Clinical and Histopathological Features of Thyroid Cancer with TERT Promoter Molecular Alterations in Isolation Versus with Concurrent Molecular Alterations: A Multicenter Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. FNAC and Molecular Testing
2.3. Definition of High Risk
2.4. Definition of Aggressive Histology
2.5. Data Collection
2.6. Data Analysis
3. Results
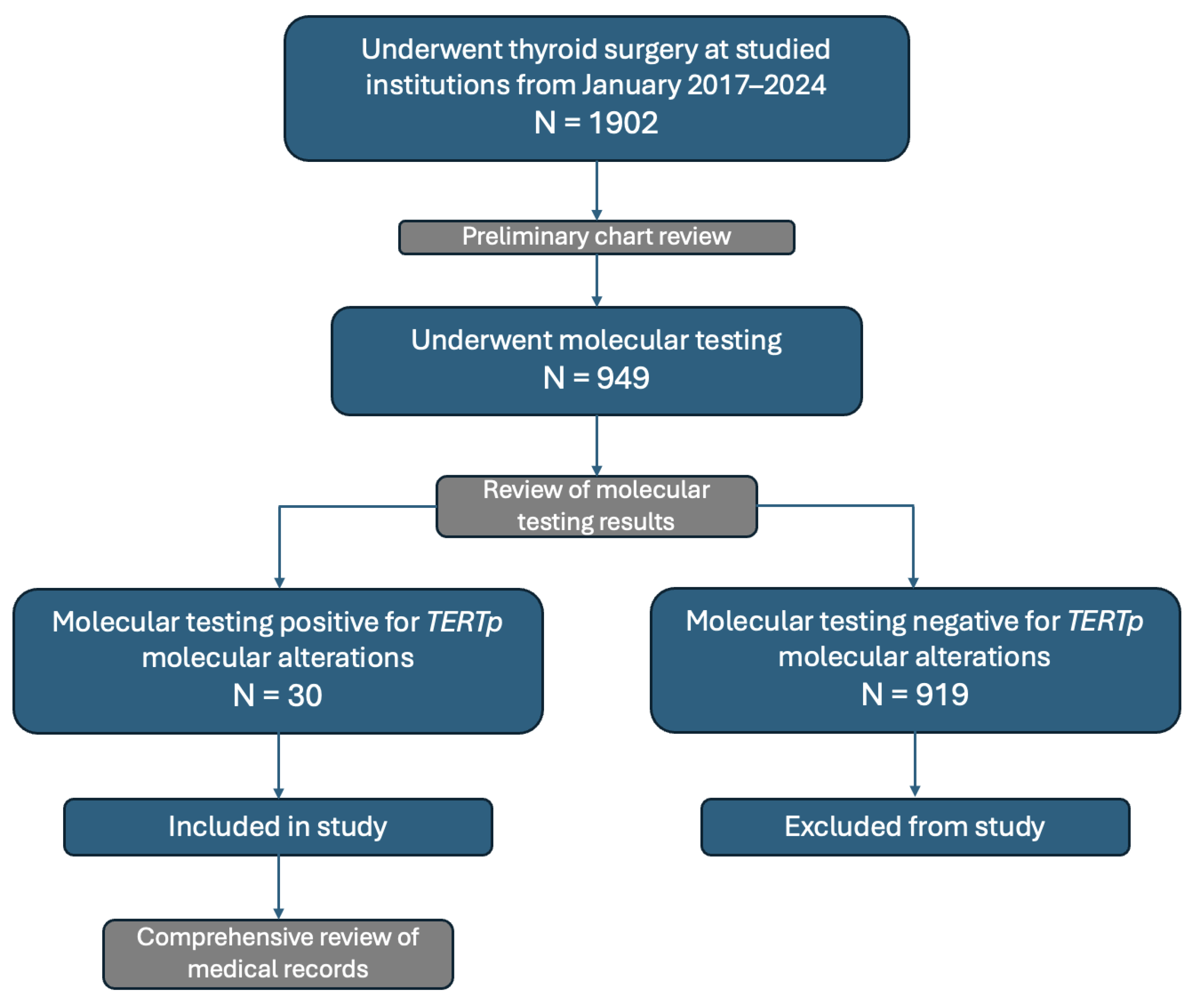
3.1. Participant Recruitment and Patient Demographics
3.2. Descriptive Statistics of TERTp Molecular Profile, Histopathological Features, and Treatment
3.3. Association between TERTp Molecular Profile and Demographics and Nodule Size
3.4. Association between TERTp Molecular Profile and Bethesda Classification
3.5. Effect of Molecular Profile on Risk Stratification
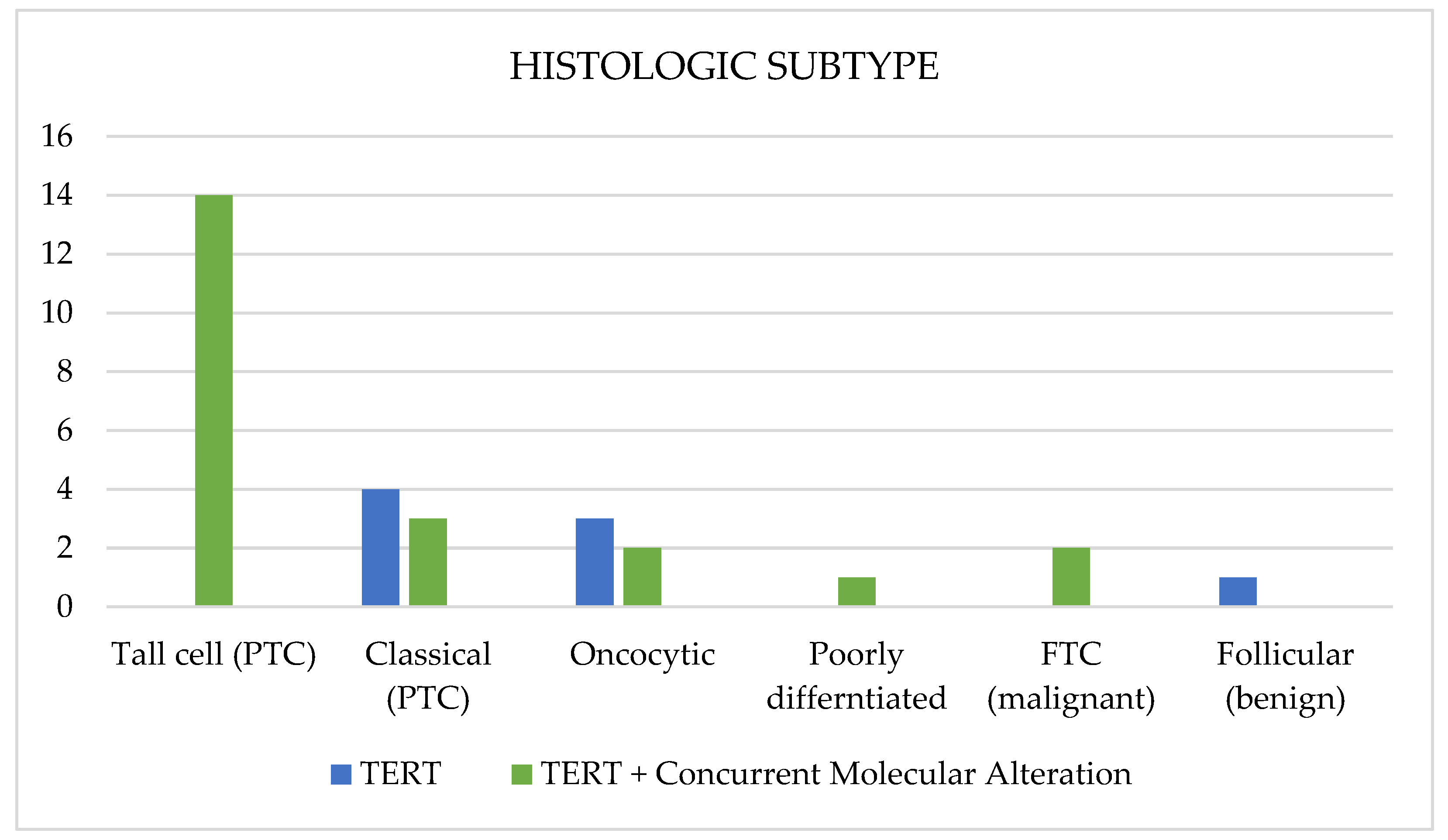
3.6. Association between TERTp Molecular Profile and Histological Subtype
3.7. Effect of Molecular Profile on Gross ETE
3.8. Effect of Molecular Profile on Distant Metaseses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Streinu, D.R.; Neagoe, O.C.; Borlea, A.; Icma, I.; Derban, M.; Stoian, D. Enhancing diagnostic precision in thyroid nodule assessment: Evaluating the efficacy of a novel cell preservation technique in fine-needle aspiration cytology. Front. Endocrinol. 2024, 15, 1438063. [Google Scholar] [CrossRef]
- Maqbali, T.A.; Tedla, M.; Weickert, M.O.; Mehanna, H. Malignancy Risk Analysis in Patients with Inadequate Fine Needle Aspiration Cytology (FNAC) of the Thyroid. PLoS ONE 2012, 7, e49078. [Google Scholar] [CrossRef]
- Ceresini, G.; Corcione, L.; Morganti, S.; Milli, B.; Bertone, L.; Prampolini, R.; Petrazzoli, S.; Saccani, M.; Ceda, G.P.; Valenti, G. Ultrasound-Guided Fine-Needle Capillary Biopsy of Thyroid Nodules, Coupled with On-Site Cytologic Review, Improves Results. Thyroid 2004, 14, 385–389. [Google Scholar] [CrossRef]
- Rossi, E.D.; Vielh, P. Thyroid and Molecular Testing. Advances in Thyroid Molecular Cytopathology. J. Mol. Pathol. 2021, 2, 77–92. [Google Scholar] [CrossRef]
- Low, K.C.; Tergaonkar, V. Telomerase: Central regulator of all of the hallmarks of cancer. Trends Biochem. Sci. 2013, 38, 426–434. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocr. Int. J. Basic Clin. Endocrinol. 2019, 67, 44–57. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, K.; Park, H.; Ki, C.-S.; Oh, Y.L.; Shin, J.H.; Kim, J.S.; Kim, S.W.; Chung, J.H.; et al. TERT promoter mutations and the 8th Edition TNM Classification in Predicting the Survival of Thyroid Cancer Patients. Cancers 2021, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Alohali, S.; Payne, A.E.; Pusztaszeri, M.; Rajab, M.; Forest, V.-I.; Hier, M.P.; Tamilia, M.; Payne, R.J. Effect of Having Concurrent Mutations on the Degree of Aggressiveness in Patients with Thyroid Cancer Positive for TERT Promoter Mutations. Cancers 2023, 15, 413. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer-Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and Their Association with BRAF V600E Mutation and Aggressive Clinicopathological Characteristics of Thyroid Cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130–E1136. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Jia, X.; Hu, X.; Pang, P.; Zhao, S.; Wang, Y.; Wang, J.; Zhang, Y.; Lyu, Z. The coexistence of genetic mutations in thyroid carcinoma predicts histopathological factors associated with a poor prognosis: A systematic review and network meta-analysis. Front. Oncol. 2020, 10, 540238. [Google Scholar] [CrossRef]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A.; et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J.; et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef]
- Mehta, V.; Nikiforov, Y.E.; Ferris, R.L. Use of molecular biomarkers in FNA specimens to personalize treatment for thyroid surgery. Head Neck 2013, 35, 1499–1506. [Google Scholar] [CrossRef]
- Bhaijee, F.; Nikiforov, Y.E. Molecular Analysis of Thyroid Tumors. Endocr. Pathol. 2011, 22, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mascarella, M.A.; Peeva, M.; Forest, V.-I.; Pusztaszeri, M.P.; Avior, G.; Tamilia, M.; Mlynarek, A.M.; Hier, M.P.; Payne, R.J. Association of Bethesda category and molecular mutation in patients undergoing thyroidectomy. Clin. Otolaryngol. 2022, 47, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Dasyam, A.K.; Carty, S.E.; Nikiforova, M.N.; Ohori, N.P.; Armstrong, M.; Yip, L.; LeBeau, S.O.; McCoy, K.L.; Coyne, C.; et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J. Clin. Endocrinol. Metab. 2013, 98, E914–E922. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.L.; Schmidt, A.; Ghuzlan, A.A.; Lacroix, L.; Vathaire, F.d.; Chevillard, S.; Schlumberger, M. Radiation exposure and thyroid cancer: A review. Arch. Endocrinol. Metab. 2017, 61, 180–187. [Google Scholar] [CrossRef]
- Liu, R.; Xing, M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr.-Relat. Cancer 2014, 21, 825–830. [Google Scholar] [CrossRef]
| n | Frequency (%) | ||
|---|---|---|---|
| Age, mean | 66.43 | ||
| Sex | Female | 23 | 76.7 |
| Male | 7 | 23.3 | |
| Histological subtype | Tall-cell (PTC) | 14 | 46.7 |
| Classical (PTC) | 7 | 23.3 | |
| Follicular (benign) | 1 | 3.3 | |
| Follicular (FTC) | 2 | 6.7 | |
| Oncocytic | 5 | 16.7 | |
| Poorly differentiated | 1 | 3.3 | |
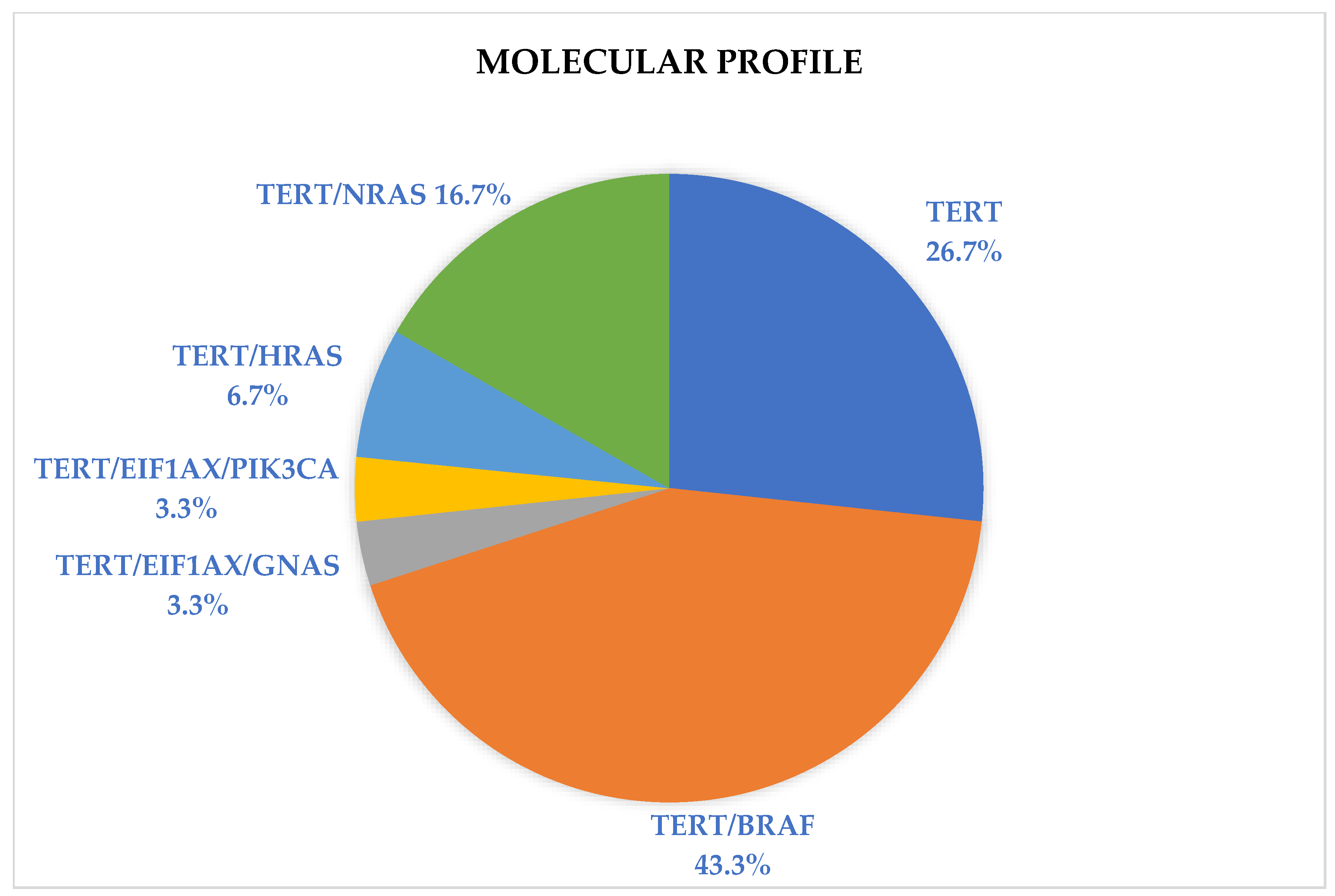
| Molecular profile | TERTp | 8 | 26.7 |
| TERTp/BRAF | 13 | 43.3 | |
| TERTp/RAS | 7 | 23.4 | |
| TERTp/EIF1AX/GNAS | 1 | 3.3 | |
| TERTp/EIF1AX/PIK3CA | 1 | 3.3 | |
| Bethesda classification | Bethesda III | 5 | 16.7 |
| Bethesda IV | 10 | 33.3 | |
| Bethesda V | 1 | 3.3 | |
| Bethesda VI | 14 | 46.7 | |
| Positive lymph nodes, mean | 1.70 | ||
| Nodule size, mean | 3.663 | ||
| Final pathology | Benign | 1 | 3.3 |
| Malignant | 29 | 96.7 | |
| High risk | No | 14 | 46.7 |
| Yes | 16 | 53.3 | |
| Surgical procedure | Total thyroidectomy | 26 | 86.7 |
| Hemi-thyroidectomy | 4 | 13.3 | |
| Other treatment | Radioactive iodine | 20 | 66.7 |
| External beam radiation | 3 | 10 | |
| Targeted treatment | 3 | 10 | |
| Molecular Profile | Age | Bethesda Classification | High Risk | Histology | Nodule Size (cm) | Positive Lymph Nodes |
|---|---|---|---|---|---|---|
| TERTP | 75 | IV | No | Follicular (benign) | 2.6 | 0 |
| TERTP | 71 | III | No | Classical PTC | 4.6 | 0 |
| TERTP | 83 | IV | No | Classical PTC | 5 | 0 |
| TERTP | 82 | III | Yes | Oncocytic | 2.8 | 0 |
| TERTP | 75 | IV | No | Classical PTC | 2.3 | 0 |
| TERTP | 75 | VI | No | Oncocytic | 7.8 | 0 |
| TERTP | 82 | IV | No | Oncocytic | 4 | 0 |
| TERTP | 58 | IV | No | Classical PTC | 4 | 3 |
| TERTP/BRAF V600E | 68 | VI | Yes | Tall-cell PTC | 2.2 | 0 |
| TERTP/BRAF V600E | 78 | VI | Yes | Tall-cell PTC | 3.4 | 4 |
| TERTP/BRAF V600E | 73 | VI | Yes | Tall-cell PTC | 5.2 | 5 |
| TERTP/BRAF V600E | 53 | VI | Yes | Tall-cell PTC | 2.3 | 1 |
| TERTP/BRAF V600E | 67 | VI | Yes | Tall-cell PTC | 1.3 | 0 |
| TERTP/BRAF V600E | 56 | VI | Yes | Tall-cell PTC | 5 | 1 |
| TERTP/BRAF V600E | 70 | V | Yes | Tall-cell PTC | 5.6 | 2 |
| TERTP/BRAF V600E | 65 | VI | Yes | Tall-cell PTC | 1.9 | 2 |
| TERTP/BRAF V600E | 58 | VI | Yes | Tall-cell PTC | 2.8 | 1 |
| TERTP/BRAF V600E | 71 | VI | Yes | Tall-cell PTC | 3.5 | 1 |
| TERTP/BRAF V600E | 75 | VI | Yes | Tall-cell PTC | 2.6 | 10 |
| TERTP/BRAF V600E | 53 | VI | No | Tall-cell PTC | 2 | 11 |
| TERTP/BRAF V600E | 86 | VI | No | Tall-cell PTC | 1.1 | 0 |
| TERTP/HRAS | 53 | IV | Yes | Tall-cell PTC | 4 | 4 |
| TERTP/HRAS | 57 | III | No | FTC | 4.2 | 0 |
| TERTP/NRAS | 66 | IV | No | Classical PTC | 3.4 | 0 |
| TERTP/NRAS | 53 | IV | Yes | Classical PTC | 6 | 0 |
| TERTP/NRAS | 53 | VI | Yes | Classical PTC | 6.5 | 6 |
| TERTP/NRAS | 52 | IV | Yes | Oncocytic | 5.1 | 0 |
| TERTP/NRAS | 45 | III | No | FTC | 2 | 0 |
| TERTP/EIF1AX/GNAS | 64 | IV | No | Poorly differentiated | 2.7 | 0 |
| TERTP/EIF1AX/PIK3CA | 76 | III | No | Oncocytic | 4 | 0 |
| TERTp Alone (n = 8) | TERTp + Concurrent Molecular Alteration (n = 22) | Pearson Chi-Squared | ||
|---|---|---|---|---|
| Bethesda classification | BIII | 2 (25.0%) | 3 (13.6%) | p = 0.097 |
| BIV | 5 (62.5%) | 5 (22.7%) | ||
| BV | 0 (0%) | 1 (4.5%) | ||
| BVI | 1 (12.5%) | 13 (59.1%) | ||
| Final pathology | Benign | 1 (12.5%) | 0 (0%) | p = 0.092 |
| Malignant | 7 (87.5%) | 22 (100%) | ||
| High risk | No | 7 (87.5%) | 7 (31.82%) | p = 0.006 |
| Yes | 1 (12.5%) | 15 (68.18%) | ||
| Aggressive histology | No | 8 (100%) | 7 (31.82%) | p = 0.003 |
| Yes | 0 (0%) | 15 (68.18%) | ||
| Gross extrathyroidal extension | No | 8 (100%) | 13 (59.1%) | p = 0.097 |
| Yes | 0 (0%) | 8 (36.4%) | ||
| Not reported | 0 (0%) | 1 (4.5%) | ||
| Distant metastases | No | 7 (87.5%) | 12 (54.5%) | p = 0.192 |
| Yes | 0 (0%) | 6 (27.3%) | ||
| Not reported | 1 (12.5%) | 4 (18.2%) |
| TERTp/BRAF (n = 13) | TERTp/RAS (n = 7) | TERTp/EIF1AX/GNAS (n = 1) | TERTp/EIF1AX/PIK3CA (n = 1) | ||
|---|---|---|---|---|---|
| Bethesda classification | BIII | 0 (0%) | 2 (28.6%) | 0 (0%) | 1 (100%) |
| BIV | 0 (0%) | 4 (57.1%) | 1 (100%) | 0 (0%) | |
| BV | 1 (7.7%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| BVI | 12 (92.3%) | 1 (14.3%) | 0 (0%) | 0 (0%) | |
| Final pathology | Tall-cell (PTC) | 13 (100%) | 1 (14.3%) | 0 (0%) | 0 (0%) |
| Classical (PTC) | 0 (0%) | 3 (42.9%%) | 0 (0%) | 0 (0%) | |
| FTC | 0 (0%) | 2 (28.5%) | 0 (0%) | 0 (0%) | |
| Oncocytic | 0 (0%) | 1 (14.3%) | 0 (0%) | 1 (100%) | |
| Poorly differentiated | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | |
| High risk | No | 2 (15.4%) | 3 (42.9%) | 1 (100%) | 1 (100%) |
| Yes | 11 (84.6%) | 4 (57.1%) | 0 (0%) | 0 (0%) | |
| Aggressive histology | No | 0 (0%) | 6 (85.7%) | 0 (0%) | 1 (100%) |
| Yes | 13 (100%) | 1 (14.3%) | 1 (100%) | 0 (0%) | |
| Gross Extrathyroidal extension | No | 6 (46.15%) | 5 (71.4%) | 1 (100%) | 1 (100%) |
| Yes | 6 (46.15%) | 2 (28.6%) | 0 (0%) | 0 (0%) | |
| Not reported | 1 (7.7%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Distant metastases | No | 7 (53.8%) | 4 (57.1%) | 0 (0%) | 1 (100%) |
| Yes | 3 (23.1%) | 3 (42.9%) | 0 (0%) | 0 (0%) | |
| Not reported | 3 (23.1%) | 0 (0%) | 1 (100%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinberg, E.; Dimitstein, O.; Morand, G.B.; Forest, V.-I.; da Silva, S.D.; Pusztaszeri, M.; Alohali, S.; Payne, R.J. Clinical and Histopathological Features of Thyroid Cancer with TERT Promoter Molecular Alterations in Isolation Versus with Concurrent Molecular Alterations: A Multicenter Retrospective Study. Cancers 2024, 16, 3446. https://doi.org/10.3390/cancers16203446
Steinberg E, Dimitstein O, Morand GB, Forest V-I, da Silva SD, Pusztaszeri M, Alohali S, Payne RJ. Clinical and Histopathological Features of Thyroid Cancer with TERT Promoter Molecular Alterations in Isolation Versus with Concurrent Molecular Alterations: A Multicenter Retrospective Study. Cancers. 2024; 16(20):3446. https://doi.org/10.3390/cancers16203446
Chicago/Turabian StyleSteinberg, Emily, Orr Dimitstein, Grégoire B. Morand, Véronique-Isabelle Forest, Sabrina D. da Silva, Marc Pusztaszeri, Sama Alohali, and Richard J. Payne. 2024. "Clinical and Histopathological Features of Thyroid Cancer with TERT Promoter Molecular Alterations in Isolation Versus with Concurrent Molecular Alterations: A Multicenter Retrospective Study" Cancers 16, no. 20: 3446. https://doi.org/10.3390/cancers16203446
APA StyleSteinberg, E., Dimitstein, O., Morand, G. B., Forest, V.-I., da Silva, S. D., Pusztaszeri, M., Alohali, S., & Payne, R. J. (2024). Clinical and Histopathological Features of Thyroid Cancer with TERT Promoter Molecular Alterations in Isolation Versus with Concurrent Molecular Alterations: A Multicenter Retrospective Study. Cancers, 16(20), 3446. https://doi.org/10.3390/cancers16203446








