MUC16 Retention after Neoadjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Ethics
2.2. Acquisition of Normal Pancreatic Tissue
2.3. Acquisition of Pancreatic Ductal Adenocarcinoma Patient Sample Slides
2.4. Immunohistochemistry and Hematoxylin and Eosin Staining of Samples
2.5. Sample Analysis by Pathologist
2.6. Statistical Analysis
3. Results
3.1. Patient Sample Characteristics
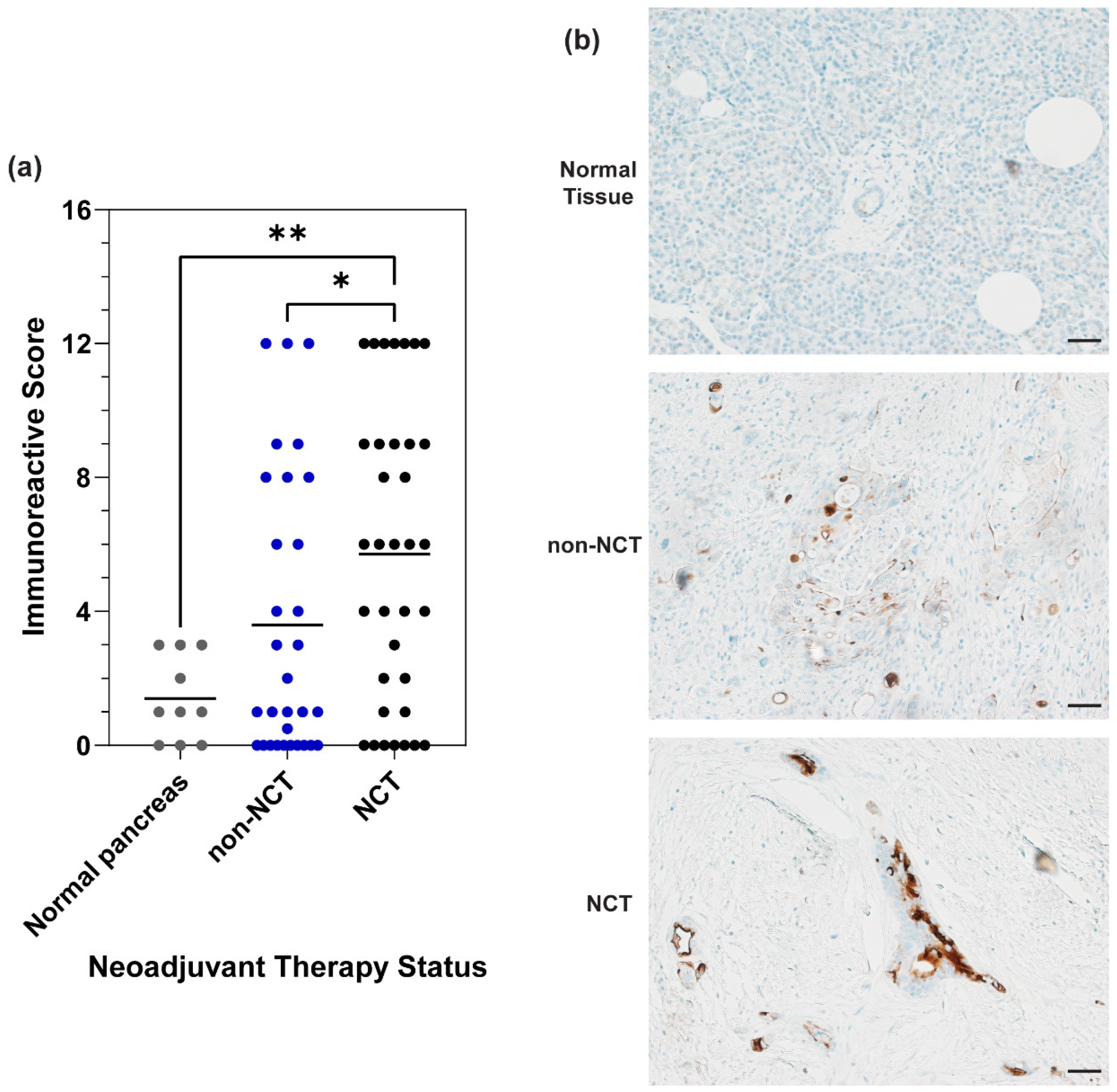
3.2. MUC16 Expression in PDAC Patient Samples
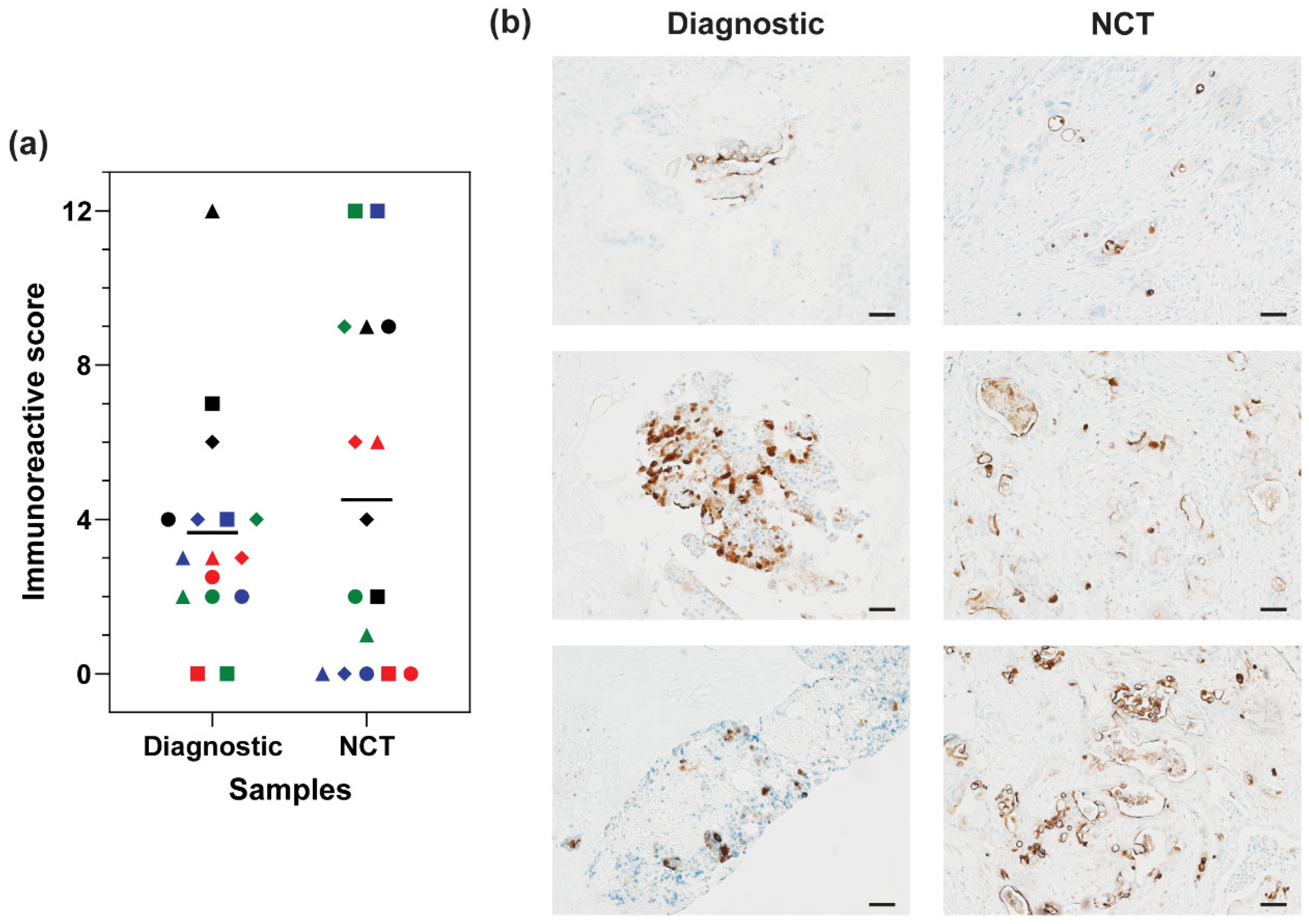
3.3. MUC16 Retention after Chemotherapy Treatment
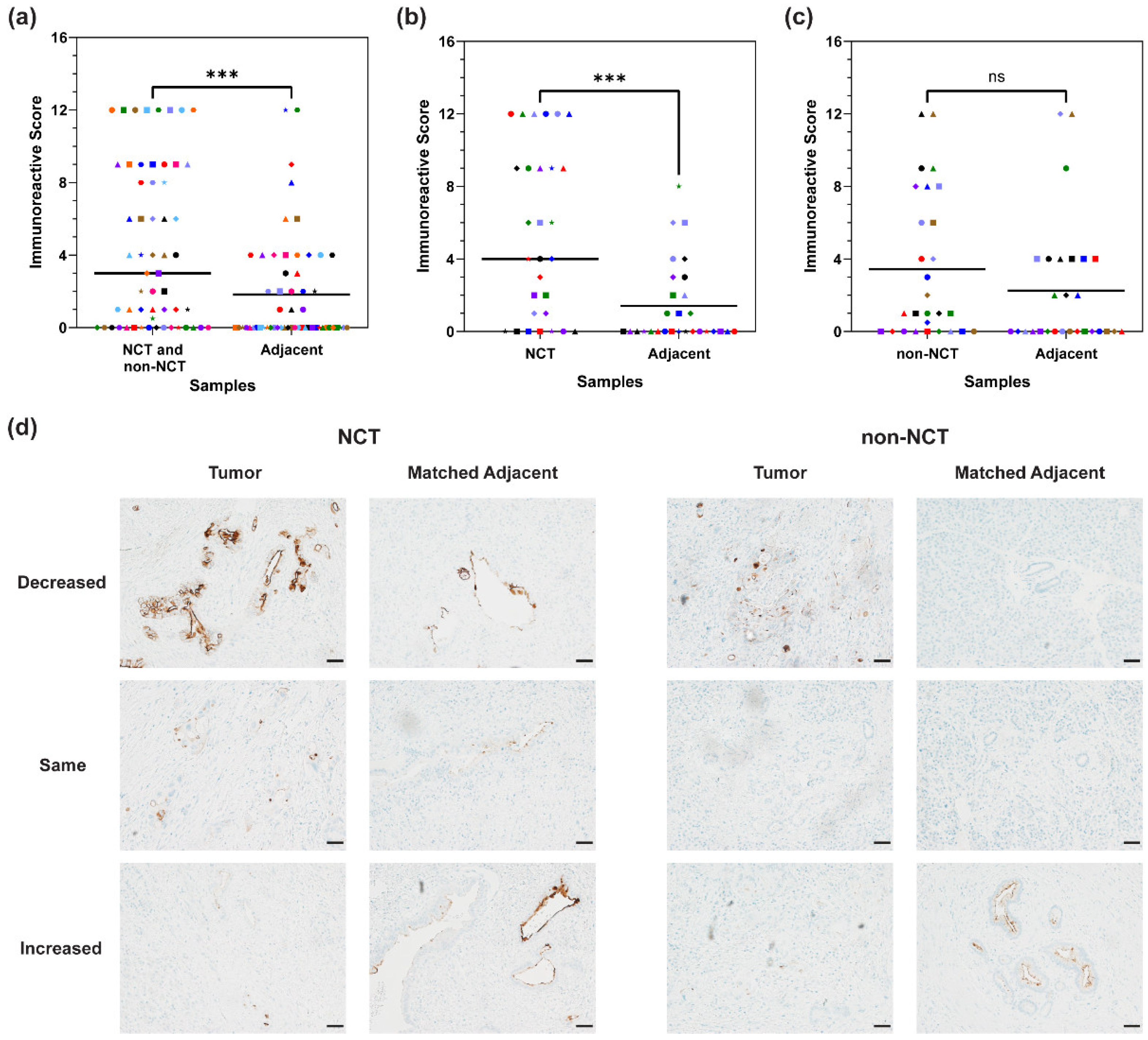
3.4. MUC16 Expression in Matched Adjacent Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Shen, C.; Santry, H.; Bridges, J.; Dillhoff, M.; Ejaz, A.; Pawlik, T.M.; Tsung, A. Disparities in the use of neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma. J. Natl. Compr. Cancer Netw. 2020, 18, 556–563. [Google Scholar] [CrossRef]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Damarla, V.; Seixas, T.; Dobrosotskaya, I.; Wollner, I.; Kwon, D.; Winters, K.; Raoufi, M.; Li, J.; Siddiqui, F.; et al. Neoadjuvant phase II trial of chemoradiotherapy in patients with resectable and borderline resectable pancreatic cancer. Am. J. Clin. Oncol. 2020, 43, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.T.; Aguilar, E.N.; Brooks, C.L.; Isder, C.C.; Muilenburg, K.M.; Talmon, G.A.; Ly, Q.P.; Carlson, M.A.; Hollingsworth, M.A.; Mohs, A.M. Preclinical evaluation of a humanized, near-infrared fluorescent antibody for fluorescence-guided surgery of MUC16-expressing pancreatic cancer. Mol. Pharm. 2022, 19, 3586–3599. [Google Scholar] [CrossRef]
- Tringale, K.R.; Pang, J.; Nguyen, Q.T. Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1412. [Google Scholar] [CrossRef]
- de Gooyer, J.M.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.S.; Frielink, C.; Desar, I.M.E.; de Wilt, J.H.W.; Flucke, U.; Rijpkema, M. Immunohistochemical selection of biomarkers for tumor-targeted image-guided surgery of myxofibrosarcoma. Sci. Rep. 2020, 10, 2915. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Groen, J.V.; Sibinga Mulder, B.G.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; van de Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.; et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br. J. Surg. 2019, 106, 1055–1065. [Google Scholar] [CrossRef]
- Rau, B.M.; Moritz, K.; Schuschan, S.; Alsfasser, G.; Prall, F.; Klar, E. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery 2012, 152, S103–S111. [Google Scholar] [CrossRef]
- Alfano, M.S.; Garnier, J.; Palen, A.; Ewald, J.; Piana, G.; Poizat, F.; Mitry, E.; Delpero, J.R.; Turrini, O. Peak Risk of Recurrence Occurs during the First Two Years after a Pancreatectomy in Patients Receiving Neoadjuvant FOLFIRINOX. Cancers 2023, 15, 5151. [Google Scholar] [CrossRef]
- Olson, M.T.; Ly, Q.P.; Mohs, A.M. Fluorescence guidance in surgical oncology: Challenges, opportunities, and translation. Mol. Imaging Biol. 2019, 21, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Ghaneh, P.; Palmer, D.; Cicconi, S.; Jackson, R.; Halloran, C.M.; Rawcliffe, C.; Sripadam, R.; Mukherjee, S.; Soonawalla, Z.; Wadsley, J.; et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): A four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The current treatment paradigm for pancreatic ductal adenocarcinoma and barriers to therapeutic efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef]
- Barenboim, A.; Lahat, G.; Geva, R.; Nachmany, I.; Nakache, R.; Goykhman, Y.; Brazowski, E.; Rosen, G.; Isakov, O.; Wolf, I.; et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: An intention to treat analysis. Eur. J. Surg. Oncol. 2018, 44, 1619–1623. [Google Scholar] [CrossRef]
- van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; van Santvoort, H.C.; van Tienhoven, G.; de Wilde, R.F.; Wilmink, J.W.; van Eijck, C.H.J.; Groot Koerkamp, B.; et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Villalobos, P.; Behrens, C.; Jiang, M.; Pataer, A.; Swisher, S.G.; William, W.N., Jr.; Zhang, J.; Lee, J.; Cascone, T.; et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J. Immunother. Cancer 2018, 6, 48. [Google Scholar] [CrossRef]
- Tummers, W.S.; Farina-Sarasqueta, A.; Boonstra, M.C.; Prevoo, H.A.; Sier, C.F.; Mieog, J.S.; Morreau, J.; van Eijck, C.H.; Kuppen, P.J.; van de Velde, C.J.; et al. Selection of optimal molecular targets for tumor-specific imaging in pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 56816–56828. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Turner, M.A.; Hollandsworth, H.M.; Nishino, H.; Amirfakhri, S.; Lwin, T.M.; Lowy, A.M.; Kaur, S.; Natarajan, G.; Mallya, K.; Hoffman, R.M.; et al. Fluorescent anti-MUC5AC brightly targets pancreatic cancer in a patient-derived orthotopic xenograft. In Vivo 2022, 36, 57–62. [Google Scholar] [CrossRef]
- Olson, M.T.; Wojtynek, N.E.; Talmon, G.A.; Caffrey, T.C.; Radhakrishnan, P.; Ly, Q.P.; Hollingsworth, M.A.; Mohs, A.M. Development of a MUC16-targeted near-infrared fluorescent antibody conjugate for intraoperative imaging of pancreatic cancer. Mol. Cancer Ther. 2020, 19, 1670–1681. [Google Scholar] [CrossRef]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.R.; Hasan, A.; Ertsey, R.; et al. Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef]
- Muilenburg, K.M.; Isder, C.C.; Radhakrishnan, P.; Batra, S.K.; Ly, Q.P.; Carlson, M.A.; Bouvet, M.; Hollingsworth, M.A.; Mohs, A.M. Mucins as contrast agent targets for fluorescence-guided surgery of pancreatic cancer. Cancer Lett. 2023, 561, 216150. [Google Scholar] [CrossRef]
- van Dam, M.A.; Vuijk, F.A.; Stibbe, J.A.; Houvast, R.D.; Luelmo, S.A.C.; Crobach, S.; Shahbazi Feshtali, S.; de Geus-Oei, L.F.; Bonsing, B.A.; Sier, C.F.M.; et al. Overview and future perspectives on tumor-targeted positron emission tomography and fluorescence imaging of pancreatic cancer in the era of neoadjuvant therapy. Cancers 2021, 13, 6088. [Google Scholar] [CrossRef]
- Gutowski, M.; Framery, B.; Boonstra, M.C.; Garambois, V.; Quenet, F.; Dumas, K.; Scherninski, F.; Cailler, F.; Vahrmeijer, A.L.; Pelegrin, A. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017, 26, 153–162. [Google Scholar] [CrossRef]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Sibinga Mulder, B.G.; Mieog, J.S.D.; Swijnenburg, R.J.; van de Velde, C.J.H.; Farina Sarasqueta, A.; Bonsing, B.A.; Framery, B.; Pelegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357. [Google Scholar] [CrossRef]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef]
- Mulder, B.G.S.; Koller, M.; Duiker, E.W.; Sarasqueta, A.F.; Burggraaf, J.; Meijer, V.E.; Vahrmeijer, A.L.; Hoogwater, F.J.H.; Bonsing, B.A.; van Dam, G.M.; et al. Intraoperative Molecular Fluorescence Imaging of Pancreatic Cancer by Targeting Vascular Endothelial Growth Factor: A Multicenter Feasibility Dose-Escalation Study. J. Nucl. Med. 2023, 64, 82–89. [Google Scholar] [CrossRef]
- Vahrmeijer, A. FLUOPANC-Trial—Fluorescence-Guided Surgery of Pancreatic and Bileduct Tumors Using cRGD-ZW800-1 (FLUOPANC); Leiden University Medical Center: Leiden, The Netherlands, 2024. [Google Scholar]
- Rehman, S.; Brennan, P.M.; Lilienkampf, A.; Bradley, M. Approved and investigational fluorescent optical imaging agents for disease detection in surgery. Int. J. Surg. 2023, 109, 2378–2387. [Google Scholar] [CrossRef]
- Fujita, K.; Urano, Y. Activity-Based Fluorescence Diagnostics for Cancer. Chem. Rev. 2024, 124, 4021–4078. [Google Scholar] [CrossRef]
- Anand, A.; Satapathy, P.; Sharma, R.K.; Sharma, D.; Arora, M.; Khatib, M.N.; Gaidhane, S.; Zahiruddin, Q.S.; Rustagi, S. Pegulicianine: A game changer for image-guided breast cancer surgery. Int. J. Surg. Open 2024, 62, 452–453. [Google Scholar] [CrossRef]
- Haridas, D.; Chakraborty, S.; Ponnusamy, M.P.; Lakshmanan, I.; Rachagani, S.; Cruz, E.; Kumar, S.; Das, S.; Lele, S.M.; Anderson, J.M.; et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS ONE 2011, 6, e26839. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.M.; Vincent, A.; Mukherjee, R.; Campbell, N.R.; Chen, S.H.; Konstantopoulos, K.; Goggins, M.G.; Van Seuningen, I.; Maitra, A.; Montgomery, E.A. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum. Pathol. 2012, 43, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, L.; Stroppa, E.M.; Citterio, C.; Mordenti, P.; Di Nunzio, C.; Peveri, S.; Orlandi, E.; Vecchia, S. Modified FOLFIRINOX for unresectable locally advanced/metastatic pancreatic cancer. A real-world comparison of an attenuated with a full dose in a single center experience. Onco Targets Ther. 2019, 12, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Kasumova, G.G.; Verheij, J.; Najarian, R.M.; Maggino, L.; de Pastena, M.; Malleo, G.; Marchegiani, G.; Salvia, R.; Ng, S.C.; et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018, 153, e183617. [Google Scholar] [CrossRef]
- Shin, D.W.; Kim, J. The American Joint Committee on Cancer 8th edition staging system for the pancreatic ductal adenocarcinoma: Is it better than the 7th edition? Hepatobiliary Surg. Nutr. 2020, 9, 98–100. [Google Scholar] [CrossRef]
- Shi, S.; Hua, J.; Liang, C.; Meng, Q.; Liang, D.; Xu, J.; Ni, Q.; Yu, X. Proposed modification of the 8th edition of the AJCC staging system for pancreatic ductal adenocarcinoma. Ann. Surg. 2019, 269, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Roalso, M.; Aunan, J.R.; Soreide, K. Refined TNM-staging for pancreatic adenocarcinoma—Real progress or much ado about nothing? Eur. J. Surg. Oncol. 2020, 46, 1554–1557. [Google Scholar] [CrossRef]
- Boogerd, L.S.; van der Valk, M.J.; Boonstra, M.C.; Prevoo, H.A.; Hilling, D.E.; van de Velde, C.J.; Sier, C.F.; Farina Sarasqueta, A.; Vahrmeijer, A.L. Biomarker expression in rectal cancer tissue before and after neoadjuvant therapy. Onco Targets Ther. 2018, 11, 1655–1664. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Specht, E.; Kaemmerer, D.; Sanger, J.; Wirtz, R.M.; Schulz, S.; Lupp, A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 2015, 67, 368–377. [Google Scholar] [CrossRef]
- Travo, A.; Piot, O.; Wolthuis, R.; Gobinet, C.; Manfait, M.; Bara, J.; Forgue-Lafitte, M.E.; Jeannesson, P. IR spectral imaging of secreted mucus: A promising new tool for the histopathological recognition of human colonic adenocarcinomas. Histopathology 2010, 56, 921–931. [Google Scholar] [CrossRef]
- Trabbic, K.R.; Whalen, K.; Abarca-Heideman, K.; Xia, L.; Temme, J.S.; Edmondson, E.F.; Gildersleeve, J.C.; Barchi, J.J., Jr. A tumor-selective monoclonal antibody from immunization with a tumor-associated mucin glycopeptide. Sci. Rep. 2019, 9, 5662. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, K.S.; Jensen, H.; Clayton, N.P.; Herbert, R.A. Immunohistochemistry in investigative and toxicologic pathology. Toxicol. Pathol. 2018, 46, 488–510. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, F.; El-Shakankery, K.H. Desmoplasia and Biophysics in Pancreatic Ductal Adenocarcinoma: Can We Learn From Breast Cancer? Pancreas 2020, 49, 313–325. [Google Scholar] [CrossRef]
- Vuijk, F.A.; de Muynck, L.; Franken, L.C.; Busch, O.R.; Wilmink, J.W.; Besselink, M.G.; Bonsing, B.A.; Bhairosingh, S.S.; Kuppen, P.J.K.; Mieog, J.S.D.; et al. Molecular targets for diagnostic and intraoperative imaging of pancreatic ductal adenocarcinoma after neoadjuvant FOLFIRINOX treatment. Sci. Rep. 2020, 10, 16211. [Google Scholar] [CrossRef]
- de Geus, S.W.; Boogerd, L.S.; Swijnenburg, R.J.; Mieog, J.S.; Tummers, W.S.; Prevoo, H.A.; Sier, C.F.; Morreau, H.; Bonsing, B.A.; van de Velde, C.J.; et al. Selecting Tumor-Specific Molecular Targets in Pancreatic Adenocarcinoma: Paving the Way for Image-Guided Pancreatic Surgery. Mol. Imaging Biol. 2016, 18, 807–819. [Google Scholar] [CrossRef]
| No Prior Neoadjuvant Chemotherapy Treatment (Non-NCT) (%) (N = 31) | Neoadjuvant Chemotherapy Treatment (NCT) (%) (N = 35) | |
|---|---|---|
| Tumor grade | ||
| Well Differentiated | 6 (19.4%) | 9 (25.7%) |
| Moderately Differentiated | 15 (48.4%) | 16 (45.7%) |
| Poorly Differentiated | 10 (32.3%) | 10 (28.6%) |
| Primary tumor designation | ||
| T1 | 5 (16.1%) | 11 (31.4%) |
| T2 | 11 (35.5%) | 20 (57.1%) |
| T3 | 14 (45.2%) | 3 (8.6%) |
| T4 | 1 (3.2%) | 1 (2.9%) |
| Lymph node designation | ||
| N0 | 7 (22.6%) | 15 (42.9%) |
| N1 | 18 (58.1%) | 16 (45.7%) |
| N2 | 6 (19.4%) | 4 (11.4%) |
| Metastatic disease designation | ||
| MX | 4 (12.9%) | 7 (20%) |
| M0 | 26 (83.9%) | 28 (80%) |
| M1 | 1 (3.2%) | 0 (0%) |
| AJCC Stage | ||
| IA | 3 (9.7%) | 6 (17.1%) |
| IB | 2 (6.5%) | 10 (28.6%) |
| IIA | 11 (35.5%) | 14 (40%) |
| IIB | 10 (32.3%) | 4 (11.4%) |
| IIIA | 3 (9.7%) | 0 (0%) |
| IIIB | 1 (3.2%) | 1 (2.9%) |
| IV | 1 (3.2%) | 0 (0%) |
| Fibrosis classification | ||
| All tumor | 1 (3.2%) | 1 (2.9%) |
| More tumor than fibrosis | 20 (64.5%) | 9 (25.7%) |
| More fibrosis than tumor | 10 (32.3%) | 25 (71.4%) |
| All fibrosis | 0 (0%) | 0 (0%) |
| Neoadjuvant therapy treatment | ||
| FOLFIRINOX-based | NA | 14 (40%) |
| FOLFIRINOX + radiation | NA | 17 (48.6%) |
| Gemcitabine-based | NA | 4 (11.4%) |
| Grade of pathological response | ||
| No response | NA | 1 (2.86%) |
| Minimal response | NA | 14 (40%) |
| Moderate response | NA | 17 (48.57%) |
| Marked response | NA | 3 (8.57%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muilenburg, K.M.; Ehrhorn, E.G.; Olson, M.T.; Isder, C.C.; Klute, K.A.; Talmon, G.A.; Carlson, M.A.; Ly, Q.P.; Mohs, A.M. MUC16 Retention after Neoadjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma. Cancers 2024, 16, 3439. https://doi.org/10.3390/cancers16203439
Muilenburg KM, Ehrhorn EG, Olson MT, Isder CC, Klute KA, Talmon GA, Carlson MA, Ly QP, Mohs AM. MUC16 Retention after Neoadjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma. Cancers. 2024; 16(20):3439. https://doi.org/10.3390/cancers16203439
Chicago/Turabian StyleMuilenburg, Kathryn M., Evie G. Ehrhorn, Madeline T. Olson, Carly C. Isder, Kelsey A. Klute, Geoffrey A. Talmon, Mark A. Carlson, Quan P. Ly, and Aaron M. Mohs. 2024. "MUC16 Retention after Neoadjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma" Cancers 16, no. 20: 3439. https://doi.org/10.3390/cancers16203439
APA StyleMuilenburg, K. M., Ehrhorn, E. G., Olson, M. T., Isder, C. C., Klute, K. A., Talmon, G. A., Carlson, M. A., Ly, Q. P., & Mohs, A. M. (2024). MUC16 Retention after Neoadjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma. Cancers, 16(20), 3439. https://doi.org/10.3390/cancers16203439






