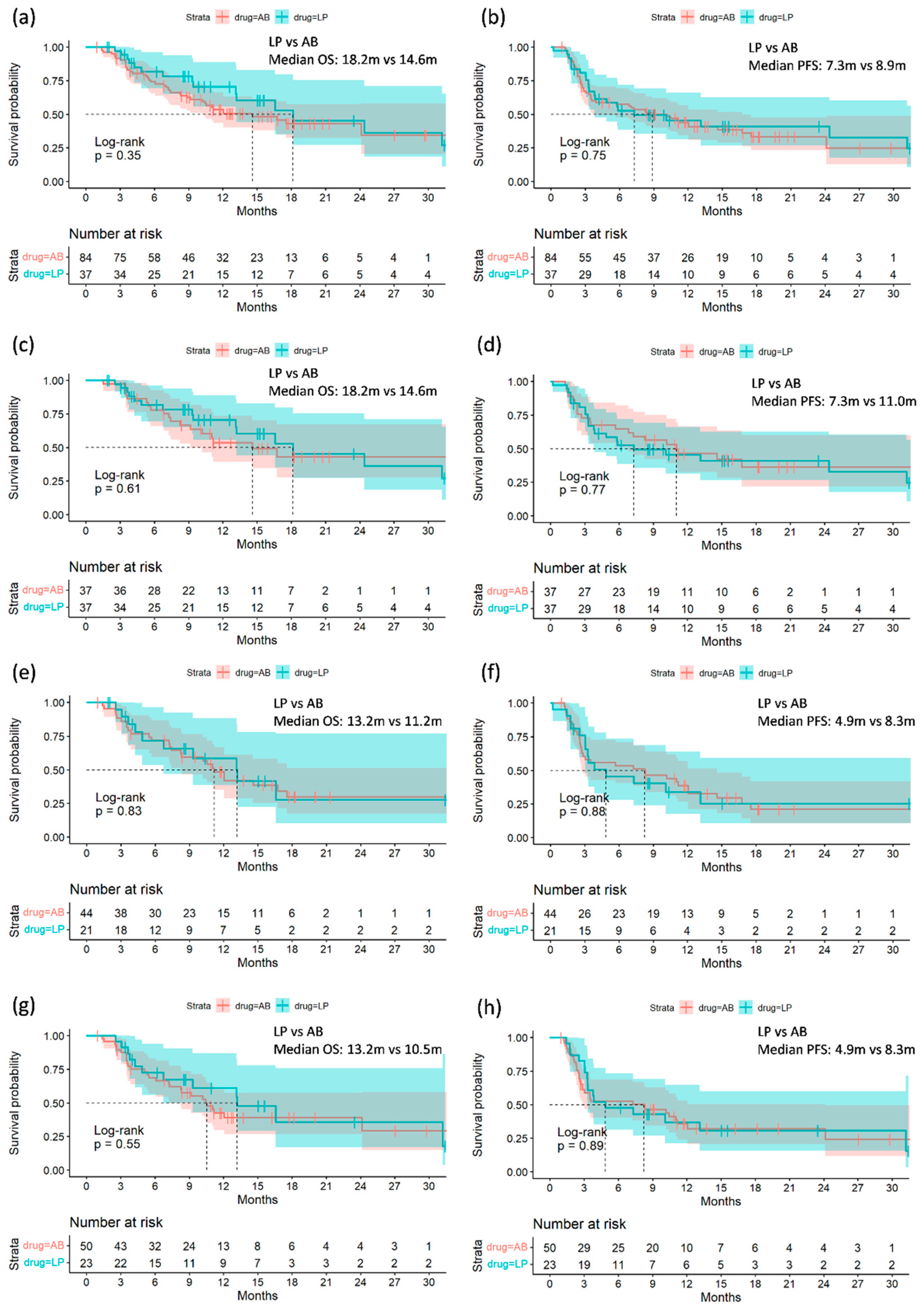
3.2. Comparing Treatment Efficacy of A+B and L+P before and after Propensity Score Matching
3.3. Clinical Outcome Predictors in HCC Patients Undergoing A+B and L+P Treatment
3.4. Efficacy across the Subgroups
3.5. Comparison of Treatment-Related Adverse Events (TRAE) between Two Treatment Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ohkawa, K.; Miyazaki, M.; Sakakibara, M.; Imanaka, K.; Tamura, T.; Sueyoshi, H.; Takada, R.; Fukutake, N.; Uehara, H.; et al. Subclassification of patients with intermediate-stage (Barcelona Clinic Liver Cancer stage-B) hepatocellular carcinoma using the up-to-seven criteria and serum tumor markers. Hepatol. Int. 2017, 11, 105–114. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, J.H.; Lee, H.C.; Sung, K.B.; Ko, H.K.; Ko, G.Y.; Gwon, D.I.; Kim, J.W.; Lim, Y.S.; Park, S.H. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int. 2017, 37, 1861–1868. [Google Scholar] [CrossRef]
- Lee, P.C.; Chao, Y.; Chen, M.H.; Lan, K.H.; Lee, C.J.; Lee, I.C.; Chen, S.C.; Hou, M.C.; Huang, Y.H. Predictors of Response and Survival in Immune Checkpoint Inhibitor-Treated Unresectable Hepatocellular Carcinoma. Cancers 2020, 12, 182. [Google Scholar] [CrossRef]
- Facciorusso, A.; Tartaglia, N.; Villani, R.; Serviddio, G.; Ramai, D.; Mohan, B.P.; Chandan, S.; Abd El Aziz, M.A.; Evangelista, J.; Cotsoglou, C.; et al. Lenvatinib versus sorafenib as first-line therapy of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2379–2387. [Google Scholar]
- Fulgenzi, C.A.M.; Scheiner, B.; Korolewicz, J.; Stikas, C.V.; Gennari, A.; Vincenzi, B.; Openshaw, M.R.; Silletta, M.; Pinter, M.; Cortellini, A.; et al. Efficacy and safety of frontline systemic therapy for advanced HCC: A network meta-analysis of landmark phase III trials. JHEP Rep. 2023, 5, 100702. [Google Scholar] [CrossRef]
- Yang, X.; Chen, B.; Wang, Y.; Wang, Y.; Long, J.; Zhang, N.; Xue, J.; Xun, Z.; Zhang, L.; Cheng, J.; et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol. Int. 2023, 17, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, R.; Li, L.; Wang, G.; Ji, S.; Zheng, X.; Jia, X.; Tao, H.; Hu, Y. Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: A real-world experience. Ther. Adv. Med. Oncol. 2023, 15, 17588359231206274. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Sun, Q.C.; Jian, Y.; He, J.Z.; Zhu, H.B.; Hong, C.; Zeng, L.; Li, R.N.; Wang, J.R.; Li, Y.; et al. Efficacy and safety of different PD-1 inhibitors in combination with lenvatinib in the treatment of unresectable primary liver cancer: A multicentre retrospective study. Discov. Oncol. 2023, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Lee, P.C.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Hou, M.C.; Huang, Y.H. Lenvatinib plus pembrolizumab for systemic therapy-naive and -experienced unresectable hepatocellular carcinoma. Cancer Immunol. Immunother. 2022, 71, 2631–2643. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Mei, J.; Yang, Z.; Chen, M.; Liang, T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: An exploration for expanded indications. BMC Cancer 2022, 22, 293. [Google Scholar] [CrossRef]
- Chen, K.; Wei, W.; Liu, L.; Deng, Z.J.; Li, L.; Liang, X.M.; Guo, P.P.; Qi, L.N.; Zhang, Z.M.; Gong, W.F.; et al. Lenvatinib with or without immune checkpoint inhibitors for patients with unresectable hepatocellular carcinoma in real-world clinical practice. Cancer Immunol. Immunother. 2022, 71, 1063–1074. [Google Scholar] [CrossRef]
- Tao, Z.W.; Cheng, B.Q.; Zhou, T.; Gao, Y.J. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: A narrative review. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 134–144. [Google Scholar] [CrossRef]
- Chuma, M.; Uojima, H.; Hiraoka, A.; Kobayashi, S.; Toyoda, H.; Tada, T.; Hidaka, H.; Iwabuchi, S.; Numata, K.; Itobayashi, E.; et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: A multicenter analysis. Hepatol. Res. 2021, 51, 201–215. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Anticancer. Res. 2020, 40, 2283–2290. [Google Scholar] [CrossRef]
- Breder, V.V.; Vogel, A.; Merle, P.; Finn, R.S.; Galle, P.R.; Zhu, A.X.; Cheng, A.-L.; Feng, Y.-H.; Li, D.; Gaillard, V.E.; et al. IMbrave150: Exploratory efficacy and safety results of hepatocellular carcinoma (HCC) patients (pts) with main trunk and/or contralateral portal vein invasion (Vp4) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in a global Ph III study. J. Clin. Oncol. 2021, 39, 4073. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Z.; Chen, S.; Luo, Z.; Tu, J.; Qiao, L.; Wu, J.; Fan, W.; Peng, Z. Sintilimab plus bevacizumab combined with radiotherapy as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A multicenter, single-arm, phase 2 study. Hepatology 2024, 80, 807–815. [Google Scholar] [CrossRef]
- Pasta, A.; Calabrese, F.; Jaffe, A.; Labanca, S.; Marenco, S.; Pieri, G.; Plaz Torres, M.C.; Strazzabosco, M.; Giannini, E.G. Safety and Efficacy of Atezolizumab/Bevacizumab in Patients with Hepatocellular Carcinoma and Impaired Liver Function: A Systematic Review and Meta-Analysis. Liver Cancer 2023, 13, 235–245. [Google Scholar] [CrossRef]
| Entire | L+P | Pre-Matched A+B | p Value | |
|---|---|---|---|---|
| Number | 121 | 37 | 84 | |
| Age (mean (SD)) | 61.50 (12.27) | 59.01 (12.78) | 62.59 (11.96) | 0.14 |
| Sex: Male (%) | 95 (78.5) | 29 (78.4) | 66 (78.6) | 1 |
| HBV (%) | 83 (68.6) | 26 (70.3) | 57 (67.9) | 0.96 |
| HCV (%) | 20 (16.7) | 6 (16.2) | 14 (16.9) | 1 |
| Viral etiology (%) | 99 (81.8) | 31 (83.8) | 68 (81.0) | 0.91 |
| Child–Pugh A/B (%) | 107/14 (88.4/11.6) | 30/7 (81.1/18.9) | 77/7 (91.7/8.3) | 0.17 |
| Baseline ALBI grade I/II+III (%) | 48/73 (39.7/60.3) | 14/23 (37.8/62.2) | 34/50 (40.5/59.5) | 0.94 |
| BCLC C (%) | 95 (78.5) | 29 (78.4) | 66 (78.6) | 1 |
| Out of up-to-seven criteria (%) | 103 (85.1) | 30 (81.1) | 73 (86.9) | 0.58 |
| Macrovascular invasion (%) | 0.28 | |||
| No MVI | 54 (44.6) | 17 (45.9) | 37 (44.0) | |
| Vp2 and Vp3 stage | 37 (30.6) | 14 (37.8) | 23 (27.4) | |
| Vp4 stage | 30 (24.8) | 6 (16.2) | 24 (28.6) | |
| Extrahepatic spread (%) | 52 (43.0) | 18 (48.6) | 34 (40.5) | 0.52 |
| Platelets (mean (SD)), 1000/uL | 221.88 (106.18) | 222.09 (110.52) | 221.80 (105.00) | 0.99 |
| INR (mean (SD)) | 1.17 (0.16) | 1.17 (0.18) | 1.18 (0.15) | 0.86 |
| AST (mean (SD)), U/L | 84.00 (76.45) | 98.94 (87.19) | 77.60 (70.96) | 0.16 |
| ALT (mean (SD)), U/L | 68.04 (62.28) | 64.14 (43.65) | 69.78 (69.16) | 0.65 |
| Albumin (mean (SD)), g/dL | 3.76 (0.50) | 3.76 (0.44) | 3.76 (0.52) | 1 |
| Total bilirubin (mean (SD)), mg/dL | 1.20 (1.61) | 1.49 (2.64) | 1.07 (0.82) | 0.18 |
| AFP (median [range]), ng/mL | 555.40 [2.00, 333,387.70] | 1030.20 [2.80, 252,632.80] | 463.50 [2.00, 333,387.70] | 0.14 |
| AFP greater than 400 ng/mL (%) | 65 (53.7) | 21 (56.8) | 44 (52.4) | 0.8 |
| Combine locoregional therapy | 47 (38.8) | 11 (29.7) | 36 (42.9) | 0.24 |
| Entire (n = 121) | L+P (n = 37) | Pre-Matched A+B (n = 84) | p Value | Post-Matched (n = 74) | Matched A+B (n = 37) | p Value | |
|---|---|---|---|---|---|---|---|
| Tumor response at the first evaluation (%) | 0.93 | 0.52 | |||||
| CR | 1 (0.8) | 0 (0.0) | 1 (1.2) | 1 (1.4) | 1 (2.7) | ||
| PR | 38 (31.4) | 11 (29.7) | 27 (32.1) | 24 (32.4) | 13 (35.1) | ||
| SD | 38 (31.4) | 13 (35.1) | 25 (29.8) | 26 (35.1) | 13 (35.1) | ||
| PD | 36 (29.8) | 11 (29.7) | 25 (29.8) | 21 (28.4) | 10 (27.0) | ||
| No image evaluation | 8 (6.6) | 2 (5.4) | 6 (7.1) | 2 (2.7) | 0 (0.0) | ||
| ORR | 32% | 30% | 33% | 0.7 | 34% | 38% | 0.47 |
| DCR | 64% | 65% | 63% | 0.85 | 69% | 73% | 0.46 |
| Follow-up duration, months (median [range]) | 9.90 [1.00, 39.42] | 9.39 [1.90, 39.42] | 10.12 [1.00, 33.33] | 0.9 | 10.12 [1.50, 39.42] | 10.90 [1.50, 33.33] | 0.67 |
| Treatment duration, months (median [range]) | 3.00 [0.61, 32.58] | 2.84 [0.68, 13.87] | 3.17 [0.61, 32.58] | 0.88 | 3.31 [0.68, 32.58] | 4.07 [0.68, 32.58] | 0.53 |
| Mortality | 57 (47.1%) | 15 (40.5%) | 42 (50.0%) | 0.45 | 33 (44.6%) | 18 (48.6%) | 0.64 |
| Cancer-related mortality | 53 (43.8%) | 15 (40.5%) | 38 (45.2%) | 0.78 | 32 (43.2%) | 17 (45.9%) | 0.81 |
| (a) | |||||
| Variables | Contrast | Hazard Ratio (95% CI) | p Value | Adjusted Hazard Ratio | p Value |
| Age | ≥65 vs. <65 | 0.78 (0.44, 1.39) | 0.40 | ||
| Sex | Male vs. Female | 0.76 (0.41, 1.41) | 0.38 | ||
| Systemic therapy | L+P vs. A+B | 0.75 (0.41, 1.37) | 0.35 | 0.79 (0.44, 1.45) | 0.45 |
| Viral etiology | Yes vs. No | 0.91 (0.47, 1.76) | 0.78 | ||
| Child–Pugh class | B vs. A | 2.21 (1.11, 4.39) | 0.02 | 1.67 (0.82, 3.44) | 0.16 |
| ALBI grade | II/III vs. I | 1.90 (1.09, 3.31) | 0.02 | ||
| BCLC | C vs. B | 2.33 (1.09, 5.00) | 0.03 | ||
| Seven criteria | Beyond vs. Within | 1.78 (0.71, 4.46) | 0.22 | ||
| Vp4 stage | Yes vs. No | 2.32 (1.31, 4.09) | <0.01 | 2.08 (1.17, 3.72) | 0.01 |
| Extrahepatic spread | Yes vs. No | 1.12 (0.66, 1.88) | 0.68 | ||
| AFP > 400 | Yes vs. No | 1.77 (1.03, 3.04) | 0.04 | 1.58 (0.90, 2.76) | 0.11 |
| Early AFP response | Yes vs. No | 0.58 (0.33, 1.02) | 0.06 | ||
| Combine LRT | Yes vs. No | 0.82 (0.48, 1.40) | 0.47 | ||
| (b) | |||||
| Variables | Contrast | Hazard Ratio (95% CI) | p Value | Adjusted Hazard Ratio | p Value |
| Age | ≥65 vs. <65 | 0.65 (0.40, 1.08) | 0.10 | ||
| Sex | Male vs. Female | 1.15 (0.64, 2.06) | 0.65 | ||
| Systemic therapy | L+P vs. A+B | 0.92 (0.55, 1.53) | 0.75 | ||
| Viral etiology | Yes vs. No | 0.84 (0.47, 1.51) | 0.57 | ||
| Child–Pugh class | B vs. A | 2.10 (1.13, 3.93) | 0.02 | 2.77 (1.45, 5.29) | <0.01 |
| ALBI grade | II/III vs. I | 1.54 (0.95, 2.50) | 0.08 | ||
| BCLC | C vs. B | 2.02 (1.05, 3.86) | 0.03 | ||
| Seven criteria | Beyond vs. Within | 1.34 (0.64, 2.80) | 0.43 | ||
| Vp4 stage | Yes vs. No | 1.58 (0.95, 2.64) | 0.08 | ||
| Extrahepatic spread | Yes vs. No | 1.01 (0.63, 1.60) | 0.97 | ||
| AFP > 400 | Yes vs. No | 1.64 (1.02, 2.64) | 0.04 | ||
| Early AFP response | Yes vs. No | 0.40 (0.24, 0.68) | <0.001 | 0.40 (0.24, 0.67) | <0.001 |
| Combine LRT | Yes vs. No | 0.57 (0.35, 0.92) | 0.02 | 0.56 (0.34, 0.92) | 0.02 |
| Lenvatinib Plus Pembrolizumab (n = 37) | Atezolizumab Plus Bevacizumab (n = 84) | |||
|---|---|---|---|---|
| Adverse events, n (%) | Any grade | Grade 3 or above | Any grade | Grade 3 or above |
| Any adverse events | 30 (81.1%) | 10 (27.0%) | 62 (73.8%) | 16 (19.0%) |
| Diarrhea | 9 (24.3%) | 2 (5.4%) | 7 (8.3%) | 0 (0.0%) |
| Anorexia | 6 (16.2%) | 0 (0.0%) | 10 (11.9%) | 0 (0.0%) |
| Fatigue | 4 (10.8%) | 0 (0.0%) | 7 (8.3%) | 0 (0.0%) |
| Elevated aspartate aminotransferase | 10 (27.0%) | 1 (2.7%) | 32 (38.1%) | 3 (3.6%) |
| Elevated alanine transaminase | 12 (32.4%) | 1 (2.7%) | 23 (27.4%) | 1 (1.2%) |
| Rash | 5 (13.5%) | 1 (2.7%) | 7 (8.3%) | 1 (1.2%) |
| Esophageal variceal bleeding | 1 (2.7%) | 1 (2.7%) | 9 (10.7%) | 9 (10.7%) |
| Palmar-plantar erythrodysesthesia | 8 (21.6%) | 3 (8.1%) | 0 (0.0%) | 0 (0.0%) |
| Hypertension | 5 (13.5%) | 1 (2.7%) | 9 (10.7%) | 0 (0.0%) |
| Proteinuria | 13 (35.1%) | 1 (2.7%) | 10 (11.9%) | 0 (0.0%) |
| Adrenal insufficiency | 0 (0.0%) | 0 (0.0%) | 4 (4.8%) | 0 (0.0%) |
| Sepsis | 2 (5.4%) | 2 (5.4%) | 2 (2.4%) | 2 (2.4) |
| Nausea | 1 (2.7%) | 0 (0.0%) | 2 (2.4%) | 0 (0.0%) |
| Dysphonia | 2 (5.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Pancreatitis | 1 (2.7%) | 0 (0.0%) | 1 (1.2%) | 0 (0.0%) |
| Acute kidney injury | 0 (0.0%) | 0 (0.0%) | 1 (1.2%) | 0 (0.0%) |
| Pneumonitis | 1 (2.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Lin, P.-T.; Teng, W.; Hsieh, Y.-C.; Chen, W.-T.; Su, C.-W.; Wang, C.-T.; Chai, P.-M.; Lin, C.-C.; Lin, C.-Y.; et al. Comparing Lenvatinib/Pembrolizumab with Atezolizumab/Bevacizumab in Unresectable Hepatocellular Carcinoma: A Real-World Experience with Propensity Score Matching Analysis. Cancers 2024, 16, 3458. https://doi.org/10.3390/cancers16203458
Hsu Y-C, Lin P-T, Teng W, Hsieh Y-C, Chen W-T, Su C-W, Wang C-T, Chai P-M, Lin C-C, Lin C-Y, et al. Comparing Lenvatinib/Pembrolizumab with Atezolizumab/Bevacizumab in Unresectable Hepatocellular Carcinoma: A Real-World Experience with Propensity Score Matching Analysis. Cancers. 2024; 16(20):3458. https://doi.org/10.3390/cancers16203458
Chicago/Turabian StyleHsu, Yu-Chun, Po-Ting Lin, Wei Teng, Yi-Chung Hsieh, Wei-Ting Chen, Chung-Wei Su, Ching-Ting Wang, Pei-Mei Chai, Chen-Chun Lin, Chun-Yen Lin, and et al. 2024. "Comparing Lenvatinib/Pembrolizumab with Atezolizumab/Bevacizumab in Unresectable Hepatocellular Carcinoma: A Real-World Experience with Propensity Score Matching Analysis" Cancers 16, no. 20: 3458. https://doi.org/10.3390/cancers16203458
APA StyleHsu, Y.-C., Lin, P.-T., Teng, W., Hsieh, Y.-C., Chen, W.-T., Su, C.-W., Wang, C.-T., Chai, P.-M., Lin, C.-C., Lin, C.-Y., & Lin, S.-M. (2024). Comparing Lenvatinib/Pembrolizumab with Atezolizumab/Bevacizumab in Unresectable Hepatocellular Carcinoma: A Real-World Experience with Propensity Score Matching Analysis. Cancers, 16(20), 3458. https://doi.org/10.3390/cancers16203458






